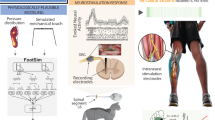
Abstract
Study design
Proof of concept.
Objectives
Standard Functional Electrical Stimulation (FES) systems can enhance motor learning in people with tetraplegia and are widely delivered by self-adhesive electrodes. Their limitations are dexterity, specific knowledge to place the electrodes on muscles, need to fix electrodes when they lose the gel layer, and time. We designed a new FES system, using an existing protocol of drinking-like movements, to the upper limb of a person with tetraplegia C5 that fits in any anthropometry and can be easily produced. Furthermore, we tested the system to assess its effectiveness and users’ perception during FES rehabilitation.
Setting
São Carlos, SP, Brazil.
Methods
A shell was designed with parametric design and fast-fabrication methods, and a stimulation unit and a smartphone application were developed. Questionnaires assessed the perceptions of a patient and a physiotherapist, about the usability of the new system in relation to standard FES. Kinematic data of drinking-like movements were collected from the patient wearing both systems and compared with data from an aged-matched control subject.
Results
The results are a personalized shell and an intuitive FES system, overcoming the limitations of standard FES. The new system suggested better wrist-flexion control shown by the mean angles (−18.93°), then the other system (−59.35°), and compared with the control (−10.97°).
Conclusions
Fast-fabrication with parametric design offers a promising alternative for personalizing FES systems, with potential for home use. Further studies are required including randomized clinical trials.

Similar content being viewed by others
Introduction
Functional Electrical Stimulation (FES) is one of the techniques adopted for the rehabilitation of people with spinal cord injury (SCI) in clinical and home settings towards a better level of physical and psychological functioning. It has been widely used for non-invasively inducing muscle contraction by Neuromuscular Electrical Stimulation, thereby enhancing the recovery of patients’ selected functions [1,2,3,4,5] and stimulating motor learning through frequent and repetitive movements [6, 7].
Over the past two decades, Activities of Daily Living (ADLs) based on FES with surface electrodes have been investigated and improved towards upper extremity home rehabilitation [8,9,10,11,12,13,14,15,16].
Surface electrodes have been used in FES as textile electrodes [17], knitted electrodes [15], electrode arrays [13, 18], and self-adhesive gel electrodes [19,20,21,22,23]. However to being affordable, surface self-adhesive electrodes have been widely applied in clinical environments [24]. Although FES with self-adhesive electrodes, henceforth called standard FES, works relatively well, the way it is used is hindered by limitations such as time-consuming manual positioning, dexterity and anatomical knowledge for a proper electrode positioning [25], loss of adhesion to the skin, which demands strips for fixation and compromises FES’ effectiveness, causing skin burns [24], large number of cables, and size of some products [26]. Consequently, a system that fits anyone and is easy to handle, comfortable, effective, and affordable is desired.
Therefore, a proof-of-concept of a wearable FES system was developed with the aim of being a potential solution to the mentioned demands, such as being a portable and intuitive system, being personalized in the sense of serving in any user anthropometry, providing total adherence of self-adhesive electrodes, including those without the gel layer, function properly and be easily produced. Inspired in the wearable’s features [27], the new system is mass-personalized in an automated way and fits any anthropometry, thus becoming affordable and easy of donning/doffing by a non trained person. It delivers ADL from an existing FES protocol of drinking-like movements developed by our research group—Laboratory of Biomechanics and Rehabilitation of the Locomotive System, at Unicamp [7, 20], for repetitive long-term training towards motor learning of people with tetraplegia. The wearable FES was developed with parametric design and fast-fabrication methods [28, 29], and consists of a shell, a stimulation unit, and a smartphone application (App).
