Abstract
Study design
Longitudinal study.
Objective
To explore whether individuals with traumatic spinal cord injury (TSCI) and non-traumatic SCI (NTSCI) experience different trajectories in changes of cardiometabolic disease (CMD) factors during initial rehabilitation stay.
Setting
Multicenter Swiss Spinal Cord Injury Cohort (SwiSCI) study.
Methods
Individuals without history of cardiovascular diseases were included. CMD factors and Framingham risk score (FRS) were compared between TSCI and NTSCI. Linear mixed models’ analysis was employed to explore the trajectory in CMD factors changes over rehabilitation period and a multivariate linear regression analysis was used at discharge from inpatient rehabilitation to explore factors associated with CMD risk profile in TSCI and NTSCI. We performed age and sex-stratified analyses.
Results
We analyzed 530 individuals with SCI (64% with TSCI and 36% NTSCI). The median age was 53 years (IQR:39-64) with 67.9% (n = 363) of the study cohort being male. The median rehabilitation duration was 4.4 months (IQR 2.4-6.4). At admission to rehabilitation, FRS (9.61 vs. 5.89) and prevalence of hypertension (33.16% vs. 13.62%), diabetes (13.68% vs. 4.06%), and obesity (79.05% vs. 66.67%) were higher in NTSCI as compared to TSCI, No difference was observed in cardiometabolic syndrome between the groups (around 40% in both groups). Overall, we observed longitudinal increases in total cholesterol, HDL-C and HDL/total cholesterol ratio, and a decrease in fasting glucose over the rehabilitation period. No differences in longitudinal changes in cardiovascular risk factors were observed between TSCI and NTSCI.
Conclusions
There was no deterioration in cardiometabolic risk factors over rehabilitation period, at discharge from initial rehabilitation stay. Both TSCI and NTSCI experienced high burden of cardiometabolic syndrome components with NTSCI experiencing more disadvantageous risk profile. The effectiveness of therapeutic and lifestyle/behavioral strategies to decrease burden of cardiometabolic disease and its components in early phase should be explored in future studies.
Similar content being viewed by others

Introduction
In the past decades, respiratory problems, renal failure and urinary complications were among the leading causes of death in the spinal cord injured (SCI) population [1]. With prolonged life-expectancy and improved acute care, mortality trends in SCI population in developed countries increasingly mimic those of the general population [2], with cardiovascular diseases (CVD) and diabetes being among the leading causes of death in both, traumatic (TSCI) and non-traumatic SCI (NTSCI) [1, 3, 4]. Increased cardiovascular risk post-injury has been driven by autonomic dysfunction, chronic inflammation and prolonged oxidative stress post-injury and has been shown to worsen within weeks post-injury [5]. In the early injury phase, a person participates in specialized rehabilitation program aimed to improve one’s independence in performing activities and to minimize limitations of physical impairments [6]. Factors such as injury severity, secondary health conditions (e.g., urinary tract infections, respiratory complications and pressure ulcers), injury management (e.g., surgical decompression) and medication use (e.g., opioids or steroids’ use) influence patients’ recovery, functioning and metabolic profile [7,8,9,10]. Thus, initial rehabilitation stay may be a critical time window to integrate early screening and preventive strategies targeting cardiometabolic risk factors to overcome accelerated functional and metabolic decline following the injury [11].
Only a few studies in the literature have explored changes in the cardiometabolic risk profile during the early phases of TSCI; whereas, there is a clear gap in evidence on cardiometabolic disease (CMD) burden in NTSCI [10, 12,13,14]. Individuals with traumatic injury may experience different trajectories of CMD risk profile post-injury as compared to those suffering non-traumatic injury due to phenotypic differences between TSCI and NTSCI. For instance, the incidence of NTSCI increases with age, and there is a higher proportion of affected females as compared to TSCI; both age and sex are established CMD risk determinants [15, 16]. Due to the presence of more complete injuries and a higher frequency of complications, patients with TSCI are hospitalized for an average of 3.4 weeks longer than patients with NTSCI, which may further impact cardiometabolic risk profile [17]. Thus, the underrepresentation of NTSCI in research limits the generalizability of findings for the most important determinants of increased CMD risk in SCI and complicates development of effective personalized preventive strategies.
