Abstract
Epigenetic modifications influenced by environmental exposures are molecular sources of phenotypic heterogeneity found in schizophrenia and bipolar disorder and may contribute to shared etiopathogenetic mechanisms of these two disorders. Newborns who experienced perinatal asphyxia have suffered reduced oxygen delivery to the brain around the time of birth, which increases the risk of later psychiatric diagnosis. This study aimed to investigate DNA methylation in blood cells for associations with a history of perinatal asphyxia, a neurologically harmful condition occurring within the biological environment of birth. We utilized prospective data from the Medical Birth Registry of Norway to identify incidents of perinatal asphyxia in 643 individuals with schizophrenia or bipolar disorder and 676 healthy controls. We performed an epigenome wide association study to distinguish differentially methylated positions associated with perinatal asphyxia. We found an interaction between methylation and exposure to perinatal asphyxia on case–control status, wherein having a history of perinatal asphyxia was associated with an increase of methylation in healthy controls and a decrease of methylation in patients on 4 regions of DNA important for brain development and function. The differentially methylated regions were observed in genes involved in oligodendrocyte survival and axonal myelination and functional recovery (LINGO3); assembly, maturation and maintenance of the brain (BLCAP;NNAT and NANOS2) and axonal transport processes and neural plasticity (SLC2A14). These findings are consistent with the notion that an opposite epigenetic response to perinatal asphyxia, in patients compared with controls, may contribute to molecular mechanisms of risk for schizophrenia and bipolar disorder.
Similar content being viewed by others
Introduction
Schizophrenia (SZ) and bipolar disorders (BD) are highly heritable (60-85%) [1,2,3] and are often regarded as part of a clinical continuum [4]. Some of the heritability may be attributed to an overlapping polygenic architecture [5,6,7,8] between the disorders and to epigenetic modifications [9,10,11,12] influenced by environmental exposures throughout development, starting as early as the prenatal period [13, 14]. Adverse events during pregnancy and birth are some of the best-replicated environmental risk factors for psychosis [13], and of these adverse events, fetal hypoxia is among those most consistently implicated [15,16,17]. Perinatal asphyxia (PA) is a condition where affected newborns experience a deprivation of oxygen (i.e., hypoxia) in the whole body including the brain, with or without concomitance of reduced cerebral blood flow. Population-based studies have found that a history of PA increases the risk of developing SZ (Odds Ratio = 4.4) [13, 18] and BD (Hazard Ratio = 5.3) [19], consistent with evidence of an altered response to hypoxia and oxidative stress that influenced brain development [20,21,22]. Most individuals with a history of PA do not develop any psychiatric condition. However, factors underlying the effect of PA on a trajectory of resilience or on a trajectory of altered neurodevelopment linked with psychiatric disorders are still unknown.
Hypoxia modulates a cascade of systemic and cell/tissue specific hormonal and molecular homeostatic responses in the body. Having experienced perinatal hypoxia impacts developmental plasticity (i.e., the ability of genes to be differentially expressed according to environmental cues) and has been linked to increased risk of disease later in life such as diabetes, inflammation, cardiac dysfunction, hypertension, atherosclerosis, accelerated age-related cognitive decline and other neurological conditions [23]. This indicates that PA can induce complex broad system-level responses, which could be revealed by changes at the level of peripheral tissues, such as epigenetic modifications.
Epigenetic modifications, such as DNA methylation (DNAm), influence gene expression by the recruitment of methyl-binding proteins and disruption of transcription factor binding that initiate gene silencing and chromatin compaction [24]. Both increases and decreases in gene expression are associated with DNAm [25], which has initiated a growing interest in epigenetic variation influenced by environmental exposures (i.e. non-genetic factors) in the molecular etiology of psychiatric disorders [26]. While most gene variants identified with genome-wide association studies (GWAS) do not directly code for changes affecting protein structure and function [27], it was recently shown that many of the SZ GWAS loci overlap with DNAm modifications associated with SZ [9]. Thus, DNAm can be influenced by both genetic and environmental factors and can contribute to the regulation of gene expression of GWAS loci associated with psychiatric disorders [9].
As part of the response to hypoxia, recent findings have identified modifications in DNAm, which reflect a regulation in the expression and function of several important genes including membrane receptors, ion channels, key enzymes and signaling proteins in fetal organs and tissues [23]. Given that DNAm is sensitive to environmental factors and the response to PA is usually systemic, DNAm changes in peripheral tissues can represent an important molecular marker to study the role of the response to PA in mental disorders.
The aim of the current study was to assess the putative interactions between DNAm profiles and PA, a variable obtained from prospective birth registry data, in association with case-control status. We hypothesize that PA is associated with divergent epigenetic modifications in individuals with SZ or BD compared to healthy controls.
Materials and methods
Participants
The current study used data from 598 individuals with SZ (n = 388) or BD (n = 210) from the Thematically Organized Psychosis (TOP) study, the main study protocol of the Norwegian Centre for Mental Disorders Research (NORMENT; Oslo, Norway), 14 individuals with SZ from the Youth Thematic Organized Psychosis (Youth-TOP; Oslo, Norway) study and 31 individuals with SZ (n = 28) or BD (n = 3) from the Early Diagnostic and Treatment of Psychosis (TIPS; Stavanger, Norway) study. The 676 healthy controls (HC) from the TOP or Youth-TOP study were recruited from the greater Oslo area based on random selection from the Norwegian national population registry. Participants were of European ancestry, between the ages of 13–49 years (median age = 29 years) and 45% female (589/1319). All participants gave written informed consent. The study was conducted in accordance with the Helsinki Declaration with approval from the Regional Committees for Medical Research Ethics South East Norway (REC South East) and the Norwegian Data Inspectorate.