Methods
Participants and ethics
As a proof-of-concept investigation, one patient and one control subject of matching ages and one health professional participated. The participants were a 45-year-old male with a traumatic tetraplegia C5 AIS A, under standard FES ADL (clinical routine of 40 min total twice-weekly on both upper limbs) since August 2007, a physiotherapist who had been working with standard FES rehabilitation with a patient with SCI for 3 years, and a 46-year-old healthy female as a control subject. The exclusion criteria were lesions on the skin of the upper limbs, cognitive deficit, inability to remain seated freely in a wheelchair with back support, history of tendon transfer surgery involving upper limbs, and musculoskeletal disorders in the upper limbs. The tests, approved by the Research Ethics Committee of the University of Campinas under registration number CAAE: 16552219.8.0000.5404, were conducted at the spinal cord outpatient clinic of Clinical Hospital of Campinas (Brazil). All subjects signed a written consent to participate.
Development of the wearable FES system
The new system was developed according to the Participatory Design [30] method and by a focus group that included healthcare professionals, patients with tetraplegia, engineers, and an architect. An automated computational design methodology was created for personalizing the shell (composed by 3-piece of hand, forearm and arm) for each user. The user’s dominant upper limb with the electrodes was three-dimensionally scanned by iSense (3D Systems, USA), according to a scanning protocol developed by our research group Laboratory at the Biocybernetics and Rehabilitation Engineering at USP. The shell was designed in a Grasshopper 3D (David Rutten at Robert McNeel & Associates, USA) graphical algorithm editor associated with Rhinoceros 3D software (TLM Inc., USA).
The shell and the stimulation unit box were 3D printed with flexible Thermoplastic Polyurethane and polylactic acid, respectively, by Flash Forge—Guider II printer (Flashforge3D technology Co., China) with Fused Deposition Modeling technology.
A color scheme was developed for stimulation unit LEDs, electrode cables, and channels in the App for corresponding activations. The self-adhesive electrodes are fixed only once inside the shell with double-sided tapes and replaced after losing their function.
The stimulation unit used a four-channel electrical stimulator powered by two rechargeable lithium ion batteries 18,650 (4.2 V, 9.8 A-h) to generate stimuli via shell with self-adhesive electrodes. Four 100 μs long pulses for each burst with a 100 μs interval from a monophasic square waveform generator of adjustable amplitude (between 30 V and 70 V, 1 kΩ load) were delivered at 25 Hz. The App set the ADL channel sequences and activation of the stimulation unit via Wi-Fi router.
Qualitative analysis
Evaluations of the usability of the wearable FES compared with standard FES were obtained from both physiotherapist and patient through individualized questionnaires (see Supplementary Material) according to Usability methods such as Think Aloud, observation, and application of questionnaires [31, 32]. The assessments were conducted for 8 days during the patient´s FES clinical routine at the outpatient clinic, with 20 min sessions per day.
Kinematic analysis
Kinematic variables were collected from the patient during the drinking-like ADL’s FES training first by standard FES and then by the wearable FES with the same self-adhesive electrodes (32 mm circular and 90 × 50 mm rectangular ones—ValuTrode, Axelgaard, USA). The data were compared with results from the control, which run the routine with no system.
The kinematic analysis comprised seven cycles divided into 4 steps of ADL with the dominant right arm. Figure 1 shows (a) Step 1—Reach (from A to B), (b) Step 2—Grasp and Raise (take the object to the mouth), (c) Step 3—Return the object to point B, and (d) Step 4—Release and Return (from B to A). Steps 1 and 4 were performed without the object.
Data were collected by MotionMonitor™ equipment (Innsports, USA) with 12 cameras (Vicon, USA) placed on the walls of the room (Fig. 4), and markers reflecting infrared light placed on the chest, arm, forearm, hand, and the object captured the movements. Kinematic variables were measured at 100 Hz sampling frequency. Figure 2 displays the coordinate system defined according to MotionMonitor equipment with Y-axis directed forward, X-axis directed sideways, and Z-axis directed upward.
