Abstract
Given the unpredictable rapid onset and ubiquitous consequences of weight gain induced by antipsychotics, there is a pressing need to get insights into the underlying processes at the brain system level that will allow stratification of “at risk” patients. The pathophysiological hypothesis at hand is focused on brain networks governing impulsivity that are modulated by neuro-inflammatory processes. To this aim, we investigated brain anatomy and functional connectivity in patients with early psychosis (median age: 23 years, IQR = 21–27) using anthropometric data and magnetic resonance imaging acquired one month to one year after initiation of AP medication. Our analyses included 19 patients with high and rapid weight gain (i.e., ≥5% from baseline weight after one month) and 23 patients with low weight gain (i.e., <5% from baseline weight after one month). We replicated our analyses in young (26 years, IQR = 22–33, N = 102) and middle-aged (56 years, IQR = 51–62, N = 875) healthy individuals from the general population. In early psychosis patients, higher weight gain was associated with poor impulse control score (β = 1.35; P = 0.03). Here, the observed brain differences comprised nodes of impulsivity networks - reduced frontal lobe grey matter volume (Pcorrected = 0.007) and higher striatal volume (Pcorrected = 0.048) paralleled by disruption of fronto-striatal functional connectivity (R = −0.32; P = 0.04). Weight gain was associated with the inflammatory biomarker plasminogen activator inhibitor-1 (β = 4.9, P = 0.002). There was no significant association between increased BMI or weight gain and brain anatomy characteristics in both cohorts of young and middle-aged healthy individuals. Our findings support the notion of weight gain in treated psychotic patients associated with poor impulse control, impulsivity-related brain networks and chronic inflammation.
Similar content being viewed by others
Introduction
Current advances in studying the drivers of antipsychotics-induced weight gain and associated metabolic dysfunction in patients with mental disorders brought evidence of a plethora of potential mechanisms. There is strong empirical evidence about the role of underlying psychopathology, genetic factors, pharmacological treatment, and/or lifestyle factors (i.e., unhealthy diet and/or lack of physical activity) [1, 2]. Studies report a 2 to 3 fold increase in mortality rates among psychiatric patients compared with the general population, corresponding to a 10- to-15-year reduction in life expectancy [3]. Two-thirds of the increased mortality risk is attributed to cardiovascular disease [4]. Patients treated with psychotropic drugs (all antipsychotics, some antidepressants and mood stabilizers) frequently show disproportional weight gain, which can affect their psychological well-being leading to treatment interruption and to a relapse of the illness [5]. Additionally, there is the notion that antipsychotics change the appetite regulation (i.e., excessive food consumption) with a large interindividual variation in the susceptibility to such effects [6, 7].
Obesity [8,9,10,11], binge eating disorder [12] and food addiction [8, 13] are associated with impaired impulse control. Grey matter (GM) volume loss and neural processing biases across network nodes involved in impulse inhibition, such as the striatum and frontal lobe, are among the most frequent observations in structural and functional imaging studies in overweight and obese participants [14,15,16,17,18,19,20,21]. Along these lines, also drug-naïve patients experiencing their first psychotic episode [22,23,24,25] and pharmacologically treated patients with schizophrenia [26,27,28,29], show differences in GM volume and structural brain connectivity.
Up to date, there are only a handful of studies that have examined the associations between brain morphology or function and metabolic changes induced by antipsychotics [30, 31]. One region-of-interest study reported a higher striatal volume and decreased striatal functional connectivity that correlated with weight gain in 81 early-phase psychosis patients treated for 12 weeks with risperidone or aripiprazole [30]. With reference to brain activation related to presentation of food stimuli, another study showed that olanzapine treatment enhanced both the anticipatory and consummatory reward responses to food [31]. A decrease in responsivity to food consumption in areas associated with inhibition of feeding behaviour was also noted [31].
Recent evidence in patients with schizophrenia supports the notion of vulnerability due to interaction between aberrant inflammatory response and the presence of metabolic syndrome. Plasminogen activator inhibitor-1 (PAI-1) is the principal inhibitor of tissue plasminogen activator (tPA) and urokinase, and is therefore an inhibitor of fibrinolysis [32]. High plasma levels of PAI-1 have been associated with an increased risk of suffering from cardiovascular disease [33]. Moreover, pathways depending on PAI-1 are also thought to play a role in the development of obesity, insulin resistance and type 2 diabetes [34]. Interestingly, the SERPINE-1 gene, which encodes for PAI-1 is overexpressed in the monocytes of patients with schizophrenia [35]. Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine involved in the regulation of innate and adaptive immunity [36] and higher levels of MIF were found in metabolic disease [37]. Normal MIF expression was found to be linked to metabolic dysfunction and insulin resistance induced by olanzapine, when compared to low MIF expression [38]. Several studies have also identified MIF level as a potential biomarker for schizophrenia [39,40,41]. These findings regarding PAI-1 and MIF levels lead to the hypothesis that they may be involved in the difference in weight gain in schizophrenia.
A plethora of factors could account for the interindividual variability in weight gain among patients treated with antipsychotics; however, the association with impulsivity, brain structure and function has not been clearly established yet. Here, we first sought to investigate, in a cohort of patients in the early phase of psychosis (Early Psychosis Patients; EPP), the associations between interindividual variability in weight gain following the introduction of antipsychotics and the structural and functional brain characteristics. We predicted that impulsivity and the anatomical and functional properties of brain regions which are relevant to impulsivity control would be linked to weight gain (i.e., frontal lobe and striatum). Additionally, we aimed to determine whether patients who had put on more weight had increased plasma levels of PAI-1 and/or MIF. By repeating these analyses in both a cohort of healthy individuals (cohort A) and a population-based cohort (cohort B), we sought to determine whether the observed associations were specific to EPP treated with weight gain-inducing antipsychotics.