Between October 2002 and 2013, adult TOP and adolescent Youth-TOP individuals with SZ and BD spectrum disorders, respectively, were consecutively recruited from psychiatric units (outpatient and inpatient units) of public hospitals in the Oslo region and Stavanger area for the TIPS participants. All participants with SZ and BD underwent thorough clinical investigation by trained psychologists and physicians. Blood samples were drawn from fasting participants and within a narrow time window in the morning. Clinical diagnoses were assessed using the Structured Clinical Interview for DSM-IV axis 1 disorder (SCID-I) module A-E [28]. For the Youth-TOP sample, we used the Norwegian version of the Kiddie-Schedule for Affective Disorders and Schizophrenia for School Aged Children (6–18 years): Present and Lifetime Version (K-SADS-PL) [29]. Psychosocial function was assessed with the Global Assessment of Functioning scale, split version (GAF) [30]. Current psychotic symptoms were rated by the use of the Positive and Negative Syndrome Scale (PANSS) [31]. The Alcohol Use Disorders Identification Test (AUDIT) [32] and Drug Use Disorders Identification Test (DUDIT) [33] were used to evaluate alcohol and drug use. HC were interviewed by trained research assistants and examined with the Primary Care Evaluation of Mental Disorders (Prime-MD) to ensure no current or previous psychiatric disorders [34].
Exclusion criteria for both patients (PT) and HC were organic disorders (substance-induced psychotic disorder, somatic health condition, brain damage or head trauma with unconsciousness over 5 min, neurological diseases and autism spectrum disorder). Additional exclusion criteria for HC were current or previous somatic illness and substance misuse disorders or dependency within the last 6 months. HC were excluded if they or a first-degree relative had a lifetime history of a severe psychiatric disorder. Additionally, TOP and Youth-TOP participants underwent magnetic resonance imaging (MRI), and scans were assessed by a neuroradiologist using a graded scheme (See TOP MRI Grading Scheme) [35]. If brain pathology was detected, the participant was also excluded.
A flow diagram of the selection of participants for this study is found in Fig. 1. Individuals with one of the following DSM-IV diagnoses were included in this study: [1] within the SZ spectrum, n = 430 [schizophrenia (DSM-IV 295.1, 295.2, 295.3, 295.6, and 295.9; n = 254), schizophreniform disorder (DSM-IV 295.4; n = 29), and schizoaffective disorder (DSM-IV 295.7; n = 48) or other psychosis (psychosis not otherwise specified, DSM-IV 298.9, n = 72; brief psychotic disorder, DSM-IV 298.8, n = 7; delusional disorder, DSM-IV 297.1, n = 20)] and [2] within the BD spectrum, n = 213 [Bipolar I disorder (DSM-IV 296.0–7; n = 133), Bipolar II disorder (DSM-IV 296.89; n = 63) or bipolar disorder not otherwise specified (DSM-IV 296.80; n = 17)].
Perinatal asphyxia data from the Medical Birth Registry of Norway (MBRN) is available on births from 1967; therefore, if the participant was not born in Norway or is >50 years, they would not have birth registry information in the MBRN. Quality control (QC); European ancestry (EUR); schizophrenia (SZ); bipolar disorder (BD); major depressive disorder (MDD).
Perinatal asphyxia (PA)
The birth data was collected from the Medical Birth Registry of Norway (MBRN), which has mandatory reporting on all births after 16-weeks gestational age. The PA variable included complications coded AS53 (asphyxia without other signs), AS54 (asphyxia with poor sound), AS55 (asphyxia and discolored amniotic fluid), AS61 (asphyxia), P211 (mild or moderate birth asphyxia) in the registry.
DNAm analysis
Methylation quantification was completed using the Illumina Infinium® Methylation EPIC BeadChip (Illumina, Inc. San Diego, USA).
Pre-processing and quality control (QC)
Epigenome-wide DNA methylation was measured in a total of 2365 blood samples. The measurements were performed in three batches of 1000, 283 and 1082 samples, respectively. Quality control (QC) and preprocessing were performed on the data from each batch separately using the following pipeline: (1) removal of sites with a detection p-value > 0.01 in at least 1% of the samples, and removal of samples where at least 1% of the sites had a detection p-value > 0.01, together with removal of sites with a bead count <3 in 5% of the samples; (2) removal of problematic probes that are known to cross-hybridize or contain single nucleotide polymorphisms (SNPs) that are close to target cytosine-phosphate-guanine sites (CpGs) [36]; (3) samples from individuals that were predicted to be of European ancestry with a probability of <0.9 were removed. Ancestry was predicted from genetic data, using a Random Forest classifier. Populations from the 1000 genome project were used to train the model; (4) functional normalization of the data was performed to reduce non-biological variation. The number of principal components (PCs) used in the normalization was 20 PCs for the first and second batches, and 25 PCs for the third batch; (5) removal of samples with a mismatch between SNP genotypes from the EPIC array and genotype data from the same samples; (6) removal of samples where the predicted and the reported sex did not match; (7) removal of sex chromosome probes; and (8) removal of batch effects from AMP plate, Sentrix ID, Sentrix Position, run date and scanner ID using ComBat [37] as implemented in the bioconductor package sva. The statistical programming software R (version 4.0.0) and Bioconductor (version 3.1.1) packages minfi, wateRmelon, sva and ChAMP were used during the QC and preprocessing of the data. After the QC and preprocessing procedures, the data from the three batches were merged, and ComBat was used to remove the batch effects.
Finally, the subset of samples for which information about PA status is available was extracted and used for the analyses in this study. The final dataset had 1319 samples and 760668 probes.
