Abstract
Preclinical rodent models suggest that psychosis involves alterations in the activity and glutamatergic function in the hippocampus, driving dopamine activity through projections to the striatum. The extent to which this model applies to the onset of psychosis in clinical subjects is unclear. We assessed whether interactions between hippocampal glutamatergic function and activity/striatal connectivity are associated with adverse clinical outcomes in people at clinical high-risk (CHR) for psychosis. We measured functional Magnetic Resonance Imaging of hippocampal activation/connectivity, and 1H-Magnetic Resonance Spectroscopy of hippocampal glutamatergic metabolites in 75 CHR participants and 31 healthy volunteers. At follow-up, 12 CHR participants had transitioned to psychosis and 63 had not. Within the clinical high-risk cohort, at follow-up, 35 and 17 participants had a poor or a good functional outcome, respectively. The onset of psychosis (ppeakFWE = 0.003, t = 4.4, z = 4.19) and a poor functional outcome (ppeakFWE < 0.001, t = 5.52, z = 4.81 and ppeakFWE < 0.001, t = 5.25, z = 4.62) were associated with a negative correlation between the hippocampal activation and hippocampal Glx concentration at baseline. In addition, there was a negative association between hippocampal Glx concentration and hippocampo-striatal connectivity (ppeakFWE = 0.016, t = 3.73, z = 3.39, ppeakFWE = 0.014, t = 3.78, z = 3.42, ppeakFWE = 0.011, t = 4.45, z = 3.91, ppeakFWE = 0.003, t = 4.92, z = 4.23) in the total CHR sample, not seen in healthy volunteers. As predicted by preclinical models, adverse clinical outcomes in people at risk for psychosis are associated with altered interactions between hippocampal activity and glutamatergic function.
Similar content being viewed by others
Introduction
The onset of psychosis is commonly preceded by a clinical high-risk (CHR) phase, characterised by ‘attenuated’ psychotic symptoms and a marked decline in social and occupational functioning [1]. This syndrome is associated with a 20–30% risk of developing psychosis in the following 2–3 years [1,2,3].
Data from preclinical studies in rats suggest that the onset of psychosis involves an increase in resting hippocampal activity [4,5,6], which may be secondary to a dysregulation of hippocampal glutamatergic neurotransmission [5, 7]. This primary hippocampal dysfunction is then thought to drive an increase in subcortical dopamine activity, through modulatory glutamatergic projections from the hippocampus to striatum [6] (Fig. 1).
In line with preclinical studies, human neuroimaging studies in CHR populations indicate that the CHR state is associated with increased resting activity and perfusion in the hippocampus [8,9,10], and with altered hippocampal activation in response to tasks that involve salience processing [11,12,13] or verbal memory [14]. There is also evidence that the concentration of glutamatergic metabolites in the hippocampus is altered in CHR subjects [15] and is related to hippocampal activation during verbal memory tasks in this group [16]. Altered hippocampal activity and striatal functioning are associated with adverse outcomes in CHR individuals [17]. Broadly consistent with preclinical studies [4,5,6,7], neuroimaging studies in CHR individuals have identified alterations in the functional connectivity of the hippocampus [18, 19] and the striatum [20, 21] with other brain areas, and in hippocampo-striatal connectivity [12, 13]. Some of these neuroimaging findings in CHR samples have been linked to adverse clinical outcomes subsequent to scanning. For example, alterations in hippocampal activation [22] and glutamate levels [15] have each been independently linked to the later onset of psychosis.
To date, however, associations between neuroimaging measures and clinical outcomes in CHR samples have largely been identified in studies of a single neuroimaging metric. Yet, contemporary models emphasise the interrelated nature of physiological and neurochemical dysfunction in the hippocampus, and its interaction with the striatum through glutamatergic connections [6]. Given this aetiological complexity, assessing interactions between neuroimaging measures of different abnormalities might better predict clinical outcomes in CHR subjects than a single neurobiological measure. This is consistent with evidence that models, which incorporate multiple variables can predict outcomes in CHR subjects [23] and patients with psychosis [24, 25] with greater accuracy than models based on a single variable [26, 27]. The aim of the present case-control study was to use multimodal neuroimaging data to examine whether clinical outcomes in CHR participants were associated with interactions between the hippocampal activity, glutamatergic function, and hippocampo-striatal connectivity. We tested the hypothesis that alterations in the relationship between these measures would be associated with adverse subsequent clinical outcomes.
Materials and methods
Participants
One hundred and six individuals were recruited to the study. Seventy-five participants were at clinical high risk of psychosis (CHR), and 31 were healthy controls (HC). The study was approved by the National Research Ethics Service Committee of London-Camberwell St Giles, United Kingdom. All participants gave written informed consent. Data collection took place between November 1, 2011, and November 1, 2017. As no previous effect size data were available, we based our groups sizes on previous studies in Schizophrenia populations that reported significant group effects in the medial temporal lobe with n~20 in each group (e.g. Allen et al., 2011/12) and a recommendation that groups of n~20 are suitable for detecting medium effect sizes d’ ~0.05 [28]. Our control and CHR-NT groups are these suitable sizes, but our CHR-T group is underpowered. This is because we cannot ensure the size of an outcome group in a prospective study of this type. We have discussed this as a limitation (see discussion).