Methods
Subjects
Early psychosis cohort
EPP (i.e., illness duration <5 years) were recruited from the Treatment and Early Intervention in Psychosis Programme (TIPP) [42] and from the PsyMetab cohort [43].
EPP within the first 3 years of treatment for a psychotic disorder and having met psychosis threshold according to the Comprehensive Assessment of At Risk Mental States criteria [44] were selected. A total of 42 patients who had an available brain magnetic resonance imaging (MRI) scan (during the first year after the introduction of antipsychotics) and who had an assessment of impulsivity were included in the current study. Based on previous findings from our group, patients were classified into a high (HWEPP, N = 23) or low (LWEPP, N = 19) weight gain group if they gained more than 5% or less than 5% of their initial weight after one month of antipsychotic treatment, respectively [45]. Patients included in the PsyMetab and TIPP cohorts gave their written informed consent to participate in the studies. PsyMetab and TIPP protocols were approved by the local Ethics Committee.
Cohort A
A total of 102 healthy psychotropic-naïve participants with no history of psychotic or substance use disorders were recruited from the same geographic area as in the TIPP programme. Only body mass index (BMI) was available for cohort A. Therefore, groups were formed based on participants’ BMI, with 83 classified as normal BMI < 25 kg/m2 and 19 as high BMI ≥ 25 kg/m2. Informed written consent in accordance with the institutional guidelines was obtained for all participants.
Cohort B
CoLaus|PsyCoLaus is a prospective cohort study designed to investigate cardiovascular risk factors and mental disorders as well as their associations in the community. A total of 6734 individuals aged 35 to 75 years were randomly selected according to the civil register of the city of Lausanne, Switzerland, between 2003 and 2006 and underwent a physical [46, 47] and psychiatric evaluation [48]. Since the baseline assessment, three follow-up evaluations were completed which took place from 2009 to 2013 (first follow up or F1), 2014 to 2018 (second follow up or F2) and 2018 to 2021 (third follow up or F3). The present analyses included data from the F1 and F2 evaluations. Computational brain anatomy analyses were confined to participants who accepted an MRI exam (BrainLaus, N = 1145). For the present study, the included participants (N = 875) were classified as follows, based on weight gained from the first to the second follow-up. Given the larger interval between two measures in cohort B, a 7% weight gain criterion was applied [49]: (i) the high weight gain group (HWcohortB, N = 729) if they had a weight gain of ≥7% [49] or more during the period between the first and second follow-up, and (ii) the low weight gain group (LWcohortB, N = 146) if they gained <7%. A total of 98 participants with weight loss >−7% and 172 with age ≥65 years of age at the first follow-up exam were excluded to avoid brain changes due to significant weight loss [50,51,52] and aging [53,54,55,56,57]. All participants gave written informed consent, and the study was approved by the local Institutional Ethics Committee.
Clinical assessments
In EPP, weight measurements were completed at baseline, after one month of treatment, and at the time of MRI scanning. Cohort A weight was assessed only once during an interview. The consumption of cannabis was assessed with the Case Manager Rating Scale (CMRS) [58] in both EPP and cohort A. In cohort B, measures of weight, BMI and smoking were collected from standardized interviews and anthropometric assessments resulting in a comprehensive set indicative of disease history and cardiovascular risk [46].
Psychopathology and functional levels were scored with the Global Assessment of Functioning (GAF) scale, the Positive and Negative Syndrome Scale (PANSS) [59] and the Montgomery-Åsberg Depression Rating Scale (MADRS) [60].
Impulsivity in EPP was assessed in the early psychosis cohort using the PANSS score for “poor impulse control (G14)” [61]. This item assesses the degree of impulsivity on a scale from 1 to 7 (1 being the absence of impulsivity and 7 an extreme level of impulsivity). Duration of illness (DOI) was defined as the time between reaching the psychosis threshold for the first time and the time of assessment. Poor impulse control score was not available in cohort A. In cohort B, impulsivity control score was constructed using the Neuroticism and the Conscientiousness factors of the NEO-Five-Factor Inventory-Revised (NEO-FFI-R) [62] completed at F1. Impulsivity control scores were calculated as the square root of the sum of the squares of the normalized scores for Neuroticism and the normalized inversed scores for Conscientiousness.
Regarding plasma analyses, blood samples were subjected to two rounds of centrifugation. Firstly, a 10-min spin at 400 g and 4 °C was performed, and the resulting supernatant was collected in a fresh falcon tube. Secondly, the tube was centrifuged again at 3000 g for 10 min at 4 °C, and 500 μl aliquots were prepared. These aliquots were subsequently frozen at −80 °C until PAI-1 and MIF analysis. Plasma levels of PAI-1 and MIF were measured using ELISA kits (-ab157528, Abcam’s PAI-1 ELISA kits, Abcam, Cambridge, MA, USA; Human Active MIF ELISA Kit, R&D System, Abingdon, United Kingdom). All assays were performed according to the manufacturer’s instructions in EPP and cohort A.