Epigenome wide association study (EWAS)
We performed an epigenome wide association study (EWAS) to identify differentially methylated positions (DMPs) associated with PA. Because cigarette smoking and white blood cell composition can affect methylation [9], smoking scores and white blood cell ratios were used as covariates in analyses, together with age and sex. The smoking scores were calculated using a method described by Elliott and colleagues [38]. The blood cell ratios were calculated using the estimateCellCounts2 function from the bioconductor package, FlowSorted.Blood.EPIC. Differentially methylated regions (DMRs) were identified using the comb-p algorithm [34], specifying parameters as seed p-value = 0.05 and maximum distance between probes of 750 base pairs, as recommended by Mallik and colleagues [39]. The comb-p algorithm was also run with the more stringent seed p-value = 0.001. Šidák correction was used to account for multiple testing. Consistent with previous studies, a significance threshold was set to false discovery rate (FDR) < 0.05 for DMPs or Šidák p < 0.05 for DMRs [37]. DMRs were considered significant with three or more probes and a Šidák p < 0.05. DMPs and DMRs were annotated using the bioconductor package IlluminaHumanMethylation EPICanno.ilm10b4.hg19 [35].
Because we were interested in whether DNAm in blood is altered in response to PA and the relevance these alterations might have for associated loci in the brain, we used the online Blood Brain DNA Methylation Comparison Tool [40]. This online tool is a searchable database containing correlations between DNAm in blood and four brain regions (prefrontal cortex, entorhinal cortex, superior temporal gyrus and cerebellum) for each of the CpGs that are included on the Illumina 450 K DNA methylation chip.
Other statistical analyses
As we hypothesized a different response to PA in cases and controls, we used linear models to evaluate the interaction of Blood methylation status (M-values) [41] and PA on case–control status (Dx ~ M-Values*PA + covariates). Initial analyses with age, sex, smoking score and blood cell ratios as covariates inflated the QQ-plots. The first 10 principal components calculated from the DNAm data were therefore also added as covariates, which effectively dampened the inflation. Nominal p-values were converted to FDR values following the Benjamini and Hochberg approach [42]. We used summary statistics from the EWAS and Pearson’s correlation to assess the relationship of PA related DNAm between groups [43]. We re-ran significant analyses stratified by sex or patient subgroups. We also explored the relationship between M-values and PA on age of onset and current symptom severity (total PANSS score) using the model: age of onset or severity ~ M-Values*PA + covariates, stratified by sex (males/females only).
Results
PT and HC differed in age by 3 years (mean age of 28 and 31 years, respectively; F = 57.54, p < 0.001), but not in the distribution of sex (47 and 43% female; χ2 = 1.73, p = 0.19). Both age and sex were included as covariates in the analyses. The incidence of PA was also similar 12% in the PT group and 15% in the HC group (χ2 = 2.52, p = 0.13). Further, demographic and clinical data are presented in Table 1.
Genome-wide identification of differentially methylated positions
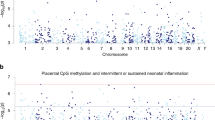
No significant associations were found for any of the models (EWAS or interaction analysis) at the single position level, after correction for multiple testing. A Manhattan plot for the main effect of PA is presented in the SI, Supplementary Fig. 1.
Genome-wide identification of differentially methylated regions
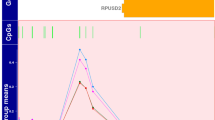
Significant interactions between four DMRs and PA on case-control status were identified in genes encoding leucine-rich repeat and immunoglobulin-like domain-containing nogo receptor-interacting protein 3 (LINGO3, Šidák corrected p = 1.71E-03), the overlapping bladder cancer-associated protein and neuronatin (BLCAP; NNAT, Šidák corrected p = 4.88E-03), nanos C2HC-type zinc finger 2 (NANOS2, Šidák corrected p = 6.92E-03) and solute carrier family 2 member 14 protein (SLC2A14, Šidák corrected p = 3.95E-02; Fig. 2 and Table 2), so that HC with PA had higher methylation at these DMRs compared with HC without PA, while the opposite was the case in patients (PT). From another point of view, HC and PT had different methylation at these DMRs if they had a history of PA exposure, with HC having higher methylation compared with PT (Fig. 2).
We analyzed the correlation of DNAm profiles associated with PA between PT and HC, to explore whether the interaction detected at the DMR in LINGO3, BLCAP; NNAT, NANOS2 and SLC2A14 may reflect a global pattern of opposite response to PA. We observed a weak, albeit significant, positive correlation of DNAm profiles associated with PA between PT and HC, r = 0.134, p < 2.2e-16 (Fig. 3). Stratified by sex, we found a weak negative correlation of DNAm profiles associated with PA between female PT and HC (r = -0.120, p < 2.2e-16) and a weak positive correlation between male PT and HC (r = 0. 088, p < 2.2e-16; Supplementary Fig. 2).
We found significant correlations between methylation in blood and methylation in previously tested brain regions, namely prefrontal cortex, entorhinal cortex, and superior temporal gyrus, for probes associated with LINGO3, BLCAP;NNAT, NANOS2 and SLC2A14 (Supplementary Figs. 3–6).
Sex, PA and differentially methylated regions
In males, we found significant interactions between DMRs and PA on case-control status in six genes encoding TNFAIP8, ADARB2, SLC35C1, SORBS2, NRIP2 and MOBP (Supplementary Table 1). In females, we identified one gene, DUSP19, with a significant interaction between DMRs and PA on case-control status (Supplementary Table 2).