Therefore, our study aims to: (i) determine the prevalence of CMD at admission to initial rehabilitation stay and (ii) explore differences in cardiometabolic risk profile changes over the rehabilitation period comparing individuals within traumatic and non-traumatic injury. We hope that our findings can assist in the identification of individuals with SCI who would benefit the most from preventive approaches to reach the metabolic equilibrium during the early injury phase.
Methods
Study design and study cohort
We used data from the inception cohort of the Swiss Spinal Cord Injury (SwiSCI) study [18]. SwiSCI study is a cohort established as a collaboration among four major rehabilitation centers across Switzerland (Swiss Paraplegic Centre, Nottwil; Klinik für Neurorehabilitation und Paraplegiologie-REHAB Basel, Basel; Clinique romande de readaptation, Sion; and Balgrist University Hospital, Balgrist) which serve as regional catchment areas for individuals requiring specialized care post-injury. SwiSCI Inception Cohort prospectively enrolled individuals with SCI who were admitted for inpatient rehabilitation in one of its participating centers in Switzerland. Data were collected in the study centers at four time points following the date of SCI diagnosis: at 28 days (range 16–40 days, T1), 84 days (70–98 days, T2), 168 days (150–186 days, T3), and at discharge (10–0 days before discharge, T4). Our analyses focused on admission to rehabilitation (T1), which represents the study baseline, and rehabilitation discharge (T4). Data are collected by extraction of routine clinical information from the medical records, by clinical assessments, and by paper-and-pencil questionnaires. A comprehensive list of commonly utilized metrics within the collaborating centers was developed, with a focus on prioritizing and standardizing established measures across all four centers. The SwiSCI Inception cohort data model is based on the International Classification of Functioning, Disability and Health (ICF), and the Brief ICF SCI Core Sets in the early post-acute context was used as a reference for the clinical setting. Additionally, whenever applicable and accessible, preference was given to incorporating the “International SCI Basic Data Sets” recommended by the International Spinal Cord Injury Society (ISCOS) (https://www.iscos.org.uk/international-sci-data-sets). Detailed information on the study design and collected data have been reported elsewhere [18, 19].
Inclusion and exclusion criteria
We enrolled all adults (≥18 years old) from May 2013 to September 2020, who were admitted to any of the four participating rehabilitation centers. Individuals with an SCI attributable to a congenital condition, neurodegenerative disorder, or Guillain–Barré syndrome, or who had a new SCI in the context of palliative care, were excluded from the study. Furthermore, individuals with SCI who had malignant neoplasms or those in palliative/end-of life care were excluded. Finally, we excluded those with previous history of CVD to create a homogenous baseline cardiovascular risk profile of our analysis population. This is also in accordance to how most studies in cardiovascular risk profiling were conducted in the literature.
Clinical measures and injury classification
The SCI characteristics included SCI lesion etiology (e.g., traumatic vs. non-traumatic, causes of the injury), level and completeness of the injury (motor complete and incomplete), and the pattern of NTSCI injury onset (including acute, sub-acute, prolonged). The level of injury was classified as tetraplegia (at level C2-C7) and paraplegia (level T1-S5), and the completeness of injury into complete motor injury (AIS A and B) and incomplete (AIS C and D) based on the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) [20]. In addition, time since injury and duration of rehabilitation at the SwiSCI rehabilitation center were derived from medical records. Further, demographic characteristics such as age at baseline, sex, information on comorbidities and medication use were obtained from the SwiSCI database and were derived from patient’s medical records.
Venous blood samples were obtained from each participant after an overnight fast. Samples were then sent to respective hospital laboratories for lipid and glucose profiles. Waist circumference (WC) was measured after bowel care. Measurement was taken at the end of a normal exhale, between the lower rib and the top of the hip bone. A flexible tape measure with a precision of 0.5 cm was used. Weight was measured using an electric wheelchair scale. The wheelchair’s weight was subtracted from the total weight of the subject with the wheelchair to determine the subject’s weight expressed in kilograms (kg). Body mass index (BMI) was computed employing the standard formula [weight in kilograms/(height in meters)2].
