Abstract
Study design
Retrospective cohort study of consecutive upper limb peripheral nerve decompressions in SCI patients. All procedures were performed at a single National Spinal Injuries Centre between 2015 and 2019.
Objectives
Entrapment neuropathies in the upper limb are underdiagnosed and undertreated in patients with spinal cord injury (SCI). This cohort study represents the first published outcomes of upper limb peripheral nerve decompression in patients with SCI.
Setting
National Spinal Injuries Centre, Stoke Mandeville Hospital, Buckinghamshire, UK.
Methods
Data collected from electronic medical records included patient demographics, procedures performed, length of inpatient stay, nerve conduction studies, and patient satisfaction. Patients were also contacted by telephone to complete a questionnaire that included patient satisfaction, the NHS ‘Friends & Family Test’ and validated patient-reported outcome measures (PROMs).
Results
Thirty-four decompression procedures (24 carpal tunnel, 10 cubital tunnel) were performed in 24 patients (14 with paraplegia, 10 tetraplegia). 71% of patients had pre-operative nerve conduction studies: 71% of these were graded as severe. Mean length of stay was 14 nights. 91% of patients were satisfied with their procedure at clinic follow-up. Mean Boston Carpal Tunnel Questionnaire (BCTQ) symptom scores were reduced from 3.7 to 1.3 pre- vs. post-operatively (p < 0.001). Patient Reported Ulnar Nerve Evaluation (PRUNE) scores reduced from 49.4 to 23.0 (p = 0.01).
Conclusion
In our experience, SCI patients tend to present with severe upper limb nerve entrapment syndromes. Operative management is well tolerated with low risk of complications and can result in marked improvements in symptoms and function.
Similar content being viewed by others
Introduction
Entrapment neuropathies are common in SCI patients. The incidence of carpal tunnel syndrome is estimated at 49–73% in SCI populations [1,2,3,4], compared to less than 5% in the general population [5, 6], and that of cubital tunnel is 40% [1] compared to <5% [7]. Nerve entrapment syndromes in SCI have been found to be more prevalent with increasing age, time since injury, and male gender [1, 2, 8], but appear to be independent of other risk factors usually associated with nerve entrapment in general populations such as diabetes mellitus [8]. Individuals with SCI are heavily dependent on their upper limbs for activities of daily living, and therefore the symptoms and functional deficits associated with upper limb entrapment neuropathies are particularly disabling. Despite the increased incidence and heavy burden of morbidity associated with entrapment neuropathies in SCI, there is a paucity of literature on the topic.
Clinical diagnosis of carpal tunnel and cubital tunnel syndromes in SCI is often challenging due to pre-existing neurological deficits, particularly for patients with tetraplegia owing to cervical spinal cord injuries. Patients may present with atypical symptoms, or peripheral neuropathy may be masked by symptoms relating to spinal cord injury such as altered sensibility, spasticity, and neuropathic pain. Hence, diagnosis may be delayed and only recognized when neurophysiological impairment is severe. Nerve conduction studies have been found to be significantly more sensitive than clinical assessment in the diagnosis of nerve entrapment in SCI [1, 3, 4].
Elective upper limb surgery is often a major undertaking for patients with SCI. It is associated with a higher rate of complications [9], prolonged inpatient stays, and difficulties resting the upper limbs and engaging with rehabilitation post-operatively. Adjuncts such as hoist transfers and electric wheelchairs are often needed in the recovery period. Post-operative immobility also carries heightened risks of venous thromboembolism and pressure sores.
Here, we share our experience of peripheral nerve decompression in the upper limb in spinal cord injury patients at the National Spinal Injuries Centre, UK.
Methods
This is a retrospective case series of consecutive SCI patients who underwent peripheral nerve decompression in the upper limb at the National Spinal Injuries Centre, UK from 2015 to 2019 inclusive. Patients who met these inclusion criteria were identified by searching the electronic medical records at Buckinghamshire Healthcare NHS Trust (Institutional Research Board approval reference number 6017). Exclusion criteria included decompression that was performed as part of a more extensive upper limb reconstruction. No criteria were set for patient age, duration of spinal injury, level of spinal injury, or surgical technique (open or endoscopic) used.