Differentially methylated regions and PA on Age of onset or disease severity
One DMR for the gene encoding TACSTD2 in male PT, and one in female PT—LGALS8, had a significant interaction between PA and methylation on disease severity (Supplementary Table 3). In male PT, we identified one DMR for the gene encoding TMEM232 that showed a significant interaction between PA and methylation on age of onset (Supplementary Table 4). No associations between PA and methylation on age of onset were observed in female PT.
Differentially methylated regions and PA on SZ and BD subgroups
Significant interactions between six DMRs and PA on SZ case-control status were identified for the HOXA4, SLC2A14, CTBP1;C4orf42, MOBP, BAHCC1 and NANOS2 genes (Supplementary Table 5). Significant interactions between two DMRs and PA on BD case-control status were identified for the HKR1 and LRRC34 genes (Supplementary Table 6). We observed a significant positive correlation of DNAm profiles associated with PA between SZ and BD, r = 0.295, p < 2.2e-16 (Supplementary Fig. 7).
Discussion
By analyzing the interaction between DNAm and PA on case-control status, we identified DMRs in genes encoding LINGO3, BLCAP;NNAT, NANOS2 and SLC2A14. In the absence of PA, PT and HC have the same methylation level; while PA exposure corresponded to an increase of methylation in HC and a decrease of methylation in PT. Consistently, we found a weak global correlation in methylation profiles associated with PA between groups.
The increase of methylation at these DMRs in HC may be part of an adaptive epigenetic response to PA, which could be absent in PT, who indeed have lower DNAm at these DMRs, in the context of PA. PA may induce different responses in the epigenome, and some of these paths may increase the risk for psychiatric disorders. Since previous studies have found alterations with LINGO3 [44,45,46,47], BLCAP;NNAT [48,49,50,51] and SLC2A14 [52, 53] genes or associated functions in SZ and BD, it might be possible that an altered response to PA at the DNAm and/or gene expression levels may disrupt brain development and function in PT. DNAm increases in HC might represent epigenetic modifications influenced by environmental exposure of PA, which may be associated with increased susceptibility to somatic diseases later in life, but not necessarily the transition to severe mental disorders. It may also represent protective-adaptive alterations that may increase resilience in healthy individuals later in life.
Due to its availability and ease of collection, this EWAS used blood as a surrogate tissue rather than disease specific tissue; however, methylation levels in blood and brain-tissue have a concordance [54]. To further address this issue, we performed secondary analyses on the probes associated with LINGO3, BLCAP;NNAT, NANOS2 and SLC2A14 to investigate the relationship between DNAm in blood and at the same genomic loci in four brain regions (prefrontal cortex, entorhinal cortex, superior temporal gyrus and cerebellum) [40] and found significant correlations. However, because the response to PA is systemic and given the stability of DNAm profiles, our findings have relevance to psychiatric disorder even in the absence of a proven brain-blood correlation. In other words, changes in DNAm detected in blood may reveal an epigenetic mechanism of response to PA which is relevant for the development of psychiatric disorders.
Nonetheless, we detected significant interactions between PA and DNAm at the level of DMR mapping to loci containing genes with relevant brain functions. Particularly, LINGO3, a paralog to LINGO1, is expressed in neurons and a highly restricted population of Olig2-expressing oligodendroglia cells. A functional overlap between LINGO3 and LINGO1 has been suggested [55]. The more studied, LINGO1, is a potent negative regulator of neuron and oligodendrocyte survival, axon regeneration, neurite extension, oligodendrocyte differentiation, axonal myelination and functional recovery; these processes are highly involved in numerous brain functions [56]. Reduced oligodendrocyte densities have been reported in both SZ and BD [44,45,46,47]. In SZ, LINGO1 has been found to be upregulated in the dorsolateral prefrontal cortex and hippocampus [57] and associated with the dysregulation in apoptosis of neurons [58], which might explain previously observed reductions in mean total neuron number in the putamen and caudate nucleus of individuals with SZ [59]. So, if the DNAm changes that we detected in blood are mirrored by DNAm changes in the brain, as our results suggest, we could speculate that PA may lead to a decrease of methylation in individuals who later in life developed SZ or BD. Such DNAm decrease may be associated with an increased LINGO3 expression, consistent with the findings of increased LINGO1 expression and reduced oligodendrocyte densities in SZ and BD.
For the overlapping BLCAP;NNAT genes, the BLCAP gene encodes a protein that reduces cell growth by stimulating apoptosis, and the imprinted gene NNAT encodes a protein associated with brain development, assembly, maturation and maintenance of the central nervous system (CNS) [60]. A recent EWAS study found that the DNAm of BLCAP;NNAT were strongly associated with left-handedness [61], a trait with low heritability and where epigenetic mechanisms have been proposed as an underlying etiological mechanism. Numerous scientific studies have demonstrated a higher occurrence of left-handedness in an array of psychiatric disorders, supporting the view that there is a genetic link between handedness and brain lateralization [48,49,50,51]. It is also known that left-handedness is associated with complications in the perinatal period [48, 62]. Consistent with these studies, we report here a higher frequency of non-right handers among PT who experienced PA compared to PA in the HC.
Of note, NNAT is an imprinted gene [60], and alterations in the DNAm of imprinted genes (i.e. genes that carry parental allele-specific methylation profiles) have been documented in studies of in utero exposure to dietary micronutrients [63, 64], caloric restriction [65,66,67], protein restriction [68] and cigarette smoking [69]. The PA related differences in the DNAm of the NNAT gene reported here may be an example of a methylation alteration in an imprinted gene that can serve as a useful biosensor of adverse environmental exposures that also include PA [70].