Outcome measures
We identified individuals with CMD using the criteria provided by the SCI-specific clinical guideline [21]. CMD factors included blood pressure, fasting lipid profile, fasting glucose, and anthropometric measures, that was also used individually for longitudinal modeling. The risk of developing the first cardiovascular event within the next 10 years was assessed using the Framingham risk score (FRS) [22]. The FRS of each study participant was computed at discharge from initial rehabilitation stay using the following variables: (a) age, (b) sex, (c) systolic blood pressure (SBP), (d) total cholesterol (mg/dL), (e) high-density lipoprotein cholesterol (mg/dL), (f) diabetes, and (g) current smoking [22].
Statistical analyses
We summarized continuous variables using median and interquartile range (IQR) as prescribed by the International Spinal Cord Society (ISCOS) Standards of Data Analysis and Reporting [23]. We log-transformed all non-normally distributed continuous variables. Categorical variables were presented as numbers and percentages. To compare the differences in demographic characteristics, injury characteristics, clinical parameters, lifestyle factors, and comorbidities at baseline between TSCI and NTSCI, we used Wilcoxon signed rank test and chi-square test, as appropriate.
We used a paired t-test to compute the longitudinal changes in cardiometabolic parameters from beginning to end of rehabilitation for individuals with TSCI and NTSCI. We also used a multilevel mixed model using random slope of each individual trajectory by residual maximum likelihood estimation. The longitudinal model was adjusted for age, sex, smoking history, alcohol use, time since injury, prevalent and incident CMD, injury completeness and injury level. Furthermore, we included an interaction term (injury etiology and rehabilitation time) to account for time specific changes in CVD risk factors.
According to the level of injury, we explored the longitudinal changes in cardiovascular risk of the study participants. We similarly used multilevel mixed model using random intercept and individuals as clusters. We used anthropometric measures, blood pressure, fasting lipid profile, and fasting glucose as outcome variable. We used injury etiology (TSCI versus NTSCI) as our predictor variable, with similar model adjustments as previously mentioned.
Finally, we investigated on risk factors for changes in the components of CMD. For this, we performed multivariable linear regression using discharge values (anthropometric measures, blood pressure, fasting lipid profile, and fasting glucose), that were fitted among individuals with TSCI and NTSCI separately. Model adjustments were done as previously mentioned. This was done to explore the longitudinal association between age, sex, injury severity, rehabilitation duration and lifestyle factors and CMD factors.
All statistical analyses were performed using the Stata 16.1 (StataCorp LLC, College Station, TX) for Windows. All computations were done using two-tailed tests, and a p-value of < 0.05 was considered statistically significant.
Sensitivity analyses
We performed sex-stratified analyses to determine sex-specific associations. We performed age-stratified analysis to determine age-specific associations based on median population age (55 years). To detect selection bias, we also compared the excluded and the included study cohort. For missing data, we tabulated missing exposures and outcomes, and performed list-wise deletion in our regression analyses.
Results
Baseline characteristics
The cohort invited 1225 acutely injured individuals from all participating centers. We excluded the following individuals: 570 individuals did not provide full consent for participation, 6 individuals did not meet the age criterion ( < 18 years old), 35 individuals had malignant neoplasms, 79 individuals had prior cardiovascular disease, and 5 individuals had pathologically or physiologically impossible values (erroneous data). Overall, 530 individuals with SCI were included in analyses (Fig. 1).
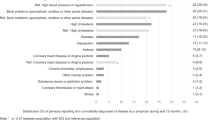
Among included participants 340 (64%) had traumatic and 190 (36%) non-traumatic injury. The study cohort had a median age of 53 years (IQR 39-64) and the majority were males 363 (67.9%). The median time since injury was 14 days (IQR 9-24) while the median length of rehabilitation stay was 4.4 months (IQR 2.4-6.4). In Table 1 we present the most important clinical characteristics of study participants at admission to rehabilitation stratified by injury etiology (TSCI and NTSCI). In brief, at baseline/admission to rehabilitation, individuals with NTSCI were significantly older (58 years vs. 50 years), had a lower proportion of men (59.5% vs. 72.5%), and had a lower proportion of cervical (23% vs. 36%) and motor complete injuries (10.8% vs. 29.9%). Further, individuals with NTSCI were more likely to have diabetes (13.7% vs. 4.1%), hypertension (33.2% vs. 13.62%), obesity (79.1% vs. 66.7%) and had significantly higher FRS at baseline (9.6% vs. 5.9%). Whereas, individuals with TSCI were more likely to be treated with opioid medications (40.0% vs. 25.8%) and individuals with NTSCI were more likely to be treated with steroids (14.2% vs. 4.7%). In Supplemental Table 1, we present the causes of NTSCI.