Source medical records including inpatient notes, discharge summaries, and clinic letters were reviewed to obtain the following data: (1) demographics; (2) nature of spinal cord injury including level and ASIA Impairment scale (3) procedure(s) performed; (4) length of inpatient stay; (5) nerve conduction study results; and (6) patient satisfaction and symptomatology at clinic follow-up.
Patients with up-to-date contact information were asked to complete a further questionnaire by telephone. This questionnaire included overall patient satisfaction and the National Health Service (UK) ‘Friends & Family Test’, in which respondents are asked ‘How likely are you to recommend our service to friends and family if they needed similar care or treatment’. Finally, patients contacted by telephone completed validated patient-reported outcome measures (PROMs) including the Boston Carpal Tunnel Questionnaire (BCTQ) (Fig. 1), Modified Bishop Score (Fig. 2), and Patient Rated Ulnar Nerve Evaluation (PRUNE) score (Fig. 3). Scores were generated at this time for both pre- and post-operative condition, and therefore the pre-operative questionnaires were completed retrospectively. All questionnaire responses were anonymized, and all telephone consultations were conducted by an independent investigator (EV) blinded to other outcome data.
For the BCTQ, both the symptom severity and functional assessment sections were completed by paraplegic patients undergoing carpal tunnel decompression. For tetraplegic patients, most of the functional questions were not relevant and therefore only the symptom severity section was utilized. Patients undergoing cubital tunnel decompression also completed the Modified BISHOP Score and the symptom severity section of the PRUNE Score. All patients who underwent cubital tunnel release and were contactable for further follow-up had tetraplegia, and therefore the functional assessment section of the PRUNE score was not relevant.
For nerve conduction studies, patients with median nerve entrapment had measurement of the sensory nerve action potential (SNAP) from their second digit (D2) to wrist. Motor function of the median nerve was assessed through abductor policis brevis (APB). Ulnar nerve function was assessed by SNAP from the fifth digit (D5) across elbow, and motor conduction was assessed via abductor digiti minimi (ADM) across elbow. Severity was graded by the neurophysiologist performing these studies.
For both the PRUNE score and BCTQ, statistical analysis was with a two-tailed paired student’s T-test. P values of <0.05 were considered statistically significant.
Results
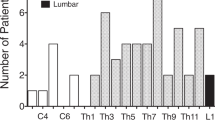
Thirty-four procedures performed in 24 patients (34 hands) over 5 years were included (Fig. 4).
Patient demographics
Of the 24 patients included, 14 had paraplegia and 10 tetraplegia. The mean age was 53 (median 52, range 30–85) years. The mean age for patients with paraplegia was 56 (36–78) years, while for those with tetraplegia this was 49 (30–85) years. For patients with median nerve entrapment, the mean duration of spinal cord injury was 21.5 (SD14.8, median 18, range 1–57) years. For patients with ulnar neuropathies this was 26.2 (SD 16.5, median 26, range 1–53) years. Demographic data including the spinal level and AIS grading for these patients is summarized in Fig. 4.
Carpal tunnel decompression
Twenty carpal tunnel decompressions were performed using the standard open approach, and four were performed endoscopically. Fourteen were performed in paraplegic patients, 10 in tetraplegic. Two patients underwent simultaneous bilateral carpal tunnel decompressions, while a further eight patients underwent decompression surgery in both upper limbs at different times. One tetraplegic patient presented with recurrent carpal tunnel symptoms 1 year post-operatively and underwent exploration and re-release of the carpal tunnel 3 months later, which led to symptomatic improvement. The median length of stay was 12 nights (range 1–35 nights).
Cubital tunnel decompression
Ten cubital tunnel decompressions were performed: four in paraplegic and six in tetraplegic patients. All cubital tunnel decompressions were unilateral and performed using the standard open approach. There was no recurrence or re-operation. For three of the ulnar nerve decompressions (performed in two patients with tetraplegia), the patients had undergone prior reconstructive surgery: One patient had prior bilateral deltoid to triceps tendon transfer. The other patient had deltoid to triceps transfer, brachioradialis to flexor policis longus transfer, and release of supinator from radius. The median length of stay was 15 nights (range 3–75 nights).