Clinical high-risk (CHR) participants were recruited through four early detection services for people at clinical high risk for psychosis: Outreach and Support in South London (OASIS), the West London Early Intervention service, the Cambridge Early Onset service (CAMEO), and the Coventry and Warwickshire Partnership NHS trust. CHR participants were assessed using the Comprehensive Assessment of At Risk Mental States (CAARMS) [1, 29]. Individuals were excluded from the CHR group based on the following criteria: past/present diagnosis of psychotic disorders, past/present familiar history of neurological illness, substance abuse/dependence as defined using DSM-5 criteria [30], or contraindication to MRI scanning.
Healthy control (HC) participants were recruited from the same geographical locations as CHR participants. HC participants were native English speakers, did not have a personal or familial history of psychiatric or neurological disorder and were not using the prescription medication as assessed via self-report. Further exclusion criteria were self-reported illicit substance use in the week before MRI scanning or alcohol use in the 24 h before MRI scanning.
Premorbid IQ was assessed using the National Adult Reading Test (NART) [31], and handedness using the Annett Handedness Scale [32]. Participants self-reported information on gender, tobacco use (number of cigarettes smoked per day), and cannabis use (0 indicated no use; 1 indicated experimental use; 2 indicated occasional use; 3 indicated moderate use; 4 indicated severe use).
The main outcome measures of the study were hippocampal 1H-MRS glutamatergic and fMRI data acquired from the same participants during the same MRI scanning sessions. These two datasets have previously been analysed and reported separately [13, 15]. The current study included all participants where both 1H-MRS and fMRI data were available. As such, the final sample comprised 75 CHR participants and 31 HCs. See Table 1 for reported demographic and clinical outcome characteristics.
Clinical measures
After recruitment, the following clinical measures were collected at King’s College London on the day of MRI scanning by trained assessors: psychopathology using the CAARMS [29]; overall functioning using the Global Assessment of Function (GAF) [33], and anxiety and depression symptoms using the Hamilton Anxiety and Depression Scale (HAM-A/HAM-D) [34].
Clinical follow-up
CHR participants were followed-up at a mean of 18.4 months (SD = 12.8 months) after MRI scanning to determine clinical and functional outcomes. Transition to psychosis was assessed using the CAARMS Psychosis Threshold criteria [29] and confirmed with the Structured Clinical Interview for Diagnosis [30], administered by a psychiatrist trained in its use. Of the 75 CHR individuals included in the current analysis, 23 were not assessed at follow-up because they were too unwell, declined to be interviewed, or were not contactable. In these CHR participants, transition or nontransition to psychosis was determined from their clinical records, but it was not possible to assess their level of functioning. To ensure that the exclusion of three participants that were too unwell to be followed-up did not affect the results, we conducted independent samples t tests to compare baseline CAARMS scores in these participants with those who were assessed at follow-up (Supplementary Table 1). We assessed whether the follow-up period differed between clinical (transition vs. nontransition) and functional (poor vs. good functioning) subgroups using independent samples t tests.
MRI data acquisition and preprocessing
The present study analysed task-based fMRI data and 1H-MRS data acquired from the same participants during the same MRI session. Details of the fMRI novelty salience task used, scan acquisition parameters, preprocessing, modelling of fMRI data, and the acquisition and analysis of hippocampal 1H-MRS data have previously been described in detail in publications that report each data modality separately [13, 15] (Supplementary Fig. 1). We include the MRS scan quality parameters for the current cohort in Supplementary Tables 2, 3 and 4. In the present study, fMRI data analysis focused on the task contrast of novel > neutral oddball trials during the novelty salience task, which was used as a measure of ‘pure stimulus novelty’ [13, 35].
Statistical analysis
Demographic and behavioural data
Differences between the HC and CHR participants for age, gender and handedness were assessed using independent sample t tests (for continuous data) or chi-square (for categorical data) in SPSS 23 (https://www.ibm.com/uk-en/products/spss-statistics). Alongside group comparisons of all CHR vs. HC participants, the CHR group was subdivided according to clinical and functional outcomes at follow-up. Clinical outcome groups (transition to psychosis) comprised CHR participants that had developed psychosis (CHR-T) and those that had not (CHR-NT). Functional outcome was defined as the GAF score at the end of the follow-up period, with a score of >65 corresponding to a good level of functioning (CHR-good), and a score of <65 corresponding to a poor level of functioning (CHR-poor) [15, 36]. Group differences during the fMRI novelty salience task, for reaction time, target recognition and error rate were assessed using independent sample t tests in SPSS 23. Significant results are reported at p < 0.05.