The forearm was the reference axis for wrist movements and the arm was the reference one for the elbow movement. Sagittal, transverse, and coronal planes were used as reference axes for shoulder movements. Negative and positive angles were measured clockwise and counterclockwise, respectively, according to the polar coordinate system.
The angle values of the shoulder (flexion/extension, adduction/abduction, and rotation), elbow (flexion/extension), and wrist (ulnar/radial deviation and flexion/extension) joints were measured during the tests, and the 3D coordinates of the object were acquired. The measurements enabled the calculation of angular speeds of the joints, trajectory, and tangential speed of the object. Data were filtered by a 4th-order low-pass zero-phase Butterworth filter at 6 Hz cutoff frequency.
Experimental setup
Figure 3 displays the patient during the drinking-like ADL and an overview of the wearable FES system composed of a 3-piece shell (a, b, and c), the stimulation unit (d), a smartphone (e) and a screenshot of channel setting in the App (f). During the FES training, the control sat in a conventional armchair and the patient sat in his wheelchair. Both individuals were provided with a “table-like” base on the chairs, which held the forearm, hand, and the object. The back was supported on the chair’s backrest at a right angle. Four electrodes intended for the triceps, radial carpal extensor, superficial flexor of the fingers, and thenars (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis, and opponens pollicis) muscle groups, plus two reference electrodes were positioned on the wearable FES and set during the training sequence in the App. The cup was simulated by a plastic cylindrical object of 11 cm height, 4 cm diameter, and 200 g mass. The initial joint angles of the participant’s shoulder, elbow, and wrist were calibrated to zero.
Table 1 shows a summary of the FES stimulation protocol [7, 20] in each step and the respective channels in the stimulation unit. The last row shows the stimulation time used in each step.
The FES cycles lasted 9 s each with a 10 s break between consecutive cycles. A 30 min rest with no stimulation was taken between the standard FES test and the test with wearable FES.
Results
The result of the shell was three pieces (hand, forearm, and arm) with 1 mm thick (Fig. 4) to integrate self-adhesive electrodes and cables. No industrial mold and processes were necessary, and the shape resulted in the user’s upper limb with openings for ventilation, tubes for the embedding of the electrode cables, and 2 mm thick points with the shape of the electrodes for their fixation at the exact position of the muscle groups to be stimulated (Fig. 4).
According to the questionnaires, the physiotherapist and the patient reported wearable FES as intuitive, safe, personalized, useful, free to move, and esthetically accepted, as detailed in what follows and summarized in Table 2.
The color signaling in the cables, stimulation unit, and App facilitated the assembly of the system. The positioning of the shell on the patient was easy, since the shape of the three parts was similar to that of his upper limb. Tubes outside the shell and the embossed points inside it provided physiotherapist with clear clues about the passage of cables and the attachment of the electrodes in the exact positions of the muscle groups, enabling a full contact of the electrodes with the skin. Used electrodes with no gel layer were employed in usability tests in both systems. Unlike the wearable FES, the standard one required the electrodes to be securely attached to the skin with straps, causing its edges to rise and reducing the area of contact of the electrode with the skin. Such properties led both users to consider the wearable FES intuitive, useful, and safe, since the movement would be performed with no failures or interruptions, thus minimizing the chances of skin burns. Although the physiotherapist was used to the standard FES setup and had no experience with the new system, a 50 s (s) time-saving was achieved for the total setup, compared with the standard FES (120 s).
A shell with embedded components (electrodes and cables), and an appropriate stimulation unit sized resulted in a portable system of 401.60 g weight. The new system promoted freedom of movement of the upper limb during FES, lightness of the shell (147.26 g), and good appearance, stimulating its more frequent use at home.
During the kinematic tests, the period of movements ranged from cycle to cycle for the subjects, hindering a straightforward comparison among them. Therefore, the time span of each cycle was normalized to the unit and the variables were resampled towards matching each other.