Nerve conduction studies
Twenty-four of 34 procedures had associated pre-operative nerve conduction studies. Seventeen of 24 pre-operative nerve conduction studies were graded as ‘severe’ (11 median, six ulnar), five were ‘moderate’ (four median, one ulnar), two were ‘mild’ (one median, one ulnar). The mean pre-operative conduction velocity (CV) for median nerve SNAP (D5-wrist) was 11.8 ms−1 (SD 20.8), while for motor conduction (APB-wrist) the mean CV was 38.6 ms−1 (SD 11.8). For sensory conduction in the ulnar nerve (D2 across elbow), mean CV was 6.8 ms−1 (SD 18.1), while mean motor CV in the ulnar nerve (ADM across elbow) was 28 ms−1 (SD 15.8).
Three patients had persistent symptoms post-operatively and had underwent further nerve conduction studies. All three demonstrated neurophysiological improvement compared to pre-operative studies. Pre- and post-operative nerve conduction study results for these patients are shown in Fig. 5.
PROMs
Ten of the 15 patients contacted for telephone follow-up had undergone carpal tunnel decompression and therefore completed the BCTQ symptom severity score. The BCTQ asks patients to rate symptoms and difficulty with daily activities on a scale of 1–5. For each section, the score is an average of these ratings where one represents no symptoms or functional impairment at all, and five corresponds to the most severe symptoms and functional impairment. The mean BCTQ symptom severity score pre-operatively was 3.7 (SD 0.38, 3.3–4.2). Post-operatively, this was reduced to 1.3 (SD 0.33, 1.0–1.9) (p < 0.001), which represents an almost complete resolution of symptoms. Paraplegic patients who underwent carpal tunnel decompression were also asked to complete the functional assessment section: mean scores were 2.75 (SD 0.87, 1.5–4.5) and 1.43 (SD 0.77, 1.0–3.3) pre- and post-operatively, respectively (p = 0.01). These results are shown in Fig. 6.
The remaining five patients contacted for telephone follow-up were patients with tetraplegia who had undergone cubital tunnel decompression, and therefore completed the symptom severity section of the PRUNE score as well as the Modified BISHOP score. Mean PRUNE symptom severity score was reduced from 49.4 (SD 17.6, 23–65) pre-operatively to 23.0 (SD 16.9, 10–52) post-operatively (p = 0.01). These results are shown in Fig. 7. The mean Modified Bishop Score in this cohort was 6 (SD 1.9, 3–8), representing a good operative outcome.
Complications
There were no documented complications in the immediate post-operative period. However, two admissions were prolonged due to the development of a sacral pressure sore and a hospital-acquired pneumonia. Most prolonged inpatient stays were due to unrelated factors such as bladder/bowel management or opportunistic input from other medical specialties.
Patient satisfaction
Twenty-eight of 34 procedures had associated clinic follow-up with a mean duration to follow-up of 3.2 months. At clinic follow-up, 31 of 34 reported satisfaction with their procedure. All 20 paraplegic patients, and 11 of 14 tetraplegic patients, were satisfied with their procedure.
Fifteen patients were later successfully contacted by telephone for further follow-up, with a mean time to follow-up of 2.7 years. At this time, 14 of 15 patients contacted reported being satisfied with their procedures.
Discussion
Preservation of upper limb function is of paramount importance in patients with SCI. We found that patients already had severe neurological impairment at presentation as evidenced by both the pre-operative neurophysiological studies and PROMs. Our experience is that peripheral nerve decompression is a beneficial procedure for these patients who reported high rates of satisfaction at both clinic and telephone follow-up. Furthermore, earlier diagnosis and intervention in these patients, whether steroid injection or surgical decompression, would likely be beneficial.
Mean pre-operative symptom severity (3.7) and functional assessment (2.75) scores from the BCTQ demonstrate the significant burden of symptoms and functional impairment in this cohort, in keeping with their severe neurophysiological impairment. Post-operatively these scores were reduced to 1.3 and 1.25, respectively; this represents near-complete resolution of symptoms and functional impairment after surgical intervention. The relative reduction in mean score pre- versus post-operatively was marked, which is likely more important than absolute scores [10]. Similarly marked improvements were seen in the ulnar nerve decompression cohort, as demonstrated by the relative reduction in mean PRUNE score and the modified BISHOP score of six, representing a ‘good’ operative outcome.