fMRI and 1H-MRS data analysis
Group x 1H-MRS interaction (effects during novel > neutral oddball trial)
To test our a-priori hypothesis, we used multivariate random-effects GLM in SPM 12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Specifically, we tested interaction effects between Group (CHR-NT vs. CHR-T, CHR-good vs. CHR-poor, and HC vs. CHR) and 1H-MRS Glx metabolite concentrations on hippocampal functional activity during novel>neutral oddball trials. We chose to use hippocampal Glx metabolite concentrations (combined glutamate and glutamine) as i) the composite Glx peak has been widely used as a marker of glutamatergic function because it predominantly reflects glutamate levels, which are typically 5–6 times higher than those of glutamine [37] and ii) a previous meta-analysis reports robust alterations in 1H-MRS Glx metabolite concentrations in schizophrenia [38]. Given our previous findings in this CHR cohort [15] additional interaction analysis using 1H-MRS glutamate metabolite concentrations are also reported (see Supplementary Table 5).
Current tobacco use and age were included as nuisance covariates, as in previous analyses [13, 15]. We conducted ANOVA using hippocampal Glx metabolite concentration as a covariate of interest, restricting the search area to our a-priori region-of-interest within the bilateral hippocampus (AAL in WFU Pickatlas toolbox; https://www.nitrc.org/projects/wfu_pickatlas).
The initial alpha was set to 0.005, before applying a small volume correction (SVC) for the hippocampal region-of-interest (ROI) analysis, at a voxel-wise threshold of peak level family-wise error (FWE) p < 0.017 [26] to correct for three group tests, i.e. i) CHR-T vs. NT ii) CHR-good vs. CHR-poor and iii) CHR vs. HC.
Psychophysiological Interaction
To test our a-priori hypothesis regarding the effects of Group and 1H-MRS glutamatergic metabolite concentration on hippocampal– striatal functional connectivity, we used a Psychophysiological Interaction (PPI) analysis [39]. Based on novel>neutral oddball trials we included all subjects who showed significant activity within the hippocampal ROI. First, eigenvariates from the hippocampal seed region were extracted from the subject-specific model of the Group x 1H-MRS Glx analyses described above. The subject-specific response peak was required to be within a 6 mm radius sphere of the right hippocampal region [x, y, z = 36, -34, -4], i.e., within the group peak to be included in the PPI analysis. This was the case in 39 subjects (14 HC, 25 CHR). Subsequently, for each subject a PPI regressor was created via deconvolution of the eigenvariate time series by weighting the resultant time series with the task contrast time series (novel > neutral oddball trials), adjusted for the effect of interest, and reconvolved with the hemodynamic response function. The resulting contrast was submitted to second-level random-effects GLM to test the interaction effect between the Group and 1H-MRS Glx metabolite concentrations on hippocampal functional connectivity. ROIs were created using an atlas composed of functional subdivisions of the striatum (ventral, associative), which is commonly applied in Positron Emission Tomography (PET) research [40].
We investigated functional connectivity between the hippocampus and two striatal ROIs (ventral and associative striatum subdivisions) based on a preclinical model highlighting ventral striatal changes in psychosis [8, 10, 41, 42], and on reports of dopaminergic dysregulation in the associative striatum in psychosis [43]. The SVC results were considered significant at alpha = 0.005 and peak level FWE p (ppeakFWE) < 0.025 to adjust for the two striatal ROI (ventral and associative striatum) comparisons.
Results
Demographic, clinical and medication data
All demographic, clinical and medication data categorised by the group are summarised in Table 1. At baseline, the CHR group were younger and smoked more cigarettes, had higher levels of anxiety (HAM-A scores) and depression (HAM-D scores), and a lower level of functioning (GAF scores) than HC. At clinical follow-up, 12 CHR individuals (16% of the total CHR sample) had transitioned to psychosis (CHR-T) and 63 (84%) had not (CHR-NT). At baseline, the CHR-T group smoked fewer cigarettes than the CHR-NT group but did not differ on any other measures (see Table 1). At follow-up, GAF scores were available in 52 CHR participants. Seventeen CHR participants had a follow-up GAF score >65 indicating a good functional outcome, and 35 had a GAF score <65 indicating a poor functional outcome. The functional outcome group did not differ on any demographic measure (see Table 1). CAARMS and GAF score significantly correlated (n = 49, p < 0.001, r = −0.51). The follow-up period did not differ between subgroups: days until follow-up in CHR-NT subjects (N = 44, M = 20 months, SD = 13.5 months) did not differ significantly from that in CHR-T subjects (N = 11, M = 15.5 months, SD = 6.1 months) (p = 0.11), and days until follow-up in subjects with a good functional outcome (N = 17, M = 17 months, SD = 13.4 months) did not differ significantly from that in subjects with a poor functional outcome (N = 33, M = 18 months, SD = 8.8 months) (p = 0.78).