MRI acquisition and analysis
In both EPP and cohort A, MRIs were performed with 3 Tesla magnetic resonance scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with 32-channel head coil. Each scanning session included a magnetization-prepared rapid acquisition gradient echo (MPRAGE) T1-weighted sequence and a 9-minute gradient echo-planar imaging (EPI) sequence that was sensitive to BOLD (blood-oxygen-level-dependent) contrast. The MPRAGE acquisition exhibited a 1 mm in-plane resolution and 1.2 mm slice thickness, encompassing 240 × 257 × 160 voxels (TR = 2.30 ms, TE = 2.98 ms, and TI = 900 ms). In contrast, the functional MRI (EPI) acquisition employed an isotropic 3.3 mm voxel size, with a 0.3 mm inter-slice gap, covering a total of 64 × 58 × 32 voxels (TR = 1920 ms and TE = 30 ms). During the resting-state fMRI (rs-fMRI) recordings, patients were instructed to lie calmly in the scanner with their eyes open, without fixating on any specific thought. The rs-fMRI sequence was initiated at the beginning of the session, immediately following the acquisition of the structural scan, with the accompaniment of an experienced psychologist for all patients throughout the scanning process. Ultimately, the acquisition procedure yielded a sequence of 280 BOLD images for each participant.
During the study, there was a routine MRI-system upgrade from the MAGNETOM-Trio to the MAGNETOM-Prisma system. Imaging parameters were precisely matched before and after the upgrade, and the same 32-channel head coil was used.
In order to obtain the sample size for a robust study and avoid scan bias, the MRI data was harmonized using the empirical Bayes approach ComBat - Combining Batches [63]. The global network analyses were repeated on data acquired on the MAGNETOM-Trio or on the MAGNETOM-Prisma system only to further exclude major effects of the scanner upgrade on the results. Additional information on the scanner effect could be found in supplementary material.
fMRI data were processed according to a state-of-the-art pipeline that involved several steps. These steps included the removal of the initial 5 time points to ensure signal stability, addressing slice-timing discrepancies, correcting for motion artifacts by regressing out six motion parameters, averaging signals from white matter and cerebrospinal fluid, performing linear detrending, and applying bandpass filtering within the frequency range of 0.01–0.1 Hz. All of these processing steps were carried out using the CMTK software [64]. Time series have been averaged over the Freesurfer (v.6.0.0, https://surfer.nmr.mgh.harvard.edu/) cortical regions. Functional connectivity has been obtained by computing the Pearson’s correlation between the mean temporal signals from each pair of brain regions. Four regions of interest have been selected: the inferior frontal gyrus, the putamen, the pallidum, and the primary motor cortex.
All imaging data for cohort B were acquired on the very same 3 T whole‐body MRI system (MAGNETOM Prisma; Siemens Medical Systems, Erlangen, Germany) using a 64‐channel radiofrequency receive head coil and body coil for transmission. Methods regarding the acquisition of cohort B were described somewhere else [65]. We sampled regional volume average values in individuals’ native space using factorization-based image labelling [66] after performing automated tissue classification using the multi-channel option of SPM12s “unified segmentation”. Aiming to adjust all regional values for the global effect of head size, we estimated its proxy – the total intracranial volume (TIV) from the sum of GM, WM, and CSF volumes [67].
Statistical analysis
Descriptive data are shown as numbers and percentages for categorical variables or median and interquartile range (IQR) for continuous variables. To compare between groups, Wilcoxon-Mann-Whitney rank-sum test or the Chi-squared test were used, depending on the variable type.
The association between weight gain and the poor impulse control score was tested using linear regression model adjusted for covariates (age, sex, smoker status and weight at baseline for EPP or at F1 for cohort B).
Weight gain differences (HWEPP vs. LWEPP or HWcohortB vs. LWcohortB) or the correlation between weight gain (%) for EPP, or BMI (<25 kg/m2 vs. ≥25 kg/m2) for cohort A, and brain volumes of region of interest (ROI), were independently tested using the general linear model at each voxel and the multiple regression analyses respectively, as implemented in Randomise (http://fsl.fmrib.ox.ac.uk/). Age, gender, and TIV were set as nuisance factors in the model. All results were corrected for multiple comparison Type I error with a non-parametric cluster-size based procedure [68, 69].
In cohort B, the associations between weight gain group or BMI status (<25 vs. ≥25 kg/m2) and GM volumes were tested with general linear regression analyses, adjusted for age, sex, and TIV.
The relationship between weight gain (%) and the fronto-striatal functional connectivity (RSFC) (i.e., the inferior frontal gyrus, putamen, pallidum and primary motor cortex) was assessed with the linear regression model in EPP and in cohort A. RSFC values were converted into Z-scores.
For inflammatory biomarker analyses, linear models were used to examine the association between plasma levels of PAI-1, or MIF and weight gain (%) in the EPP cohort, or BMI in the cohort A, adjusted for age and sex. Associations between inflammatory biomarkers and poor impulse scores were analysed. Considering that inflammatory markers have been associated with impulsive behaviour [70,71,72], we sought to investigate whether there is an association between PAI-1 or MIF levels and poor impulse control.
Analyses were performed in R (version 4.0.2; RStudio, Inc; Boston, Massachusetts). The statistical significance was set at a P ≤ 0.05.