NANOS2 and Nanos genes are expressed in fetal and adult testis and ovary and the adult brain, particularly the hippocampus [71], and are known for their evolutionarily preserved role in germ cell pluripotency and survival [72]. Nanos proteins are important in the development of the CNS [71, 73], but also their overexpression is associated with various human cancers [72, 74], consistent with the link between cancer and gene regulatory network acting during development. Specifically, alterations due to DNAm of Nanos genes have been associated with hepatocellular carcinoma [75], prostate cancer [76], thyroid carcinomas [77], adult [78] and childhood [79] asthma, type II diabetes [80] and metabolic syndrome [81]. Of interest, an animal study has detected increased NANOS2 expression in the hypothalamus following exposures to maternal deprivation, consistent with the possibility that other early life stressors, in addition to PA, may alter the epigenetic status of this gene, whose function as a zinc finger protein may contribute to regulate the translation of genes relevant for development [82].
SLC2A14 gene, a paralog of SLC2A3, is highly expressed in the brain and other tissues: cardiomyocytes, placenta, white blood cells, and others [83]. In both the neocortex and in deeper cortical structures, SLC2A3 seems to be primarily localized to axonal and dendritic processes, suggesting an important role for SLC2A3 in ATP‐dependent axonal transport processes and synaptic plasticity [84]. Furthermore, SLC2A3 has been shown to strongly respond to hypoxic stress [85] and it has been shown that hypoxia-induced chromatin conformation changes influence SLC2A3 expression and functions in multiple cell types [86]. Consistent with our findings, it has been suggested that alterations in SLC2A3 gene dosage interfere with neurodevelopment of individuals later diagnosed with a neuropsychiatric disorders, such as ADHD [87], SZ [52] and BD [53].
A strength of this study is the prospective birth registry information on PA that was available on a large sample of individuals with SZ or BD and HC. A limitation to the study is represented by our sample size, which reduces the power to detect changes at the differentially methylated position level. Stratifying by sex reduced the sample size considerably, so we interpret these findings and associations with disease severity and age of onset with caution.
Another limitation to this study is that we define PA based on codes from the MBRN, which may not fulfill modern criteria for PA [88]. Indeed, most of the participants were born before 1990, thus before the definition of current criteria for PA [88]. Additionally, the use of encoded data from the MBRN may not accurately describe the clinical picture of the condition and may account for the over-registration of PA (>10%) in this study. However, given that MBRN data are collected from maternity wards in Norwegian hospitals, we can assume that the conditions classified as PA in the MBRN (asphyxia without other signs, asphyxia with poor sound, asphyxia and discolored amniotic fluid, asphyxia, mild or moderate birth asphyxia) were defined based on the ICD classification of asphyxia (e.g., “failing to initiate and sustain breathing at birth”), which should also capture more recent strict definition of this important complication. Inflammatory presentation is unclear in the MBRN. Since inflammation together with PA increases the risk for neural damage [89], milder insults may cause injury or alterations in those with a genetic liability for SZ development [90]. Given that PA is associated with neurodevelopmental deficits later in life, the exclusion criteria of the TOP study (i.e., exclusion based neurological diseases and intellectual impairments) might not fully capture the full range of PA exposure in our patient sample. Additionally, the exclusion criteria might be the reason for the lower percentage of PA in the SZ group, which have a higher genetic liability for SZ but may also have a lower tolerance for PA.
Since common genomic variants and epigenetic modifications influenced by environmental risk factors are frequent in a population and in part shared with other psychiatric disorders [91], future studies might include larger and more diverse cohorts of mental and neurodevelopmental disorders to investigate the specificity of these findings. Given the polypharmacy among patients, a potential effect of medication was unavoidable and is a limitation of any clinical study, including ours. Future studies addressing the influence of psychiatric medication and simultaneous use of several psychiatric medications on DNAm, also in combination with early life complications, might be important. Since PA commonly co-occurs with other severe obstetric complications [35], we cannot rule out that other underlying conditions like placental pathophysiology, in which placental failure can lead to asphyxia and subsequent growth restriction or preterm births [92] (see Supplementary Note 1), might influence DNAm in our sample. Additionally, possible maternal risk factors, such as preeclampsia, maternal diabetes and BMI, could influence developmental outcomes in offspring and may explain both the DMRs as well as later PT status. Future work in larger samples should include additional environmental risk factors like maternal and placental conditions and exposures in combination with DNAm and offspring patient status to better define what may drive the differential methylation in response to PA.
We identified four DMRs for LINGO3, BLCAP;NNAT, NANOS2 and SLC2A14 genes that significantly interacted with PA on case-control status, where methylation patterns differed with PA exposure between groups. These findings provide further evidence of alterations in the response to hypoxia and oxidative stress and may underscore the contribution of PA as a source of the shared etiopathogenetic mechanisms leading to the abnormal brain development commonly observed in schizophrenia and bipolar disorder. Prevention strategies may benefit from understanding the possible divergent epigenetic responses to PA that give rise to a healthy brain development or to trajectories of risk for psychiatric disorders. We mainly assessed DNAm in adults but examining infants or young children might be of interest for future studies aimed at detecting epigenetic changes specific to early stages of development, which could represent biomarkers of risk.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy or ethical restrictions.
References
Song J, Bergen SE, Kuja-Halkola R, Larsson H, Landen M, Lichtenstein P. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;17:184–93.
Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97.
Bienvenu OJ, Davydow DS, Kendler KS. Psychiatric ‘diseases’ versus behavioral disorders and degree of genetic influence. Psychol Med. 2011;41:33–40.
Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar disorders. Nat Rev Dis Prim. 2018;4:18008.
Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–29.