Behavioural data
During the novelty salience task, the mean reaction time for responses to target stimuli was 544 ms (SD = 142 ms), and the mean number of errors was 1.69 (SD = 3.1). There were no significant group differences in mean reaction time or target recognition time for any comparison (CHR-NT vs. CHR-T, CHR-good vs. CHR-poor, HC vs. CHR).
MRI: Interactions between Group, hippocampal Glx and functional activity
All Group x fMRI x 1H-MRS Glx interaction results are summarised in Table 2. To ensure that variance was similar between the CHR-T, CHR-NT and HC groups, we compared parameter estimates for hippocampal fMRI activation during the main effect of the task, which were similar (HC SD = 2.81, CHR-NT SD 2.87, CHR-T SD = 1.88).
Transition to psychosis
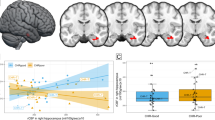
There was a significant interaction between group (CHR-T vs. CHR-NT) and 1H-MRS Glx metabolite concentrations on right hippocampal activation (ppeakFWE = 0.003, x y z = 36 −34 −4, t = 4.4, z = 4.19, k = 93). In the CHR participants who later developed psychosis, there was a negative association between Glx metabolite concentrations and right hippocampus activity that was not evident in the CHR participants who did not transition to psychosis (Fig. 2A, B).
A Scatterplot showing interaction between Group (CHR-T vs. CHR-NT) x hippocampal Glx on right hippocampal activation during novel > neutral oddball trials. B SPM brain map (coronal section) showing right hippocampal activation for Group (CHR-T vs. CHR-NT) x hippocampal Glx during novel > neutral oddball trials (P FWE < .017). C and D Scatterplots showing interaction between Group (CHR-poor vs. CHT-good) x hippocampal Glx on bilateral hippocampal activation during novel > neutral oddball trials. E) SPM brain map (coronal section) showing bilateral hippocampal activation for Group (CHR-good vs. CHR-poor) x hippocampal Glx on novel > neutral oddball trials (pFWE < .017).
CHR-good vs. poor functional outcome
There was a significant interaction between the functional outcome group (CHR-good vs. CHR-poor) and 1H-MRS Glx metabolite concentrations on hippocampal activity bilaterally (ppeakFWE < 0.001, x, y, z = 30 −12 −16, t = 5.52, z = 4.81, k = 507 & ppeakFWE = 0.001, x, y, z = −26 −28 −12, t = 5.25, z = 4.62, k = 585). In the CHR participants with a poor functional outcome, there was a negative relationship between hippocampal activity and local Glx concentration. Conversely, in the CHR subjects with a good functional outcome, this relationship was positive (Fig. 2C, D−E).
CHR vs. HC
The interaction between group (HC vs. CHR) and 1H-MRS Glx on hippocampal activation during novel>neutral oddball trials within the bilateral hippocampal ROI was non-significant (ppeakFWE = 0.052, x y z = 34 −34 −6, t = 3.46, z = 3.35, k = 146).
Interactions between Group, hippocampal Glx concentrations and hippocampal–striatal connectivity
PPI analyses focused on a right hippocampal seed region identified by the CHR-NT vs. CHR-T x 1H-MRS Glx interaction reported above (extracted eigenvariates x y z = 36 −34 −4). Analysis in relation to transition to psychosis and functional outcome did not reveal any significant PPI effects for hippocampo-striatal connectivity in either ventral or associative striatum ROIs (p > .025 for all analyses). Analysis comparing all CHR vs. HC participants, did reveal a significant interaction effect between group and Glx concentration within the a-priori ventral striatum ROI in the bilateral ventral caudate (ppeakFWE = .016, x y z = 6 12 0, t = 3.73, z = 3.39, cluster extent = 8 and ppeakFWE = 0.014, x y z = −10 12 −2, t = 3.78, z = 3.42, k = 50). An interaction was also observed in the a-priori associative striatum ROI in the dorsal bilateral caudate (ppeakFWE = .011, x y z = 18 −4 18, t = 4.45, z = 3.91, cluster extent = 570 and ppeakFWE = 0.003, x y z = -20 12 12, t = 4.92, z = 4.23, k = 352). In all these regions HC showed a positive association between hippocampal–striatal functional connectivity and hippocampal Glx metabolite concentrations. Conversely, in CHR participants, this relationship was negative (Fig. 3).
) Scatterplots showing PPI interactions between Group (CHR vs. HC) in the bilateral associative striatum and B) SPM brain map (axial section) showing bilateral functional connectivity in bilateral associative striatum for the Group (CHR vs HC) x hippocampal Glx during novel > neutral oddball trials (p FEW < 0.025). C Scatterplots showing PPI interactions between Group (CHR vs. HC) in the bilateral ventral striatum and D) SPM brain map (axial section) showing bilateral functional connectivity in bilateral ventral striatum for the Group (CHR vs. HC) x hippocampal Glx during novel > neutral oddball trails (p FWE < 0.025).