Longitudinal changes in cardiometabolic risk factors
Overall, we observed an increase in total cholesterol, HDL, and HDL-TC ratio between the beginning and end of rehabilitation period [β 0.06 (95%CI 0.03, 0.09) p < 0.01], [β 0.16 (95%CI 0.12, 0.19) p < 0.01] and [β 0.11 (95%CI 0.07, 0.15) p < 0.01], respectively. Glucose concentration decreased over rehabilitation stay [β −0.03 (95%CI −0.06, −0.01) p < 0.01], while no changes in BMI, waist circumference, SBP, DBP, triglycerides, and LDL cholesterol concentration were observed (Table 2). When comparing whether changes in CMD factors differed between individuals with traumatic and non-traumatic injury, in a fully adjusted model, we observed greater increase in mean HDL concentration in NTSCI as compared to TSCI [β 0.08 (95%CI 0.00, 0.16) p < 0.05]. We did not observe any changes among other risk factors (Table 3). Sex and age stratified analysis was in line with overall findings (Supplemental Tables 2 and 3). The Fig. 2 depicts individual trajectories in FRS score comparing the beginning and end of rehabilitation, in the overall cohort, and individuals with TSCI and NTSCI. Overall, we observed decreasing FRS over the rehabilitation stay in the overall study cohort and among those with traumatic injury, whereas for NTSCI no significant change in FRS was observed.
A–C Showing Framingham risk score at baseline and follow-up (10-year risk for first cardiovascular event). The graphs describe the course of each individual represented by a dot via plotting the baseline and follow-up values on x-y-axis. Individuals (dots) on the line mean no change across time has been observed. Individuals above the line represent an increased 10-year risk (Framingham score), whereas those below the line represent a decreased 10-year risk (Framingham score). p value is measured through sign test for matched pairs.
Cardiometabolic Risk Profile Prior to Discharge from Initial Rehabilitation
We explored the association between one’s clinical characteristics and cardiometabolic risk factors through multivariable linear regression (adjusting for age, sex, smoking history, alcohol use, time since injury, diabetes, injury etiology, injury completeness and injury level). For TSCI, we found a positive association between age and SBP, DBP, LDL, and WC [β 0.22 (95%CI 0.12, 0.31) p < 0.001], [β 0.17 (95%CI 0.10, 0.24) p < 0.001], [β 0.01 (95%CI 0.00, 0.01) p < 0.05], [β 0.36 (95%CI 0.27, 0.44) p < 0.001] respectively (Table 4). SBP, DBP and triglycerides were lower in females [β −4.34 (95%CI −7.94, −0.75) p < 0.05], [β −3.05 (95%CI −5.82, −0.28) p < 0.05], and [β −0.35 (95%CI −0.67, −0.02) p < 0.05] respectively; while HDL and HDL-total cholesterol ratio were higher in females vs. males [β 0.35 (95%CI 0.21, 0.48) p < 0.001] and [β 0.05 (95%CI 0.01, 0.08) p < 0.05], respectively. Total cholesterol was higher among individuals with paraplegia in comparison to tetraplegia [β 0.38 (95%CI 0.09, 0.68) p < 0.01]. Total cholesterol and triglycerides were higher among smokers [β 0.45 (95%CI 0.05, 0.84) p < 0.05] and [β 0.64 (95%CI 0.24, 1.04) p < 0.01], respectively.
In NTSCI, increasing age was associated with higher SBP [β 0.19 (95%CI 0.03, 0.35) p < 0.05] and females compared to males had higher HDL [β 0.25 (95%CI 0.06, 0.44) p < 0.05]. Furthermore, DBP was lower among those with paraplegia in comparison to those with tetraplegia [β−15.38 (95%CI −29.91, −0.86) p < 0.05]. Total cholesterol and LDL were lower among individuals with toxic and metabolic NTSCI injury etiology [β −0.49 (95%CI −0.91, −0.06) p < 0.05] and [β −0.40 (95%CI −0.80, −0.01) p < 0.05] respectively (Table 4). Finally, triglycerides were higher among smokers [β 0.44 (95%CI 0.02, 0.85) p < 0.05]. We did not find any further associations for the remaining cardiometabolic risk factors.