Results
Study demographics
Among 42 EPP, high (≥5%) and low (<5%) weight gain were reported for 19 and 23 patients, respectively. Both groups (Table 1) were of similar age and sex (HWEPP: 37%; LWEPP: 35% women) and did not differ in terms of illness duration or in terms of treatment. There was no difference between the groups in the PANSS negative, PANSS positive and PANSS general scores, nor for total MADRS score or GAF score. EPP were younger than cohort A (N = 102) with a median age of 23 years (IQR: 21–27) versus 26 years (IQR: 22–33) for cohort A (P = 0.002). EPP had a lower level of education (13 years vs. 16 years; P < 0.001) and were more likely to smoke (59% vs. 8%; P < 0.001) and to use cannabis (29% vs. 5%; P < 0.001) when compared to cohort A (Supplementary Table 1).
Within cohort A, there were no statistical differences for sex, smoking status and cannabis use between the high (≥25 kg/m²; N = 19) and normal BMI groups (<25 kg/m²; N = 83). There were differences in age, level of education and BMI between the two groups (Supplementary Table 2).
In cohort B, which included middle-aged individuals (N = 875, median 51 years, IQR: 46–57), there were differences for age (median 48 vs. 51 years, respectively; P < 0.001), sex (60% vs. 49% women; P = 0.01), and BMI during follow-up 2 (median 29 vs. 25 kg/m2; P < 0.001; Supplementary Table 3) between HWcohortB and LWcohortB.
Association between the poor impulse control score and weight gain
In the EPP, higher weight gain was significantly associated with a poor impulse control when corrected by age, sex, baseline weight and cigarette smoker status (β = 1.35, P = 0.03; Table 2). In cohort B, there was no association between the impulsivity control score at F1 and weight gain between F1 and F2 (β = 0.01, 95%CI = −0.36–0.38, P = 0.94, data not shown), when corrected by age, sex, smoker status and weight at F1.
Differences in brain regions related to impulsivity
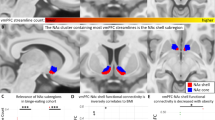
In the EPP, spatial clusters of significantly reduced GM in the frontal lobe (Pcorrected = 0.007) were found in the HWEPP group (N = 19) compared to the LWEPP group (N = 23; Fig. 1a). No significant GM volume group difference was found in the striatum. Considering the whole early psychosis group, a positive correlation was identified by multiple regression analysis between the GM volume of the striatum and weight gain (%) after 1 month of treatment (Pcorrected = 0.048; Fig. 1b).
a The clusters of voxels result from the comparison between the high weight gain group (HWEPP) and the low weight gain group (LWEPP) in the frontal lobe: the orange cluster located on the left frontal lobe represents areas of lower grey matter volume in HWEPP (contrast: HWEPP < LWEPP, MNI: 132,161,76, Pcorrected = 0.007). The colour bar representing the t statistic. b The clusters of voxels result from the correlation between weight gain and volume of grey matter in the striatum: the yellow cluster located on the right striatum represents areas of higher grey matter volume (MNI: 69,142,65, Pcorrected = 0.048). The colour bar represents the t statistic.
As no longitudinal data are available for cohort A, associations were examined between brain structures and functions with BMI at the time of the scan (normal BMI < 25 kg/m² vs. high BMI ≥ 25 kg/m²). No associations based on BMI (continuous and dichotomous), or ROIs (data not shown) were found.
No association was found in cohort B, nor between HWcohortB and the frontal lobe or the striatum volumes (Supplementary Table 4). When analysing participants according to BMI groups (BMI < 25 kg/m² vs. BMI ≥ 25 kg/m²), no statistical differences nor associations were shown with impulsivity related brain regions (data not shown).
Correlation between fronto-striatal RSFC and weight gain
Pearson correlation was performed with the RSFC between the areas of the fronto-striatal circuit areas. In the early psychosis group, we observed a negative correlation between weight gain (%) and the RSFC of the right primary motor cortex to the pallidum (R = −0.32; P = 0.04; Supplementary Fig. 1). Considering the whole cohort A, no correlations were observed between BMI and RSFC (data not shown).
PAI-1 and MIF markers
In EPP, higher PAI-1 levels were measured in the HWEPP (N = 11) as compared to the LWEPP (N = 15) group (34 vs. 14 U/mL; P = 0.02; Table 3). In linear models, after correction for age and sex, weight gain was associated with an increase in PAI-1 levels (β = 4.9; P = 0.002; Table 4). This association was absent for MIF levels (N = 12 for HWEPP, N = 12 for LWEPP; P = 0.66). The association between PAI-1 and poor impulse control was investigated, however, no significant associations were found.
In cohort A, a difference in PAI-1 levels was observed between low and high BMI groups (3.5 vs. 13 U/mL; P = 0.04; Table 3). On the other hand, after adjustment for age and sex, BMI was not associated with PAI-1 or MIF levels in linear models (Supplementary Table 5).
Higher PAI-1 levels were measured in EPP as compared to subjects from cohort A (20 vs. 4.0 U/mL; P < 0.001; Table 3), and no difference in MIF levels was observed between EPP and cohort A.
The association between PAI-1 or MIF and poor impulse control was investigated, but no significant associations were found.
Discussion
Our study on early psychosis patients treated with antipsychotics confirms associations between weight gain and poor impulse control, brain networks governing impulsivity and chronic inflammation.
In our study, we show that EPP treated with antipsychotics, the high and rapid weight gain was associated with poor impulsive control. This suggests that patients with increased impulsiveness have difficulties controlling their eating behaviour. Such an association was not found in the population-based cohort B, which suggests a specific mechanism in EPP. Then, by analysing brain regions related to impulsivity and reward, a decrease in frontal lobe GM volumes in the HWEPP gain group was observed, as well as a positive correlation between weight gain and the striatum GM volume. These results are consistent with the previously reported decreased frontal GM volume following antipsychotic therapy [73] and reported positive correlation between striatal [30] volume and weight gain induced by antipsychotic treatment. The absence of frontal and striatal volume changes in cohort A and cohort B suggests that changes of volume in these regions are specific to weight gain in the early psychosis cohort. Altogether, the present results support the hypothesis that brain region related to impulsivity and reward, namely fronto-striatal structures, and poor impulse control scores are associated with weight gain induced by antipsychotics.