Discussion
Preclinical models propose that psychosis is associated with increased resting hippocampal activity [4,5,6], and altered hippocampal glutamate activity [5, 7] which is thought to drive an increase in subcortical dopamine activity through glutamatergic projections from the hippocampus to striatum [6]. In previous studies of CHR participants, we reported reduced hippocampal activity during a novelty salience task [13], and increased hippocampal glutamatergic metabolite concentrations related to clinical outcomes [15]. The present study extends these findings and provides support for preclinical models by demonstrating that clinical outcomes in CHR participants are also associated with altered interactions between hippocampal glutamatergic metabolite concentrations and task-related hippocampal activity. These findings are the first in humans to show that hippocampal glutamatergic concentrations are associated with altered hippocampal activity and function [7] in CHR individuals with adverse clinical and functional outcomes. Also in line with preclinical models, our data indicate that the relationship between hippocampal Glx concentrations and hippocampal–striatal connectivity was altered in CHR subjects relative to controls, although this association did not influence clinical outcomes.
In the subgroup of CHR subjects that later developed psychosis, there was a negative association between hippocampal Glx and hippocampal activation. Similarly, there was a negative association between these hippocampal measures in CHR subjects who had a low level of functioning at follow-up. A link between this negative association and adverse outcomes is broadly in line with our previous findings from single modality analyses: in separate studies, reduced hippocampal activation in CHR relative to control subjects [13] and adverse outcomes linked to increased hippocampal glutamate metabolite concentrations [15]. To our knowledge, our study presents the first human data to support preclinical evidence [4,5,6,7] that the altered hippocampal activity that predates psychosis reflects dysregulation of local glutamatergic transmission. As discussed by Modinos et al., (2020) the reduced hippocampal activation we observed during novel>neutral oddball stimuli in CHR participants likely reflects a ceiling effect due to increased resting hippocampal activity in CHR subjects [8, 10, 41]. Indeed, this was confirmed by our supplementary analysis of Group x 1H-MRS interaction effects which showed a positive association between hippocampal glutamatergic metabolite concentrations and activity during neutral > standard oddball stimuli in relation to functional outcome (see Supplementary Table 6).
In contrast to single modality findings within the CHR group in relation to clinical outcome, we did not find significant differences between the whole CHR group and healthy controls. This is unlikely to reflect a lack of statistical power, as the significant effects relating to outcomes involved CHR subgroups with smaller sample sizes. Rather, it may be related to the heterogeneity of neurobiological findings in the total CHR population: previous neuroimaging studies have reported greater differences within CHR samples in relation to outcomes than between CHR subjects and controls [15, 44,45,46].
Our prediction that the relationship between hippocampal glutamatergic metabolite concentrations and hippocampo-striatal connectivity would be linked to clinical outcomes was not confirmed, although there was an effect in relation to transition that did not survive correction for multiple testing, and our subgroups in this analysis were small. We examined hippocampal connectivity with the associative (dorsal) striatum due to its role in psychosis and psychosis risk [45, 47,48,49], and the ventral striatum as hippocampal glutamatergic outputs project to this subregion [4, 6, 43]. There is also evidence for altered salience and reward processing associated with ventral striatal activity in CHR subjects [11, 12, 50]. In the present study, we did show; however, that hippocampal Glx concentrations were associated with hippocampal—striatal functional connectivity in both the ventral and the dorsal caudate, although this effect was distributed more widely in its dorsal portion. For both sub-regions, there was a positive association between hippocampal Glx concentrations and functional connectivity in controls, but the opposite association in CHR subjects. Our data thus provides evidence for an association between both the ventral and the dorsal striatum and increased risk for psychosis.
The study has some limitations. Although the total number of CHR participants that we examined was relatively large for a multimodal neuroimaging study because only a minority developed psychosis subsequent to scanning, the size of this subgroup (N = 12) was modest. We cannot therefore exclude the possibility of Type I and Type II errors due to limited statistical power. This issue may be addressed by conducting studies that involve a large number of different centres, permitting the recruitment of larger CHR samples. Further, the average follow-up period was 18.4 months. Although a longer period of follow-up would have been ideal, meta-analysis of the incidence of psychosis in CHR samples indicates that the majority of transitions occur within the first 18 months, with only a relatively small number of additional transitions occurring over the subsequent 36 months [51, 52]. A more general limitation is that conventional 1H-MRS can provide a measure of the mean concentration of glutamatergic metabolites in a given region, but cannot determine which glutamatergic synapses or receptors are involved, or whether the signal reflects the transmitter or metabolic glutamate pools. These issues may be addressed through the development of SPECT or PET ligands that are specific for particular glutamate receptors [53,54,55], and the use of novel MRS techniques [40]. As in previous studies [56], we categorised outcomes in the CHR sample according to transition status and level of function at follow-up. Although the transition to psychosis is often associated with a low level of functioning, this is not always the case. Moreover, many subjects who do not progress to psychosis still have a poor functional outcome. In the present sample, 75% of those who developed psychosis also had a poor functional outcome, while among those who did not become psychotic, 65% had a poor functional outcome. Finally, we did not observe any group behavioural differences on the task. This is not unexpected, as the study was powered to detect task-related differences in fMRI response, rather than behavioural effects. Thus, although our results are consistent with the notion that salience processing is altered in CHR subjects, our evidence was at the neural rather than the behavioural level.