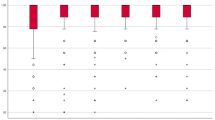
Among all tests performed with the control, standard, and wearable FES systems (see Supplementary Material) the wrist-flexion angles in steps 2–3 in both systems differed the most from each other (−18.93° mean value of wearable FES and −59.35° mean value of standard FES), as shown in Fig. 5.
Figure 6 shows a larger oscillatory behavior of the object’s mean trajectory with the standard FES in comparison with the wearable FES at the beginning of step 2 and at the end of step 3. The results of the remaining trajectories are similar (see Supplementary Material).
Figure 7 displays the object’s tangential speed in the seven cycles and the respective mean speeds in steps 2 and 3, steps in which the object is in motion (the object was at rest in steps 1 and 4, as shown in Fig. 1)—Fig. 7a shows a comparison of the average speeds between the systems. The control is shown as a reference, unlike step 3. In step 2, the object moves with the wearable FES without oscillations regularly increasing or decreasing its speed. Conversely, with the standard FES, the oscillation occurs in step 2.
This result may be misleading, since the mean alone may cause distortions in data interpretation. The normalized object’s speeds in the seven cycles with wearable and standard FES are displayed and compared with that of the control in Fig. 7b–d, respectively. The wearable FES curves (Fig. 7c) show greater speed oscillations in the seven cycles, in step 3, compared with their average, than in step 2, as in the control curve (Fig. 7b). In contrast, the standard FES curve (Fig. 7d) shows larger speed oscillations in the 7 cycles in steps 2 and 3, in relation to their average, emphasized by the standard deviation (SD) of such averages (Table 4). In steps 2 and 3, the SD of the standard FES is approximately the double of the values of the wearable FES, which are closer to the control. The speed values were normalized for a more reliable comparison.
Discussion
Three milestones of industrialization, namely mechanization of manual processes, mass-production, and mass-personalization have affected product development and production. The main feature of the latter is the large-scale production through the same procedures, but with different parameters, promoting more flexibility [33]. The parametric design associated with the fast-fabrication technique is an alternative method. A wearable FES was designed according to this feature. Although this method is used in other areas (e.g., architecture, digital art, furniture design, and fashion design), to the best of our knowledge, the literature does not report its use in the field.
This article has addressed a proof-of-concept of a wearable FES system in the design and production stages and during its use by a person with SCI C5. Its main advantages are automation for design personalization, precision, intuitiveness, simplicity, portability, speed in production, and effectiveness. However, the design of a new shell requires a trained person to change the automation data, the weight of the stimulation unit made it uncomfortable to place on the arm, and the shell finishing was satisfactory.
Regarding design automation and personalization, the computational design method solved the problem of fitting to any anthropometry in an automated way, thus showing an alternative to be explored for other parts of the body. Additionally, a trained person, instead of an expert, is required for 3D scanning and algorithm input changes for a new wearable FES design and print.
Unlike manual measurements, which are subject to errors and revisions, the method also delivered precise measurements of both upper limb and positions of the muscle groups to be stimulated. The features of the shell design and the use of colors made the whole system configuration and activation intuitive, thus saving time. The shell´s flexible material and design provided comfort, simplicity, and easiness of donning/doffing, which are fundamental characteristics for patients with tetraplegia. The design features of the shell, the size of the stimulation unit, and its activation by a smartphone App made the system portable and with potential for home use. The weight of the batteries compromised the attachment of the stimulation unit to the body; therefore, they must be replaced and tested, although they did not hamper any function or feature of the wearable FES.
The shell material, thickness, size, and number of ventilation openings offered flexibility and comfort to the users, which are highly desirable features in any FES system. However, the surfaces and tubes’ finishing had protrusions, flaws, and fine wefts, although they did not affect training and comfort.
The reports in Table 2 show progress in terms of usability properties of the new system in relation to the standard FES system, with self-adhesive electrodes, as well as confidence and comfort to the patient and practicality, effectiveness, and confidence to the physiotherapist.