Our study extends previous findings by additionally investigating the properties of fronto-striatal connectivity. A negative correlation between weight gain and RSFC in the fronto-striatal circuits in the EPP group was identified. A functional MRI study in healthy volunteers exposed to one-week treatment with olanzapine showed increased responses to cues predicting rewarding liquids, while activations of striatum activities elicited by the image of tasteless liquid decreased. This suggests that striatum activity may be one of the mechanisms leading to weight gain induced by antipsychotics [31]. Of note, no correlation between BMI and RSFC in the fronto-striatal circuits was found in healthy individuals from cohort A. In patients with schizophrenia, there appears to be a significant association between emotion-related impulsivity (positive and negative emotions) and brain connectivity. In particular, one study has shown that schizophrenia patients with higher levels of emotion-related impulsivity have reduced connectivity between the ventral prefrontal and limbic/cognitive control regions and within the ventral prefrontal areas [74]. In contrast, increased connectivity has been observed between emotion-related impulsivity and sensory regions such as the middle occipital gyrus [74]. It is also worth noting that this issue extends beyond schizophrenia, as similar patterns have been observed in conditions such as binge eating disorder, where greater impulsivity in relation to negative emotions has been found [75]. Future research should examine these multiple dimensions of impulsivity in psychotropic drug-induced weight gain in patients with early psychosis.
The present study also sought to investigate whether PAI-1 and MIF levels, as markers of inflammation, were elevated in patients with higher weight gain. In the EPP, PAI-1 levels were significantly higher in patients who had significant weight gain after one month of treatment. Prospective studies have shown that PAI-1 is a predictor for future cardiovascular events [76, 77], and this is in line with a previous study that predicted cardiovascular events in patients who gained more than 5% of their baseline weight after 1 month of the introduction of an AP at risk [45]. The present study did not identify an association between MIF levels and changes in weight, contrary to a previous study that identified such an association [38]. The previous study took into account MIF expression, whereas our study just examined plasma analyses, which could be one reason for the discrepancy. In contrast to previous studies [39,40,41], no difference was also noted between MIF in EPP and cohort A. In one study, there was a difference in MIF levels between controls and patients who were naïve or not treated with AP for more than 6 weeks prior to sample collection [39]. Our analyses included patients who were being treated with AP drugs, which could explain the contradictory results. In addition, one study with older participants, including 86 patients with schizophrenia (mean age ± SD, 54.3 ± 10.3 years) and 51 controls (48.4 ± 9.5 years), observed that MIF levels were unrelated to the schizophrenia group when compared to the control group [41] after regression analyses, which is consistent with our findings. Although no significant association was found between PAI-1 or MIF levels and poor impulse control, it is important to note that the limited number of patients with available PAI-1 or MIF samples may have contributed to these null findings. In addition, the nature of the inflammatory markers and the multidimensional aspect of the impulsivity scores may have influenced our results compared to previous studies. Future research, with larger patient cohorts and specific facets of impulsivity [78], may provide a more comprehensive exploration of potential associations with PAI-1 or MIF.
Some limitations of our study must be acknowledged. First, results are limited by the cross-sectional design of the study, the MR scan being available only after the introduction of the antipsychotic. Further longitudinal studies are required to elucidate whether or not the same associations would be observed with MR scans obtained before the treatment. Second, this is an observational study and no causal relationship can be established between weight gain, impulsivity and brain structures. Third, the MRI technique for measuring changes in frontal and striatum volumes was different in cohort B (analyses on averaged volumes from selected ROIs), with an older population (median 56 years, IQR: 51–62) and with a long duration between weight measurements (median 63 months, IQR: 63–66), which differs from the other cohorts. Fourth, the assessments of impulsivity control scores did not rely on specific scales designed to measure this personality characteristic and differed between EPP and cohort B, with the former relying on a single PANSS item and the latter using the two dimensions of the NEO-FFI-R, which may account for differential findings between the two cohorts. Fifth, there were few plasma samples available for measuring MIF and PAI-1 levels in EPP. Although further studies using larger samples of patients with early psychosis are still needed, the current results may represent the first analysis of PAI-1 levels associated with weight changes induced by antipsychotics. To better understand the impact of treatment on weight gain, additional studies are required, including pre- and post-treatment comparisons.
The strength of the present study lies in the inclusion of patients with early psychosis and the availability of two additional cohorts with similar measures, which allowed us to test whether the findings derived from EEP can be generalized to unaffected individual or mostly untreated people from the general population. In addition, analyses in both population-based cohorts support the hypothesis that these differences in the fronto-striatal GM volumes, poor impulse control scores and in PAI-1 levels are only present in EPP and absent in the general population.
In summary, the present findings suggest that inter-individual variability in rapid weight gain induced by antipsychotics in EPP is associated with poor impulse control and with differences in brain regions related to impulsivity and is specific to the psychiatric population. Future research in this area, also confirming the observed association with the inflammatory marker PAI-1, may provide new insights into the underlying neurobiology of antipsychotic-induced weight gain.