Our findings have potential clinical implications. First, they suggest that the ability of tools that are designed to help predict clinical outcomes in CHR subjects may be improved by using multiple, as opposed to single measures [57, 58]. Secondly, they add to existing evidence that hippocampal activity and glutamatergic function represent promising targets for the development of novel treatments for psychosis [6, 59,60,61,62,63].
In summary, we present the first evidence in humans, in line with rodent models of psychosis, that hippocampal activity and glutamatergic function are associated and that their interactions predict clinical and functional outcomes in CHR subjects. Future research should improve the prediction of outcomes in CHR subjects by incorporating multiple imaging measures in the predictive model, rather than using single risk factors alone.
Change history
30 November 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41398-021-01731-x
References
Yung AR, Phillips LJ, McGorry PD, McFarlane CA, Francey SM, Harrigan S, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry. 1998;172:14–20.
Häfner H. Onset and early course as determinants of the further course of schizophrenia. Acta Psychiatr Scandinavica, Suppl. 2000;102:44–8.
Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–9.
Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Psychiatry: Interpers Biol Process. 2006;60:253–64.
Lisman JE, Coyle JT, Green RW, Javitt DC, M F, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 2008. 2009;31:234–42.
Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–13.
Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018;23:1764–72.
Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93.
Allen P, Chaddock CA, Egerton A, Howes OD, Bonoldi I, Zelaya F, et al. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am J Psychiatry. 2016;173:392–9.
Allen P, Azis M, Modinos G, Bossong MG, Bonoldi I, Samson C, et al. Increased resting hippocampal and basal ganglia perfusion in people at ultra high risk for psychosis: replication in a second cohort. Schizophrenia Bull. 2018;44:1323–31.
Roiser JP, Howes OD, Chaddock CA, Joyce EM, McGuire P. Neural and Behavioral Correlates of Aberrant Salience in Individuals at Risk for Psychosis. Schizophrenia Bull. 2013;39:1328–36.
Winton-Brown T, Schmidt A, Roiser JP, Howes OD, Egerton A, Fusar-Poli P, et al. Altered activation and connectivity in a hippocampal-basal ganglia-midbrain circuit during salience processing in subjects at ultra high risk for psychosis. Transl Psychiatry. 2017;7:1–8.
Modinos G, Allen P, Zugman A, Dima D, Azis M, Samson C, et al. Neural circuitry of novelty salience processing in psychosis risk: association with clinical outcome. Schizophr Bull. 2020;46:1–10.
Allen P, Seal ML, Valli I, Fusar-Poli P, Perlini C, Day F, et al. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophrenia Bull. 2011;37:746–56.
Bossong MG, Antoniades M, Azis M, Samson C, Quinn B, Bonoldi I, et al. Association of hippocampal glutamate levels with adverse outcomes in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2019;46:1–9.
Valli I, Stone J, Mechelli A, Bhattacharyya S, Raffin M, Allen P, et al. Altered medial temporal activation related to local glutamate levels in subjects with prodromal signs of psychosis. Biol Psychiatry. 2011;69:97–9.
Modinos G, Richter A, Egerton A, Bonoldi I, Azis M, Antoniades M, et al. Interactions between hippocampal activity and striatal dopamine in people at clinical high risk for psychosis: relationship to adverse outcomes. Neuropsychopharmacol. 2021;46:1468–74.
Damme KSF, Ristanovic I, Vargas T, Mittal VA. Timing of menarche and abnormal hippocampal connectivity in youth at clinical-high risk for psychosis. Psychoneuroendocrinology. 2020;117:104672.
Benetti S, Mechelli A, Picchioni M, Broome M, Williams S, McGuire P. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132:2426–36.
Hubl D, Schultze-Lutter F, Hauf M, Dierks T, Federspiel A, Kaess M, et al. Striatal cerebral blood flow, executive functioning, and fronto-striatal functional connectivity in clinical high risk for psychosis. Schizophrenia Res. 2018;201:231–6.
Dandash O, Fornito A, Lee J, Keefe RSE, Chee MWL, Adcock RA, et al. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr Bull. 2014;40:904–13.