Three patients had persistent symptoms and underwent post-operative nerve conduction studies. Despite their ongoing symptoms post-operatively, all three cases had marked and clinically meaningful improvements on neurophysiology. However, no other patients underwent post-operative nerve conduction studies and therefore these results did not reach statistical significance.
It is also of interest whether upper limb entrapment neuropathies may cause or contribute to the development of muscle spasms and associated pain. There has been speculation that spasticity in the wrist and finger flexors could contribute to carpal tunnel syndrome [11] but there has not been any report of improvement of spasticity secondary to peripheral nerve decompression. In our cohort, we noted one patient with tetraplegia whose debilitating muscle spasms were dramatically improved immediately after an open carpal tunnel decompression.
The reliance on upper limbs for both paraplegic and tetraplegic patients likely contributes to the increased incidence of carpal and cubital tunnel syndromes in SCI. The correlation between increased hand use and carpal tunnel syndrome was noted as early as 1950 by Phalen [12]. Repetitive loading and trauma to the flexor retinaculum is likely to play a role. Increased body weight, poor transfer technique, and the use of a conventional manual wheelchair have been implicated as risk factors [2, 13]. Given the increased incidence of nerve entrapment in SCI and the apparent correlation with time since spinal injury, clinicians should have a high index of suspicion for occult nerve compression in patients with prolonged duration of SCI. In the future, there may be a role for electrophysiological screening for SCI patients.
At the National Spinal Injuries Centre, we recently started offering endoscopic carpal tunnel decompressions as an evolution of our service, in the hope that this procedure may lead to swifter recovery [14] with shorter inpatient stays. In particular, we hypothesize that the endoscopic approach has the benefit of reducing pillar pain [15] which may be particularly important for wheelchair users. However, we are not yet able to draw conclusions regarding the relative efficacy of this technique compared to the open approach.
Elective surgery, particularly of the upper limb, is a major undertaking for individuals with SCI. It has been associated with a higher complication rate in both the peri-operative and post-operative period [9], including heightened risk of pressure sores, venous thromboembolism, autonomic dysreflexia, arrhythmias, and respiratory compromise. Post-operative recovery may also be complicated by dependence on the upper limbs for activities of daily living, and short-term measures such as hoist transfers and electric wheelchairs may be necessary to allow upper limb healing and recovery.
In our cohort, patients also had prolonged lengths of stay (median = 14 nights), while our non-SCI patients routinely undergo peripheral nerve decompression as day cases. The median length of stay for ulnar nerve decompressions (15 nights) was longer than for median nerve decompressions (12 nights). It is worth noting that the prolonged stays were largely due to logistical and social, rather than clinical, circumstances. One patient’s stay was complicated by a hospital-acquired pneumonia, which resolved with a course of antibiotics. A further patient had worsening of a longstanding sacral pressure sore, which may have been exacerbated by poor mobility post-operatively. As described, one patient had recurrence of carpal tunnel syndrome and underwent successful re-operation. No other complications were described in patient records. Our patients are intensively nursed at a dedicated spinal injury unit by a specialist spinal injury team and, in this context, peripheral nerve decompression is generally safe.
There are a number of limitations of this study. First, we used of PROMs that were designed for non-SCI patients. The applicability of these scoring systems in SCI is limited, particularly for assessing functional outcomes in tetraplegic patients. There is currently no standardized, validated scoring system for outcomes following peripheral nerve decompression in SCI. Despite this, functional and symptomatic improvements were encouraging, particularly when the pre-operative disease severity is considered. Another limitation is the inherent recall bias in retrospective evaluation of pre-operative symptoms. However, the most meaningful outcome reported is patient satisfaction which was reported. Although there is no non-surgical group for comparison, the high levels of patient satisfaction is a good indicator that nerve decompression was a likely useful intervention. There was also a significant level of attrition bias as only 15 of 34 patients were successfully contacted for follow-up. A prospective study with pre- and post-operative neurophysiology and standardized outcome measures tailored to the SCI population is planned.