Khanzada NS, Butler MG, Manzardo AM. Gene analytics pathway analysis and genetic overlap among autism spectrum disorder, bipolar disorder and schizophrenia. Int J Mol Sci. 2017;18:527.
Trubetskoy V, Pardinas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8.
Hindley G, O’Connell KS, Rahman Z, Frei O, Bahrami S, Shadrin A, et al. The shared genetic basis of mood instability and psychiatric disorders: a cross-trait genome-wide association analysis. Am J Med Genet B Neuropsychiatr Genet. 2022;189:207–18.
Hannon E, Dempster EL, Mansell G, Burrage J, Bass N, Bohlken MM, et al. DNA methylation meta-analysis reveals cellular alterations in psychosis and markers of treatment-resistant schizophrenia. Elife 2021;10:e58430.
Khavari B, Cairns MJ. Epigenomic dysregulation in dchizophrenia: in search of disease etiology and biomarkers. Cells. 2020;9:1837.
Richetto J, Meyer U. Epigenetic modifications in schizophrenia and related disorders: molecular scars of environmental exposures and source of phenotypic variability. Biol Psychiatry. 2021;89:215–26.
Smigielski L, Jagannath V, Rossler W, Walitza S, Grunblatt E. Epigenetic mechanisms in schizophrenia and other psychotic disorders: a systematic review of empirical human findings. Mol Psychiatry. 2020;25:1718–48.
Davies C, Segre G, Estrade A, Radua J, De Micheli A, Provenzani U, et al. Prenatal and perinatal risk and protective factors for psychosis: a systematic review and meta-analysis. Lancet Psychiatry. 2020;7:399–410.
Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15:485–515.
Nalivaeva NN, Turner AJ, Zhuravin IA. Role of prenatal hypoxia in brain development, cognitive functions, and neurodegeneration. Front Neurosci. 2018;12:825.
Zornberg GL, Buka SL, Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry. 2000;157:196–202.
Wortinger L, Engen K, Barth C, Andreassen O, Jørgensen K, Agartz I. Asphyxia at birth affects brain structure in patients on the schizophrenia- bipolar disorder spectrum and healthy participants. Psychol Med. 2020;52:1–10.
Dalman C, Thomas HV, David AS, Gentz J, Lewis G, Allebeck P. Signs of asphyxia at birth and risk of schizophrenia. Population-based case-control study. Br J Psychiatry. 2001;179:403–8.
Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, Yin L, et al. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry. 2012;69:E1–8.
Akkouh IA, Ueland T, Hansson L, Inderhaug E, Hughes T, Steen NE, et al. Decreased IL-1beta-induced CCL20 response in human iPSC-astrocytes in schizophrenia: potential attenuating effects on recruitment of regulatory T cells. Brain Behav Immun. 2020;87:634–44.
Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–97.
Schmidt-Kastner R, Guloksuz S, Kietzmann T, van Os J, Rutten BPF. Analysis of GWAS-derived schizophrenia genes for links to ischemia-hypoxia response of the brain. Front Psychiatry. 2020;11:393.
Ducsay CA, Goyal R, Pearce WJ, Wilson S, Hu XQ, Zhang L. Gestational hypoxia and developmental plasticity. Physiol Rev. 2018;98:1241–334.
Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsycho Pharmacology. 2013;38:23–38.
Wagner JR, Busche S, Ge B, Kwan T, Pastinen T, Blanchette M. The relationship between DNA methylation, genetic and expression inter-individual variation in untransformed human fibroblasts. Genome Biol. 2014;15:R37.
Dempster E, Viana J, Pidsley R, Mill J. Epigenetic studies of schizophrenia: progress, predicaments, and promises for the future. Schizophr Bull. 2013;39:11–6.
Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–5.
Spitzer RL, Williams JB & Gibbon M. First MB structured clinical interview for DSM-III-R-patient version (SCID-P). Arch Gen Psychiatry. 1988. https://doi.org/10.1001/archpsyc.1992.01820080032005.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8.
Pedersen G, Hagtvet KA, Karterud S. Generalizability studies of the global assessment of functioning-split version. Compr Psychiatry. 2007;48:88–94.
Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88:791–804.
Berman AH, Bergman H, Palmstierna T, Schlyter F. Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. Eur Addict Res. 2005;11:22–31.
Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV 3rd, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. Jama. 1994;272:1749–56.
Wortinger LA, Barth C, Nerland S, Jorgensen KN, Shadrin AA, Szabo A, et al. Association of birth asphyxia with regional white matter abnormalities among patients with schizophrenia and bipolar disorders. JAMA Netw Open. 2021;4:e2139759.
Zhou W, Laird PW, Shen H. Comprehensive characterization, annotation and innovative use of Infinium DNA methylation BeadChip probes. Nucleic Acids Res. 2017;45:e22.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–27.
Elliott HR, Tillin T, McArdle WL, Ho K, Duggirala A, Frayling TM, et al. Differences in smoking associated DNA methylation patterns in South Asians and Europeans. Clin Epigenetics. 2014;6:4.
Mallik S, Odom GJ, Gao Z, Gomez L, Chen X, Wang L. An evaluation of supervised methods for identifying differentially methylated regions in Illumina methylation arrays. Brief Bioinform. 2019;20:2224–35.
Hannon E, Lunnon K, Schalkwyk L, Mill J. Interindividual methylomic variation across blood, cortex, and cerebellum: implications for epigenetic studies of neurological and neuropsychiatric phenotypes. Epigenetics. 2015;10:1024–32.
Du P, Zhang X, Huang CC, Jafari N, Kibbe WA, Hou L, et al. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinforma. 2010;11:587.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300.