Allen P, Luigjes J, Howes OD, Egerton A, Hirao K, Valli I, et al. Transition to Psychosis Associated With Prefrontal and Subcortical Dysfunction in Ultra High-Risk Individuals. Schizophrenia Bull. 2012;38:1268–76.
Pettersson-Yeo W, Benetti S, Marquand AF, Joules R, Catani M, Williams SCR, et al. An empirical comparison of different approaches for combining multimodal neuroimaging data with support vector machine. Front Neurosci. 2014;8:189.
Lei D, Pinaya WHL, Young J, van Amelsvoort T, Marcelis M, Donohoe G, et al. Integrating machining learning and multimodal neuroimaging to detect schizophrenia at the level of the individual. Hum Brain Mapp. 2020;41:1119–35.
Modinos G, Pettersson-Yeo W, Allen P, McGuire PK, Aleman A, Mechelli A. Multivariate pattern classification reveals differential brain activation during emotional processing in individuals with psychosis proneness. NeuroImage. 2012;59:3033–41.
Pettersson-Yeo W, Benetti S, Marquand AF, Dell’Acqua F, Williams SCR, Allen P, et al. Using genetic, cognitive and multi-modal neuroimaging data to identify ultra-high-risk and first-episode psychosis at the individual level. Psychological Med. 2013;43:2547–62.
Koutsouleris N, Dwyer DB, Degenhardt F, Maj C, Urquijo-Castro MF, Sanfelici R, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78:195–209.
Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. 2017;18:115–26.
Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust NZ J Psychiatry. 2005;39:964–71.
Diagnostic and Statistical Manual of Mental Disorders. 5th Editio. American Psychiatric Association: Washington, DC, 2013.
Nelson H. National Adult Reading Test (NART): Test Manual. Windsor, United Kingdom, 1982.
Coren S. Measurement of handedness via self -report: the relationship between brief and extended inventories. Percept Mot Skills. 1993;76:1035–42.
Hall RCW. Global assessment of functioning. Psychosomatics. 2011;36:267–75.
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–9.
Bunzeck N, Düzel E. Absolute coding of stimulus novelty in the human substantia Nigra/VTA. Neuron. 2006;51:369–79.
Allen P, Chaddock CA, Egerton A, Howes OD, Barker G, Bonoldi I, et al. Functional Outcome in People at High Risk for Psychosis Predicted by Thalamic Glutamate Levels and Prefronto-Striatal Activation. Schizophrenia Bull. 2015;41:429–39.
Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of glutamate alterations in schizophrenia a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry. 2016;73:665–74.
Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: H MR spectroscopy study at 4 T. Life Sci Part 1 Physiol Pharmacol. 2008;26:665–72.
Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–29.
Bossong MG, Mehta MA, Van Berckel BNM, Howes OD, Kahn RS, Stokes PRA. Further human evidence for striatal dopamine release induced by administration of δ9-tetrahydrocannabinol (THC): selectivity to limbic striatum. Psychopharmacology. 2015;232:2723–9.
Allen P, Chaddock CA, Egerton A, Howes OD, Bonoldi I, Zelaya F, et al. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am J Psychiatry. 2016;173:392–9.
Allen P, Moore H, Corcoran CM, Gilleen J, Kozhuharova P, Reichenberg A, et al. Emerging temporal lobe dysfunction in people at clinical high risk for psychosis. Front Psychiatry. 2019;10:298.
McCutcheon R, Beck K, Jauhar S, Howes OD. Defining the Locus of Dopaminergic Dysfunction in Schizophrenia: A Meta-analysis and Test of the Mesolimbic Hypothesis. Schizophrenia Bull. 2018;44:1301–11.
Mechelli A, Riecher-Rössler A, Meisenzahl EM, Tognin S, Wood SJ, Borgwardt SJ, et al. Neuroanatomical Abnormalities That Predate the Onset of Psychosis. Arch Gen Psychiatry. 2011;68:489.
Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR, et al. Dopamine Synthesis Capacity before Onset of Psychosis: A Prospective [18F]-dOPA Pet Imaging Study. Am J Psychiatry. 2011;168:1311–7.
Wood SJ, Kennedy D, Phillips LJ, Seal ML, Yücel M, Nelson B, et al. Hippocampal pathology in individuals at ultra-high risk for psychosis: A multi-modal magnetic resonance study. NeuroImage. 2010;52:62–8.
Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: Version III - The final common pathway. Schizophrenia Bull. 2009;35:549–62.
Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–86.
Egerton A, Chaddock C, Winton-Brown TT, Bloomfield MAP, Bhattacharyya S, Allen P, et al. Presynaptic Striatal Dopamine Dysfunction in People at Ultra-high Risk for Psychosis: Findings in a Second Cohort. Biol Psychiatry. 2013;74:106–12.