Overall, the wearable FES works as well as the standard FES and, therefore, can be considered functional and efficient. Additionally, it seems to have improved wrist-flexion angles and stability of object trajectory and slightly increased speed in comparison with the standard FES.
As shown in Fig. 5, the averages of wrist-flexion angles in steps 1 (Reach) and 4 (Release and Return) with both systems are closer to each other, with similar wrist movements compatible with those of the control. In contrast, the two systems showed considerably different performances in steps 2 (Grasp and Raise) and 3 (Return to B). The wrist-flexion mean angle with the wearable FES resembles that of the control. The standard FES induces wrist angles far from the natural ones, even with the use of the same electrodes. These analysis can be shown by the percentage difference (Δ%) between each system and the control (Table 3). Therefore, the wearable FES system improved the wrist movements where they are most necessary, i.e., for raising the object to the mouth and back to the table, outperforming the standard FES. Overall, the remaining angles (see Supplementary Material) selected by the experimental setup provided similar results for both systems, indicating the wearable FES delivers results comparable to those of the standard one.
The trajectories of the object with the use of both standard and wearable FESs, shown in Fig. 6, exhibit an akin profile. The wearable FES seems to provide more stable trajectories, at least at the beginning/ending of the movements. For example, at the very beginning of the standard FES trajectory, some difficulty in maintaining a more linear trajectory is observed, as opposed to the trajectory of the wearable FES, which is closer to the control one. Furthermore, the standard FES trajectory shows the object’s final position is slightly different from the start position. Again, the wearable FES seems to enable a better control of the movement.
The tangential speed of the object suggested higher stability to take the object to the mouth with the wearable FES, despite some instability to return it to the table. With standard FES, the object’s mean speed suggested instability in both steps (grasp/raise and return), leading to a certain difficulty in keeping the object up. According to Table 4, the SD in steps 2 and 3 is much greater for standard FES than for the wearable FES. On the other hand, the latter is very close to that of the control. In the three situations (Fig. 7b–d), the mean SD is greater in step 3 than in step 2, as can be seen in Table 4. Earlier studies showed the lack of triceps brachialis muscle function in patients with tetraplegia slows the speed of the return step; however, the speed can change when the movement is performed with a load [34, 35]. In both standard and wearable FESs, the oscillations in step 3 appeared probably due to the lack of triceps brachialis muscle contraction required to smoothen the downward movement. However, the wearable FES showed smaller oscillations.
The proof of concept is a preliminary, usual, and necessary step in the development of medical devices that informs on necessary improvements. Since the patient was used to the standard FES (11-year training) and the test cycles were performed initially with this system, the wearable FES system showed improvements in the upper limb movements. The new system promoted better wrist joint control and smoothness of movement in the drinking-like ADL and tended to induce movement patterns closer to the natural ones. These findings may be the result of the better contact between the electrodes and the skin, the shell design providing stability of movement, and the patient feeling secure and confident performing the movements wearing the new system. However, more tests are necessary for confirming such hypotheses.
Parametric design and 3D printing in the development of personal systems proved to be an unexplored and promising strategy to overcome the limitations of FES systems. The novelty of the wearable FES relies on its fast fabrication, automation development, and personalization. Furthers studies such as randomized clinical trials are necessary. Although the new system has been tested on a patient by a healthcare professional, it can potentially be used at home as a portable and intuitive system, and shows an open field for applications to other parts of the body.
References
Bradbury EJ, Mcmahon SB. Spinal cord repair strategies: why do they work? Nature 2006;7:644–53.
Peckham PH, Keith MWFA. Restoration of functional control by electrical stimulation in the upper extremity of the quadriplegic patient. J Bone Jt Surg. 1988;70-A:144–8.
Fouad K, Tetzlaff W. Rehabilitative training and plasticity following spinal cord injury. Exp Neurol. 2012;235:91–9. https://doi.org/10.1016/j.expneurol.2011.02.009.