Fieller EC, Hartley HO, Pearson ES. Tests for rank correlation coefficients. I. Biometrika. 1957;44:470–81.
Bellani M, Boschello F, Delvecchio G, Dusi N, Altamura CA, Ruggeri M, et al. DTI and myelin plasticity in bipolar disorder: integrating neuroimaging and neuropathological findings. Front Psychiatry. 2016;7:21.
Kolomeets NS, Uranova NA. Reduced oligodendrocyte density in layer 5 of the prefrontal cortex in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2019;269:379–86.
Falkai P, Malchow B, Wetzestein K, Nowastowski V, Bernstein HG, Steiner J, et al. Decreased oligodendrocyte and neuron number in anterior hippocampal areas and the entire hippocampus in schizophrenia: a stereological postmortem study. Schizophr Bull. 2016;42:S4–12.
Vostrikov VM, Uranova NA. Reduced density of oligodendrocytes and oligodendrocyte clusters in the caudate nucleus in major psychiatric illnesses. Schizophr Res. 2020;215:211–6.
Hirnstein M, Hugdahl K. Excess of non-right-handedness in schizophrenia: meta-analysis of gender effects and potential biases in handedness assessment. Br J Psychiatry. 2014;205:260–7.
Berlim MT, Mattevi BS, Belmonte-de-Abreu P, Crow TJ. The etiology of schizophrenia and the origin of language: overview of a theory. Compr Psychiatry. 2003;44:7–14.
Francks C, Maegawa S, Lauren J, Abrahams BS, Velayos-Baeza A, Medland SE, et al. LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Mol psychiatry. 2007;12:1129–39.
Wiberg A, Ng M, Al Omran Y, Alfaro-Almagro F, McCarthy P, Marchini J, et al. Handedness, language areas and neuropsychiatric diseases: insights from brain imaging and genetics. Brain A J Neurol. 2019;142:2938–47.
Dean B, Thomas N, Scarr E, Udawela M. Evidence for impaired glucose metabolism in the striatum, obtained postmortem, from some subjects with schizophrenia. Transl Psychiatry. 2016;6:e949.
Yang S, Wang K, Gregory B, Berrettini W, Wang LS, Hakonarson H, et al. Genomic landscape of a three-generation pedigree segregating affective disorder. PLoS One. 2009;4:e4474.
Braun PR, Han S, Hing B, Nagahama Y, Gaul LN, Heinzman JT, et al. Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Transl Psychiatry. 2019;9:47.
Guillemain A, Laouarem Y, Cobret L, Stefok D, Chen W, Bloch S, et al. LINGO family receptors are differentially expressed in the mouse brain and form native multimeric complexes. FASEB J. 2020;34:13641–53.
Andrews JL, Fernandez-Enright F. A decade from discovery to therapy: Lingo-1, the dark horse in neurological and psychiatric disorders. Neurosci Biobehav Rev. 2015;56:97–114.
Fernandez-Enright F, Andrews JL, Newell KA, Pantelis C, Huang XF. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4:e348.
Reis-de-Oliveira G, Zuccoli GS, Fioramonte M, Schimitt A, Falkai P, Almeida V, et al. Digging deeper in the proteome of different regions from schizophrenia brains. J Proteom. 2020;223:103814.
Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, et al. Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain A J Neurol. 2007;130:678–92.
Evans HK, Weidman JR, Cowley DO, Jirtle RL. Comparative phylogenetic analysis of blcap/nnat reveals eutherian-specific imprinted gene. Mol Biol Evol. 2005;22:1740–8.
Odintsova V, Sudermann M, Hagenbeek F, Caramaschi D, Hottenga J-J, Pool R, et al. Epigenome-wide association study of left-handedness for different tissues and ages. Res Square. 2021. https://doi.org/10.21203/rs.3.rs-375556/v1.
Sperling W, Martus P, Barocka A. Non-right-handedness and obstetrical complications in paranoid hallucinatory schizophrenics. Psychopathology. 1999;32:267–76.
Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, Steegers EA, et al. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One. 2009;4:e7845.
Hoyo C, Murtha AP, Schildkraut JM, Jirtle RL, Demark-Wahnefried W, Forman MR, et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics. 2011;6:928–36.
Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18:4046–53.
Waterland RA, Kellermayer R, Laritsky E, Rayco-Solon P, Harris RA, Travisano M, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 2010;6:e1001252.
Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–9.
Gong L, Pan YX, Chen H. Gestational low protein diet in the rat mediates Igf2 gene expression in male offspring via altered hepatic DNA methylation. Epigenetics. 2010;5:619–26.
Murphy SK, Adigun A, Huang Z, Overcash F, Wang F, Jirtle RL, et al. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene. 2012;494:36–43.
Murphy SK, Huang Z, Hoyo C. Differentially methylated regions of imprinted genes in prenatal, perinatal and postnatal human tissues. PLoS One. 2012;7:e40924.
Haraguchi S, Tsuda M, Kitajima S, Sasaoka Y, Nomura-Kitabayashid A, Kurokawa K, et al. nanos1: a mouse nanos gene expressed in the central nervous system is dispensable for normal development. Mech Dev. 2003;120:721–31.
De Keuckelaere E, Hulpiau P, Saeys Y, Berx G, van Roy F. Nanos genes and their role in development and beyond. Cell Mol Life Sci. 2018;75:1929–46.
Sabariego M, Moron I, Gomez MJ, Donaire R, Tobena A, Fernandez-Teruel A, et al. Incentive loss and hippocampal gene expression in inbred Roman high- (RHA-I) and Roman low- (RLA-I) avoidance rats. Behav Brain Res. 2013;257:62–70.