Wotruba D, Heekeren K, Michels L, Buechle R, Simon JJ, Theodoridou A, et al. Symptom dimensions are associated with reward processing in unmedicated persons at risk for psychosis. Front Behav Neurosci. 2014;8:1–9.
Salazar de Pablo G, Radua J, Pereira J, Bonoldi I, Arienti V, Besana F, et al. Probability of transition to psychosis in individuals at clinical high risk: an updated meta-analysis. JAMA Psychiatry. 2021;78:970–8.
Fusar-Poli P, Salazar de Pablo G, Correll CU, Meyer-Lindenberg A, Millan MJ, Borgwardt S, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77:755–65.
Abi-Dargham A, Meyer JM. Schizophrenia: the role of dopamine and glutamate. J Clin Psychiatry. 2014;75:274–5.
Poels EMP, Kegeles LS, Kantrowitz JT, Slifstein M, Javitt DC, Lieberman JA, et al. Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Mol Psychiatry. 2014;19:20–9.
Poels EMP, Kegeles LS, Kantrowitz JT, Javitt DC, Lieberman JA, Abi-Dargham A, et al. Glutamatergic abnormalities in schizophrenia: A review of proton MRS findings. Schizophrenia Res. 2014;152:325–32.
Lin A, Wood SJ, Yung AR. Measuring psychosocial outcome is good. Curr Opin Psychiatry. 2013;26:138–43.
McGuire P, Sato JR, Mechelli A, Jackowski A, Bressan RA, Zugman A. Can neuroimaging be used to predict the onset of psychosis? Lancet Psychiatry. 2015;2:1117–22.
Gifford G, Crossley N, Fusar-Poli P, Schnack HG, Kahn RS, Koutsouleris N, et al. Using neuroimaging to help predict the onset of psychosis. NeuroImage. 2017;145:209–17.
Mikell CB, McKhann GM, Segal S, McGovern RA, Wallenstein MB, Moore H. The hippocampus and nucleus accumbens as potential therapeutic targets for neurosurgical intervention in schizophrenia. Stereotact Funct Neurosurg. 2009;87:256–65.
De La Fuente-Sandoval C, León-Ortiz P, Azcárraga M, Stephano S, Favila R, Díaz-Galvis L, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry. 2013;70:1057–66.
Egerton A, Broberg BV, Van Haren N, Merritt K, Barker GJ, Lythgoe DJ, et al. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre 1 H-MRS study (OPTiMiSE). Mol Psychiatry. 2018;23:2145–55.
De Bartolomeis A, Sarappa C, Magara S, Iasevoli F. Targeting glutamate system for novel antipsychotic approaches: Relevance for residual psychotic symptoms and treatment resistant schizophrenia. Eur J Pharmacol. 2012;682:1–11.
Egerton A, Murphy A, Donocik J, Anton A, Barker GJ, Collier T, et al. Dopamine and glutamate in antipsychotic-responsive compared with antipsychotic-nonresponsive psychosis: a multicenter positron emission tomography and magnetic resonance spectroscopy study (STRATA). Schizophrenia Bulletin. 2020;47:505–16.
Acknowledgements
This research was funded in whole, or in part, by the Wellcome Trust [grant 091667/Z/10/Z]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. This study was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London, Maudsley NHS Foundation Trust and King’s College London. EJH is funded by the Medical Research Council (Grant No. MC- A656-5QD30, Grant No. MC_PC_17214) GM is funded by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (#202397/Z/16/Z). AG is funded by USPHS MH57440. MB was supported by a Veni fellowship from the Netherlands Organization for Scientific Research.
Author information
Authors and Affiliations
Contributions
P.A. (Conceptualisation, funding, analysis, manuscript preparation), E.J.H. (analysis, manuscript preparation), N.O. (analysis), G.M. (data collection, analysis), M.B. (data collection analysis), M.A. (data collection, analysis), O.H. (Funding, manuscript preparation), J.S. (funding, data collection, manuscript preparation), J.P. (data collection, manuscript preparation), M.B. (drafting), A.G. (manuscript preparation), P.M. (concept, funding, manuscript preparation).
Corresponding author
Ethics declarations
COMPETING INTERESTS
A.G. receives research funding from Alkermes, Lundbeck, and receives consulting fees from Takeda, Roche, Lyra, and Concert. ODH has received investigator-initiated research funding from and/ or participated in advisory/speaker meetings organised by Astra-Zeneca, Autifony, BMS, Eli Lilly, Heptares, Jansenn, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand and Roche. Neither ODH or his family have been employed by or have holdings/a financial stake in any biomedical company.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allen, P., Hird, E.J., Orlov, N. et al. Adverse clinical outcomes in people at clinical high-risk for psychosis related to altered interactions between hippocampal activity and glutamatergic function. Transl Psychiatry 11, 579 (2021). https://doi.org/10.1038/s41398-021-01705-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-021-01705-z