Sensitivity analyses
The percentage of missing data for the dependent variables included in the analysis was generally lower than 50% (Supplemental Table 4). The excluded individuals with SCI were older compared to individuals who were included in the analysis [62 years IQR (43–72) vs 53 years IQR (41–64)]. We did not find any significant differences in sex, education level, injury level nor completeness among the two comparison groups, Supplemental Table 5.
Discussion
This study provides the first comparison of cardiometabolic disease burden among individuals with TSCI and NTSCI admitted to first inpatient rehabilitation stay. At admission, we observed significantly higher proportion of individuals with tetraplegia and motor complete injury among those with TSCI as compared to NTSCI. Individuals with NTSCI were older, comprised higher proportion of females and higher burden of cardiometabolic risk factors (obesity, diabetes, hypertension). They also had significantly higher FRS, which indicates the risk of developing the first CVD events within the next 10 years. Following an average of 4.4 months in rehabilitation, we observed a borderline improvement in lipid profile (higher HDL-C and HDL-C/TC ratio) and glucose in the overall study cohort and no significant differences in changes in these factors between NTSCI and TSCI. Factors such as age, male sex, injury level, and smoking were associated with poorer risk profile at discharge.
Clinical implications of our findings and outlook
First, previous studies focusing on CMD burden during initial rehabilitation stay were conducted among individuals with traumatic injury. Our findings among individuals with TSCI are comparable with those reported in the literature [12, 24], however, our findings among individuals with NTSCI are to be confirmed in other populations.
Second, higher burden of CMD at admission among those with NTSCI is not a surprise and may be driven by older age at the time of injury, as well as the higher proportion of females (e.g., after menopause females observe undesirable changes in body composition and CMD risk profile). We used the FRS to calculate the 10-year risk of first CVD event. Individuals with NTSCI had significantly higher scores at admission. In Fig. 2, we provide individual trajectories in FRS (comparing admission and discharge) for the overall cohort and among those with TSCI and NTSCI. Over the follow-up period (on average 5.3 months in TSCI and 4.4 months in NTSCI) we observed a decrease in FRS in TSCI and no change in score among NTSCI. The FRS may underestimate the future CVD risk in individuals with SCI. Thus, the true risk may be even higher than reported in the current study. Moreover, the follow-up period may not be sufficient to detect more extensive changes in FRS. Yet, regardless of FRS, considering high burden of hypertension, diabetes, and overweight and obesity among those with NTSCI future studies are needed to determine on whether routine CVD screening should be implemented during initial rehabilitation stay.
Third, nearly every individual sustaining SCI receives multiple types of medications that may alter or modify blood glucose, lipids, and blood pressure. These drugs manage a range of problems associated with neurotrauma to the spinal cord, secondary health conditions (pain, muscle spasm, respiratory or unitary tract infections) and multimorbidity. Most prescribed medications include skeletal muscle relaxants, analgesic-narcotics or tricyclic antidepressants [25]. Simultaneous use of these medications may have a detrimental effect on neurological recovery and functioning as well as increase the risk of complications such as respiratory depression, fractures, hypothalamic–pituitary–gonadal (HPG) axis dysregulation and lead to adverse metabolic changes [25]. Among these medications, opioids, although not recommended as a first-line therapy for pain due to questionable benefit-to-risk ratio, are ubiquitously administered for pain management in humans sustaining an acute SCI during a therapeutic window of opportunity for neuroprotection and repair [25, 26]. We observed that high proportions of individuals with TSCI (40%) and NTSCI (25%) were treated with opioid medications. Another study from Canada reported that in the year following discharge from inpatient rehabilitation, 60% of individuals with NTSCI had opioid medications prescribed [27]. Older age, being female, diagnosis of osteoporosis, prior exposure to prescription opioids, higher morbidity score, and lower functional status were the main predictors of higher opioid use [27]. Thus, future studies should explore the patterns of opioid prescriptions and the clinical significance of opioid medications on modifying metabolic changes, rehabilitation outcomes and functioning post-injury. Finally, considering the high burden of CMD during initial rehabilitation stay, it is worthwhile to explore the patterns of treatment and prophylactic use of CMD medications (e.g., antihypertensives, statins, etc.) as well as lifestyle and behavioral interventions.