In summary, our case series found that individuals with SCI tend to present late with severe upper limb nerve entrapment syndromes, evidenced by severe neurophysiological impairment and a significant burden of symptoms. The outcomes are excellent with high patient satisfaction and marked improvements in symptomatology and function. Clinicians involved in the care of SCI patients should have a low threshold to diagnose and refer for specialist assessment and electrophysiological studies.
Change history
13 January 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41394-021-00468-5
References
Aljure J, Eltorai I, Bradley WE, Lin JE, Johnson B. Carpal tunnel syndrome in paraplegic patients. Spinal Cord. 1985;23:182–6.
Asheghan M, Hollisaz M, Taheri T, Kazemi H, Aghda A. The prevalence of carpal tunnel syndrome among long-term manual wheelchair users with spinal cord injury: a cross-sectional study. J Spinal Cord Med. 2016;39:265–71.
Davidoff G, Werner R, Waring W. Compressive mononeuropathies of the upper extremity in chronic paraplegia. Spinal Cord. 1991;29:17–24.
Tun CG, Upton J. The paraplegic hand: electrodiagnostic studies and clinical findings. J Hand Surg Am. 1988;13:716–9.
Atroshi I, Gummersson C, Johnsson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153–8.
de Krom MC, Knipschild PG, Kester AD, Thijs CT, Boekkooi PF, Spaans F. Carpal tunnel syndrome: prevalence in the general population. J Clin Epidemiol. 1992;45:373–6.
Osei DA, Groves AP, Bommarito K, Ray WZ. Cubital tunnel syndrome: incidence and demographics in a national administrative database. Neurosurgery. 2017;80:417–20.
Kentar Y, Zastrow R, Bradley H, Brunner M, Pepke W, Bruckner T, et al. Prevalence of upper extremity pain in a population of people with paraplegia. Spinal Cord. 2018;56:695–703.
Petsas A, Drake J. Perioperative management for patients with a chronic spinal cord injury. BJA Educ. 2015;15:123–30.
De Kleermaeker FGCM, Boogaarts HD, Meulstee J, Verhagen WIM. Minimal clinically important difference for the Boston Carpal Tunnel Questionnaire: new insights and review of literature. J Hand Surg Eur. 2019;44:283–9.
Orcutt SA, Kramer WG, Howard MW, Keenan MA, Stone LR, Waters RL, et al. Carpal tunnel syndrome secondary to wrist and finger flexor spasticity. J Hand Surg Am. 1990;15:940–4.
Phalen GS, Gardner WJ, La Londe AA. Neuropathy of the median nerve due to compression beneath the transverse carpal ligament. J Bone Jt Surg Am. 1950;32A:109–12.
Hogaboom NS, Diehl JA, Oyster ML, Koontz AM, Boninger ML. Ultrasonographic median nerve changes after repeated wheelchair transfer in persons with paraplegia: relationship with subject characteristics and in transfer skills. PM R. 2016;8:305–13.
Li Y, Luo W, Wu G, Cui S, Zhang Z, Gu X. Open versus endoscopic carpal tunnel release: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2020;272:272.
Ludlow KS, Merla JL, Cox JA, Hurst LN. Pillar pain as a postoperative compliation of carpal tunnel release: a review of the literature. J Hand Ther. 1997;10:277–82.
Acknowledgements
With thanks to Eleanor Vickers BA for assistance with data collection, and to the multi-disciplinary team at the National Spinal Injuries Centre. The authors have no conflicts of interest to declare. No funding was sought/received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thomas, M., Hinton, A., Heywood, A. et al. Peripheral nerve decompression in the upper limb in spinal cord injury: experiences at the National Spinal Injuries Centre, UK. Spinal Cord Ser Cases 7, 56 (2021). https://doi.org/10.1038/s41394-021-00423-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-021-00423-4
This article is cited by
-
Anterior interosseous nerve neuropathy in a patient with spinal cord injury: case report and literature review
Spinal Cord Series and Cases (2022)