Data availability
Data from PsyMetab cannot be publicly deposited due to participant confidentiality purposes. Data from PsyMetab can be accessed after formal application and ethical review by the Ethics Committee of the Canton of Vaud. For further details: http://www.chuv.ch/cnp-psymetab. The dataset analyzed for the Treatment and Early Intervention in Psychosis Programme (TIPP) in the current study is available from the corresponding author upon reasonable request.
References
De Hert M, Schreurs V, Vancampfort D, Van Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8:15.
Ren W, Lui S, Deng W, Li F, Li M, Huang X, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry. 2013;170:1308–16.
Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25:83–8.
Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large‐scale meta‐analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16:163–80.
Zimmermann U, Kraus T, Himmerich H, Schuld A, Pollmächer T. Epidemiology, implications and mechanisms underlying drug-induced weight gain in psychiatric patients. J Psychiatr Res. 2003;37:193–220.
Vandenberghe F, Saigí-Morgui N, Delacrétaz A, Quteineh L, Crettol S, Ansermot N, et al. Prediction of early weight gain during psychotropic treatment using a combinatorial model with clinical and genetic markers. Pharmacogenet Genom. 2016;26:547–57.
Zai CC, Tiwari AK, Zai GC, Maes MS, Kennedy JL. New findings in pharmacogenetics of schizophrenia. Curr Opin Psychiatry. 2018;31:200–12.
VanderBroek-Stice L, Stojek MK, Beach SR, MacKillop J. Multidimensional assessment of impulsivity in relation to obesity and food addiction. Appetite. 2017;112:59–68.
Mobbs O, Crépin C, Thiéry C, Golay A, Van der Linden M. Obesity and the four facets of impulsivity. Patient Educ Couns. 2010;79:372–7.
Wirt T, Hundsdörfer V, Schreiber A, Kesztyüs D, Steinacker JM. Associations between inhibitory control and body weight in German primary school children. Eat Behav. 2014;15:9–12.
Bénard M, Camilleri GM, Etilé F, Méjean C, Bellisle F, Reach G, et al. Association between impulsivity and weight status in a general population. Nutrients. 2017;9:217.
Giel KE, Teufel M, Junne F, Zipfel S, Schag K. Food-related impulsivity in obesity and binge eating disorder—a systematic update of the evidence. Nutrients. 2017;9:1170.
Meule A, Hermann T, Kübler A. Food addiction in overweight and obese adolescents seeking weight‐loss treatment. Eur Eat Disord Rev. 2015;23:193–8.
Pannacciulli N, Del Parigi A, Chen K, Le DSN, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–25.
Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52:1696–703.
Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–64.
Marqués-Iturria I, Pueyo R, Garolera M, Segura B, Junqué C, García-García I, et al. Frontal cortical thinning and subcortical volume reductions in early adulthood obesity. Psychiatry Res. 2013;214:109–15.
Bobb JF, Schwartz BS, Davatzikos C, Caffo B. Cross‐sectional and longitudinal association of body mass index and brain volume. Hum Brain Mapp. 2014;35:75–88.
Opel N, Redlich R, Grotegerd D, Dohm K, Heindel W, Kugel H, et al. Obesity and major depression: body-mass index (BMI) is associated with a severe course of disease and specific neurostructural alterations. Psychoneuroendocrinology. 2015;51:219–26.
Shott ME, Cornier MA, Mittal VA, Pryor TL, Orr JM, Brown MS, et al. Orbitofrontal cortex volume and brain reward response in obesity. Int J Obes (Lond). 2015;39:214–21.
García-García I, Michaud A, Dadar M, Zeighami Y, Neseliler S, Collins DL, et al. Neuroanatomical differences in obesity: meta-analytic findings and their validation in an independent dataset. Int J Obes. 2019;43:943–51.
Leung M, Cheung C, Yu K, Yip B, Sham P, Li Q, et al. Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta-analyses with sample size weighting. Schizophr Bull. 2011;37:199–211.
Alvarado-Alanis P, León-Ortiz P, Reyes-Madrigal F, Favila R, Rodríguez-Mayoral O, Nicolini H, et al. Abnormal white matter integrity in antipsychotic-naive first-episode psychosis patients assessed by a DTI principal component analysis. Schizophr Res. 2015;162:14–21.
Serpa M, Doshi J, Erus G, Chaim-Avancini T, Cavallet M, Van De Bilt M, et al. State-dependent microstructural white matter changes in drug-naive patients with first-episode psychosis. Psychol Med. 2017;47:2613–27.
Sagarwala R, Nasrallah HA. The effect of antipsychotic medications on white matter integrity in first-episode drug-naive patients with psychosis: a review of DTI studies. Asian J Psychiatry. 2021;61:102688.
van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–53.
Klauser P, Baker ST, Cropley VL, Bousman C, Fornito A, Cocchi L, et al. White matter disruptions in schizophrenia are spatially widespread and topologically converge on brain network hubs. Schizophr Bull. 2017;43:425–35.
Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23:1261–9.
Van Erp TG, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry. 2018;84:644–54.
Homan P, Argyelan M, Fales CL, Barber AD, DeRosse P, Szeszko PR, et al. Striatal volume and functional connectivity correlate with weight gain in early-phase psychosis. Neuropsychopharmacology. 2019;44:1948–54.
Mathews J, Newcomer JW, Mathews JR, Fales CL, Pierce KJ, Akers BK, et al. Neural correlates of weight gain with olanzapine. Arch Gen Psychiatry. 2012;69:1226–37.