Janic A, Mendizabal L, Llamazares S, Rossell D, Gonzalez C. Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science. 2010;330:1824–7.
Sun XJ, Wang MC, Zhang FH, Kong X. An integrated analysis of genome-wide DNA methylation and gene expression data in hepatocellular carcinoma. FEBS Open Bio. 2018;8:1093–103.
Aref-Eshghi E, Schenkel LC, Ainsworth P, Lin H, Rodenhiser DI, Cutz JC, et al. Genomic DNA methylation-derived algorithm enables accurate detection of malignant prostate tissues. Front Oncol. 2018;8:100.
Beltrami CM, Dos Reis MB, Barros-Filho MC, Marchi FA, Kuasne H, Pinto CAL, et al. Integrated data analysis reveals potential drivers and pathways disrupted by DNA methylation in papillary thyroid carcinomas. Clin Epigene. 2017;9:45.
Nicodemus-Johnson J, Myers RA, Sakabe NJ, Sobreira DR, Hogarth DK, Naureckas ET, et al. DNA methylation in lung cells is associated with asthma endotypes and genetic risk. JCI Insight. 2016;1:e90151.
Forno E, Wang T, Qi C, Yan Q, Xu CJ, Boutaoui N, et al. DNA methylation in nasal epithelium, atopy, and atopic asthma in children: a genome-wide study. Lancet Respir Med. 2019;7:336–46.
Florath I, Butterbach K, Heiss J, Bewerunge-Hudler M, Zhang Y, Schottker B, et al. Type 2 diabetes and leucocyte DNA methylation: an epigenome-wide association study in over 1,500 older adults. Diabetologia. 2016;59:130–8.
Chitrala KN, Hernandez DG, Nalls MA, Mode NA, Zonderman AB, Ezike N, et al. Race-specific alterations in DNA methylation among middle-aged African Americans and Whites with metabolic syndrome. Epigenetics. 2020;15:462–82.
Ding F, Li HH, Li J, Myers RM, Francke U. Neonatal maternal deprivation response and developmental changes in gene expression revealed by hypothalamic gene expression profiling in mice. PLoS One. 2010;5:e9402.
Ziegler GC, Almos P, McNeill RV, Jansch C, Lesch KP. Cellular effects and clinical implications of SLC2A3 copy number variation. J Cell Physiol. 2020;235:9021–36.
Mantych GJ, James DE, Chung HD, Devaskar SU. Cellular localization and characterization of Glut 3 glucose transporter isoform in human brain. Endocrinology. 1992;131:1270–8.
Lauer V, Grampp S, Platt J, Lafleur V, Lombardi O, Choudhry H, et al. Hypoxia drives glucose transporter 3 expression through hypoxia-inducible transcription factor (HIF)-mediated induction of the long noncoding RNA NICI. J Biol Chem. 2020;295:4065–78.
Mimura I, Nangaku M, Kanki Y, Tsutsumi S, Inoue T, Kohro T, et al. Dynamic change of chromatin conformation in response to hypoxia enhances the expression of GLUT3 (SLC2A3) by cooperative interaction of hypoxia-inducible factor 1 and KDM3A. Mol Cell Biol. 2012;32:3018–32.
Ziegler G, Jansch C, Almos P, Conzelmann A, Hahn T, Weber H, et al. SLC2A3 copy number variants in ADHD–from cellular to clinical correlates. Pharmacopsychiatry 2020;53:5.
Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ task force on neonatal encephalopathy. Obstet Gynecol. 2014;123:896–901.
Serdar M, Kempe K, Rizazad M, Herz J, Bendix I, Felderhoff-Muser U, et al. Early pro-inflammatory microglia activation after inflammation-sensitized hypoxic-ischemic brain injury in neonatal rats. Front Cell Neurosci. 2019;13:237.
Ursini G, Punzi G, Chen Q, Marenco S, Robinson JF, Porcelli A, et al. Convergence of placenta biology and genetic risk for schizophrenia. Nat Med. 2018;24:792–801.
Legge SE, Santoro ML, Periyasamy S, Okewole A, Arsalan A, Kowalec K. Genetic architecture of schizophrenia: a review of major advancements. Psychol Med. 2021;51:2168–77.
Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452–7.
Acknowledgements
We would like to thank the participants of the TOP, Youth-TOP and TIPS studies and express gratitude for the hard work of the clinicians, research assistants and referring hospital units involved in recruitment and assessment of participants. We thank Thomas Bjella for assistance with the TOP database. This work was partly performed on the TSD (Tjeneste for Sensitive Data) facilities, owned by the University of Oslo, and operated and developed by the TSD service group at the University of Oslo, IT-Department (USIT). Funding for this study was provided by the South-Eastern Norway Regional Health Authority (grant number: 2020-020) and the Research Council of Norway (grant number: 223273 and 273446).
Author information
Authors and Affiliations
Contributions
LAW, IA and SLH conceived the study. LAW primarily wrote the manuscript and performed statistical analyses. AKS pre-processed and QCed the DNAm data and performed the EWAS and other statistical analyses. GU contributed to the study design, interpretation of data and commenting on the original draft of the article. IA and OAA acquired the data. All authors contributed to revisions of the manuscript and have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
OAA has received speaker’s honorarium from Lundbeck, Janssen and Sunovion and is a consultant for cortechs.ai. IA has received speaker’s honorarium from Lundbeck. The remaining authors report no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wortinger, L.A., Stavrum, AK., Shadrin, A.A. et al. Divergent epigenetic responses to perinatal asphyxia in severe mental disorders. Transl Psychiatry 14, 16 (2024). https://doi.org/10.1038/s41398-023-02709-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02709-7