Varoto R, Cliquet Jr A. Experiencing functional electrical stimulation roots on education, and clinical developments in paraplegia and tetraplegia with technological innovation. Artif Organs. 2015;39:187–201.
Nagai MK, Marquez-Chin C, Popovic MR. Why Is Functional Electrical Stimulation Therapy Capable ofRestoring Motor Function Following Severe Injury to the Central Nervous System?. In: Tuszynski M (ed). Translational Neuroscience. Springer, Boston, MA. 2016. https://doi.org/10.1007/978-1-4899-7654-3_25.
Popovic MR, Kapadia N, Zivanovic V, Furlan JC, Craven BC, McGillivray C, et al. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: A randomized clinical trial. Neurorehabil Neural Repair. 2011;25:433–42.
Alonso K, Azevedo E, Beinotti F, Maria R AC Jr. Electromyographic assessment of the tetraplegic upper limb during functional movement. J Neurol Sci. 2013;333:e564. https://doi.org/10.1016/j.jns.2013.07.1977.
Prochazka A, Gauthier M, Wieler M, Kenwell Z. The bionic glove: an electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Arch Phys Med Rehabil. 1997;78:608–14.
Popovic D, Stojanovic A, Pjanovic A, Radosavljevic S, Popovic M, Vulovic D, et al. Clinical evaluation of the bionic glove. Arch Phys Med Rehabil. 1999;80:299–304.
Mangold S, Keller T, Curt A, Dietz V. Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord. 2005;43:1–13.
Fujii T, Seki K, Handa Y. Development of a new FES system with trained super-multichannel surface electrodes. 9th Annu Conf Int FES Soc Sept 2004. Bournemouth: UK Dev; 2004.p. 1–2.
Malesevic NM, Maneski LZP, Ilic V, Jorgovanovic N, Bijelic G, Keller T, et al. A multi-pad electrode based functional electrical stimulation system for restoration of grasp. J Neuroeng Rehabil. 2012;9:66.
Crema A, Malešević N, Furfaro I, Raschellà F, Pedrocchi A, Micera S, et al. A wearable multi-site system for NMES-based hand function restoration. IEEE Trans Neural Syst Rehabil Eng. 2018;26:428–40.
Konecny P, Jarkovsky J, Marques E, Novakova M, Palanova P, Mrkvicova V, et al. Home‐based training using neuromuscular electrical stimulation in patients on continuous ambulatory peritoneal dialysis: a pilot study. Artif Organs. 2018;1–10.
Moineau B, Marquez-chin C, Alizadeh-meghrazi M, Popovic MR. Garments for functional electrical stimulation: design and proofs of concept. J Rehabil Assist Technol Eng. 2019;6:1–15.
Pennati GV, Bergling H, Carment L, Borg J, Lindberg PG, Palmcrantz S. et al. Effects of 60?min electrostimulation with the EXOPULSE Mollii suit on objective signs of spasticity. Front Neurol. 2021;12:1–14.
Muccio P. Upper extremity biosleeve [Internet]. Vol. 1. United States; Patent: US 2016/0325090 A1, 2016. https://patents.justia.com/patent/20160325090.
Zabaleta H, Imatz E, Keller T. Device and system for functional electrical stimulation [Internet]. Vol. 1. Patent: EP 3 650 077 A1, 2020. p. 1–16. https://patents.google.com/patent/EP3650077A1/en?q=%22DEVICE+AND+SYSTEM+FOR+FUNCTIONAL+ELECTRICAL+STIMULATION%22.
Keller T, Popovic MR, Pappas IPI, Müller P. Transcutaneous functional electrical stimulator "Compex Motion.". Artif Organs. 2002;26:219–23.
Castro MCF, Cliquet A Jr. An artificial grasping evaluation system for the paralysed hand. Med Biol Eng Comput. 2000;38:275–80.
Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI, et al. Functional electrical therapy: Retraining grasping in spinal cord injury. Spinal Cord. 2006;44:143–51.
Varoto R, Barbarini ES, Cliquet AJ. A hybrid system for upper limb movement restoration in quadriplegics. Artif Organs. 2008;32:725–729.
Thorsen R, Dalla Costa D, Chiaramonte S, Binda L, Beghi E, Redaelli T, et al. A noninvasive neuroprosthesis augments hand grasp force in individuals with cervical spinal cord injury: the functional and therapeutic effects. Sci World J. 2013;2013:1–7.
Patriciu A, Yoshida K, Struijk JJ, DeMonte TP, Joy MLG, Stødkilde-jørgensen H, et al. Current density imaging and electrically induced skin burns under surface electrodes. IEEE Trans Biomed Eng. 2005;52:2024–31.
Maffiuletti NA, Alessandro M. Atlas of the muscle motor points for the lower limb: implications for electrical stimulation procedures and electrode positioning. Eur J Appl Physiol. 2011;111:2461–71.
Snoek GJ, Ijzerman MJ, In’t Groen FACG, Stoffers TS, Zilvold G. Use of the NESS Handmaster to restore handfunction in tetraplegia: Clinical experiences in ten patients. Spinal Cord. 2000;38:244–9.
Mann S. Smart clothing: the wearable computer and WearCam. Pers Technol. 1997;1:21–7.
Janssen P. Dexen: a scalable and extensible platform for experimenting with population-based design exploration algorithms. Artif Intell Eng Des Anal Manuf. 2015;29:443–55.
Kolarevic B. Towards integrative design. Int J Arch Comput. 2009;07:335–44.
Redström J. RE: definitions of use. Des Stud. 2008;29:410–23.
Jaspers MWM. A comparison of usability methods for testing interactive health technologies: Methodological aspects and empirical evidence. Int J Med Inf. 2009;78:340–53.
Maguire M. Methods to support human-centred design. Int J Hum-Computer Stud. 2001;55:587–634.
Celani G. Changing the architectural production chain in Latin America with the introduction of new technologies. Mater Arquit. 2016;13:118–21.
Walker E, Cacho A, D Oliveira R, Ortolan RL, Varoto R, Cliquet A, et al. Upper limb assessment in tetraplegia: Clinical, functional and kinematic correlations. Int J Rehabil Res. 2011;34:65–72.
Reyes-Guzmán A de los, Gil-Agudo A, Peñasco-Martín B, Solís-Mozos M, Ama-Espinosa A del, Enrique Pérez-Rizo. et al. Kinematic analysis of the daily activity of drinking from a glass in a population with cervical spinal cord injury. J Neuroeng Rehabil. 2010;7:1–12.
Acknowledgements
The authors would like to acknowledge the Renato Archer Information Technology Center in particular Jorge Vicente Lopes da Silva, Pedro Yoshito Noritomi and Leonardo Mendes Ribeiro Machado for their assistance with 3D technologies, and Janiele Rossi and Geruza Perlato Bella for assistance in physiotherapy, and Dr. Mauro Masili, from University of São Paulo, for his valuable discussions and critical reading of the article.
Funding
FAPESP (2017/06147-4 and 2016/50253-0), Capes (88882.379203/2019-01), and CNPq.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the study that led to the submission of this paper, acquired data, and played an important role in the interpretation of the results. All authors wrote, reviewed the manuscript, and approved the final version, and also agreed to be responsible for all its aspects for ensuring issues on the accuracy or completeness of any part would be properly investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ventura, A., Bataglia, J.M.P., Ginja, G. et al. Design and fast-fabrication of a system for functional electrical stimulation in upper limb of people with tetraplegia. Spinal Cord Ser Cases 8, 54 (2022). https://doi.org/10.1038/s41394-022-00519-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-022-00519-5