Declerck PJ, Gils A, editors. Three decades of research on plasminogen activator inhibitor-1: a multifaceted serpin. Seminars in thrombosis and hemostasis; 2013: Thieme Medical Publishers.
Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342:1792–801.
Festa A, Williams K, Tracy RP, Wagenknecht LE, Haffner SM. Progression of plasminogen activator inhibitor-1 and fibrinogen levels in relation to incident type 2 diabetes. Circulation. 2006;113:1753–9.
Drexhage RC, van der Heul-Nieuwenhuijsen L, Padmos RC, van Beveren N, Cohen D, Versnel MA, et al. Inflammatory gene expression in monocytes of patients with schizophrenia: overlap and difference with bipolar disorder. A study in naturalistically treated patients. Int J Neuropsychopharmacol. 2010;13:1369–81.
Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800.
Grieb G, Merk M, Bernhagen J, Bucala R. Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect. 2010;23:257.
Cui D, Peng Y, Zhang C, Li Z, Su Y, Qi Y, et al. Macrophage migration inhibitory factor mediates metabolic dysfunction induced by atypical antipsychotic therapy. J Clin Investig. 2018;128:4997–5007.
Schwarz E, Izmailov R, Spain M, Barnes A, Mapes JP, Guest PC, et al. Validation of a blood-based laboratory test to aid in the confirmation of a diagnosis of schizophrenia. Biomark Insights. 2010;5:BMI. S4877.
Chan MK, Krebs M, Cox D, Guest P, Yolken R, Rahmoune H, et al. Development of a blood-based molecular biomarker test for identification of schizophrenia before disease onset. Transl psychiatry. 2015;5:e601–e.
Okazaki S, Hishimoto A, Otsuka I, Watanabe Y, Numata S, Boku S, et al. Increased serum levels and promoter polymorphisms of macrophage migration inhibitory factor in schizophrenia. Prog Neuro Psychopharmacol Biol Psychiatry. 2018;83:33–41.
Baumann PS, Crespi S, Marion-Veyron R, Solida A, Thonney J, Favrod J, et al. Treatment and early intervention in psychosis program (TIPP-Lausanne): Implementation of an early intervention programme for psychosis in Switzerland. Early Inter Psychiatry. 2013;7:322–8.
Dubath C, Delacrétaz A, Glatard A, Vollenweider P, Preisig M, Richard-Lepouriel H, et al. Evaluation of cardiometabolic risk in a large psychiatric cohort and comparison with a population-based sample in Switzerland. J Clin Psychiatry. 2020;81:19m12796.
Yung AR, Yung AR, Pan Yuen H, Mcgorry PD, Phillips LJ, Kelly D, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust NZ J Psychiatry. 2005;39:964–71.
Vandenberghe F, Gholam-Rezaee M, Saigí-Morgui N, Delacretaz A, Choong E, Solida-Tozzi A, et al. Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. J Clin psychiatry. 2015;76:0.
Firmann M, Mayor V, Vidal PM, Bochud M, Pécoud A, Hayoz D, et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord. 2008;8:6.
Marques-Vidal P, Bochud M, Bastardot F, von Känel R, Aubry J-M, Gaspoz J-M, et al. Assessing the associations between mental disorders, cardiovascular risk factors, and cardiovascular disease: the CoLaus/PsyCoLaus study. Lausanne: Institut universitaire de médecine sociale et préventive (IUMSP); 2011.
Preisig M, Waeber G, Vollenweider P, Bovet P, Rothen S, Vandeleur C, et al. The PsyCoLaus study: methodology and characteristics of the sample of a population-based survey on psychiatric disorders and their association with genetic and cardiovascular risk factors. BMC Psychiatry. 2009;9:1–12.
Musil R, Obermeier M, Russ P, Hamerle M. Weight gain and antipsychotics: a drug safety review. Expert Opin Drug Saf. 2015;14:73–96.
Jimenez A, Pegueroles J, Carmona-Iragui M, Vilaplana E, Montal V, Alcolea D, et al. Weight loss in the healthy elderly might be a non-cognitive sign of preclinical Alzheimer’s disease. Oncotarget. 2017;8:104706.
Katzman DK, Lambe EK, Mikulis DJ, Ridgley JN, Goldbloom DS, Zipursky RB. Cerebral gray matter and white matter volume deficits in adolescent girls with anorexia nervosa. J Pediatr. 1996;129:794–803.
Titova OE, Hjorth OC, Schiöth HB, Brooks SJ. Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: a meta-analysis of VBM studies. BMC Psychiatry. 2013;13:1–11.
Raz N, Daugherty AM. Pathways to brain aging and their modifiers: free-radical-induced energetic and neural decline in senescence (FRIENDS) model-a mini-review. Gerontology. 2018;64:49–57.
Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96.
Taubert M, Roggenhofer E, Melie-Garcia L, Muller S, Lehmann N, Preisig M, et al. Converging patterns of aging-associated brain volume loss and tissue microstructure differences. Neurobiol Aging. 2020;88:108–18.
Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–8.
Ramanoël S, Hoyau E, Kauffmann L, Renard F, Pichat C, Boudiaf N, et al. Gray matter volume and cognitive performance during normal aging. A voxel-based morphometry study. Front Aging Neurosci. 2018;10:235.
Drake RE, Osher FC, Noordsy DL, Hurlbut SC, Teague GB, Beaudett MS. Diagnosis of alcohol use disorders in schizophrenia. Schizoph Bull. 1990;16:57–67.
Wallwork R, Fortgang R, Hashimoto R, Weinberger D, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137:246–50.
Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
McCrae RR, Costa PT Jr. A contemplated revision of the NEO five-factor inventory. Personal Individ Differences. 2004;36:587–96.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–27.
Mullier E, Roine T, Griffa A, Xin L, Baumann PS, Klauser P, et al. N-Acetyl-cysteine supplementation improves functional connectivity within the cingulate cortex in early psychosis: a pilot study. Int J Neuropsychopharmacol. 2019;22:478–87.
Grosu C, Trofimova O, Gholam-Rezaee M, Strippoli M-PF, Kherif F, Lutti A, et al. CYP2C19 expression modulates affective functioning and hippocampal subiculum volume—a large single-center community-dwelling cohort study. Transl Psychiatry. 2022;12:316.
Yan Y, Balbastre Y, Brudfors M, Ashburner J. Factorisation-based image labelling. Front Neurosci. 2021;15:818604.
Trofimova O, Loued-Khenissi L, DiDomenicantonio G, Lutti A, Kliegel M, Stringhini S, et al. Brain tissue properties link cardio-vascular risk factors, mood and cognitive performance in the CoLaus| PsyCoLaus epidemiological cohort. Neurobiol Aging. 2021;102:50–63.
Friston KJ, Worsley KJ, Frackowiak RS, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–20.
Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25.
Kim JS, Kang E-S, Bahk YC, Jang S, Hong KS, Baek JH. Exploratory analysis of behavioral impulsivity, pro-inflammatory cytokines, and resting-state frontal EEG activity associated with non-suicidal self-injury in patients with mood disorder. Front Psychiatry. 2020;11:124.
Isung J, Aeinehband S, Mobarrez F, Nordström P, Runeson B, Åsberg M, et al. High interleukin-6 and impulsivity: determining the role of endophenotypes in attempted suicide. Transl Psychiatry. 2014;4:e470–e.
Coccaro EF, Lee R, Coussons-Read M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry. 2014;71:158–65.
Guo F, Zhu YQ, Li C, Wang XR, Wang HN, Liu WM, et al. Gray matter volume changes following antipsychotic therapy in first-episode schizophrenia patients: a longitudinal voxel-based morphometric study. J Psychiatr Res. 2019;116:126–32.
Hoptman MJ, Antonius D, Mauro CJ, Parker EM, Javitt DC. Cortical thinning, functional connectivity, and mood-related impulsivity in schizophrenia: relationship to aggressive attitudes and behavior. Am J Psychiatry. 2014;171:939–48.
Kenny TE, Singleton C, Carter JC. An examination of emotion-related facets of impulsivity in binge eating disorder. Eat Behav. 2019;32:74–7.
Jung RG, Motazedian P, Ramirez FD, Simard T, Di Santo P, Visintini S, et al. Association between plasminogen activator inhibitor-1 and cardiovascular events: a systematic review and meta-analysis. Thromb J. 2018;16:12.
Thögersen AM, Jansson J-HK, Boman K, Nilsson TRK, Weinehall L, et al. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: evidence for the fibrinolytic system as an independent primary risk factor. Circulation. 1998;98:2241–7.
Madison AA, Kiecolt-Glaser JK. Are sick people really more impulsive?: Investigating inflammation-driven impulsivity. Psychoneuroendocrinology. 2022;141:105763.
Acknowledgements
We thank all patients and participants who take part in the studies. We thank all collaborators involved in the studies, and in particular: M. Cleusix, R. Jenni, M. Baumgartner. The authors thank L. Maw for editorial assistance.
Funding
This work has been funded in part by the Swiss National Research Foundation (CBE and PC: 320030–120686, 324730–144064, 320030–173211; 320030–200602). PK was supported by the Adrian & Simone Frutiger Foundation. DD is supported by the Adrian & Simone Frutiger Foundation. The CoLaus|PsyCoLaus study was supported by research grants from GlaxoSmithKline, the Faculty of Biology and Medicine of Lausanne, the Swiss National Science Foundation (grants 3200B0–105993, 3200B0–118308, 33CSCO-122661, 33CS30–139468, 33CS30–148401, 33CS30_177535 and 3247730_204523) and the Swiss Personalized Health Network (project: Swiss Ageing Citizen Reference). BD is supported by the Swiss National Science Foundation (project grants Nr. 32003B_212466, 324730_192755 and CRSII5–3_209510), ERA_NET iSEE project (32NE30_198552), the Swiss Personalised Health Network SACR project, the CLIMACT starting grant UNIL-EPFL, and the Leenaards Foundation. LREN is very grateful to the Roger De Spoelberch and Partridge Foundations for their generous financial support.
Author information
Authors and Affiliations
Contributions
Study concept and design: CG, PK, and CE. Acquisition and analysis of data: CG, PK, DD, IK, MP, NL, YA-G, MF, MP, and BD. Drafting of the manuscript: CG, PK, and CE. Critical revision of the manuscript for important intellectual content: All authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. CBE received honoraria for conferences from Forum pour la formation médicale, Janssen-Cilag, Lundbeck, Otsuka, Sandoz, Servier, Sunovion, Sysmex Suisse AG, Vifor-Pharma, and Zeller in the past 3 years.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grosu, C., Klauser, P., Dwir, D. et al. Associations between antipsychotics-induced weight gain and brain networks of impulsivity. Transl Psychiatry 14, 162 (2024). https://doi.org/10.1038/s41398-024-02881-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-02881-4