Study strengths and limitations
To our knowledge, this is the first study to describe cardiometabolic risk factors in individuals with NTSCI and the first to compare them to individuals with traumatic injury. Major strengths of our analysis include robust statistical estimates by applying linear mixed models for repeated measures analysis and a relatively large sample size. However, this study has some limitations. First, it is possible that because we used the SCI-specific cut-offs for defining obesity (BMI > 22 kg/m2 [21, 28] and WC ≥ 86.5 cm [29, 30]) and metabolic disease/syndrome, some individuals may be misclassified and the proportion of individuals with obesity may be overestimated. In particular, we cannot ascertain when the SCI specific cut-off should be used along the course of SCI or how early it should be used to define overweight/obesity. Second, we used the FRS that may be a suboptimal risk estimator in the SCI population. However, despite FRS underestimating the overall risk, it can still distinguish between those who are at high vs. low CVD risk in the SCI population [31]. Third, because causes for NTSCI are heterogeneous, including tumor-related, congenital/developmental, infectious, inflammatory and ischemic causes, as well as several others, interpretation of changes in CMD factors may be challenging [32]. Despite all efforts to harmonize data collection within the four rehabilitation centers, we cannot exclude the possibility of variation in clinical assessments across the involved centers. Fifth, females, the older adults, and individuals with lower functional independence were less likely to participate in the SwiSCI study [18]. Therefore, it is possible that initially, individuals with an impaired CMD risk profile were less likely to be included in the SwiSCI. Although we excluded individuals with a history of CVD from the current study, the generalizability of our results could be limited when considering individuals with a poorer CMD risk profile.
Conclusion
In conclusion, we report a high prevalence of cardiometabolic disease and its components at admission to first inpatient rehabilitation, especially in individuals with NTSCI. We did not observe differences in early changes in CMD components among TSCI and NTSCI. Moreover, there is a systematic lack of evidence on cardiometabolic diseases in NTSCI and our results should be replicated in another study whereas the potential of early preventable strategies (e.g., CMD prophylactic medication use, or lifestyle modifications) to improve metabolic health among those with NTSCI should be explored.
Data availability
The dataset generated and/or analyzed during the current study are available from the SwiSCI Study Center on reasonable request.
References
DeVivo MJ, Chen Y, Wen H. Cause of Death Trends Among Persons With Spinal Cord Injury in the United States: 1960-2017. Arch Phys Med Rehab. 2022;103:634–41.
Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86:142–52.
Chamberlain JD, Buzzell A, Gmunder HP, Hug K, Jordan X, Moser A, et al. Comparison of all-cause and cause-specific mortality of persons with traumatic spinal cord injuries to the general swiss population: results from a national cohort study. Neuroepidemiology. 2019;52:205–13.
Buzzell A, Chamberlain JD, Eriks-Hoogland I, Hug K, Jordan X, Schubert M, et al. All-cause and cause-specific mortality following non-traumatic spinal cord injury: evidence from a population-based cohort study in Switzerland. Spinal Cord. 2020;58:157–64.
Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53.
New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehab. 2005;86:250–61.
New PW, Rawicki HB, Bailey MJ. Nontraumatic spinal cord injury: Demographic characteristics and complications. Arch Phys Med Rehab. 2002;83:996–1001.
Stampas A, Dominick E, Zhu L. Evaluation of functional outcomes in traumatic spinal cord injury with rehabilitation-acquired urinary tract infections: A retrospective study. J Spinal Cord Med. 2019;42:579–85.
New PW, Jackson T. The costs and adverse events associated with hospitalization of patients with spinal cord injury in Victoria, Australia. Spine (Phila Pa 1976). 2010;35:796–802.
Raguindin PF, Stoyanov J, Eriks-Hoogland I, Stucki G, Jordan X, Schubert M, et al. Cardiometabolic risk profiling during spinal cord injury rehabilitation: A longitudinal analysis from Swiss Spinal Cord Injury cohort (SwiSCI). Pm r. 2022.
Pili R, Gaviano L, Pili L, Petretto DR. Ageing, disability, and spinal cord injury: some issues of analysis. Curr Gerontol Geriatrics Res. 2018;2018:4017858.
de Groot S, Dallmeijer AJ, Post MW, Angenot EL, van den Berg-Emons RJ, van der Woude LH. Prospective analysis of lipid profiles in persons with a spinal cord injury during and 1 year after inpatient rehabilitation. Arch Phys Med Rehab. 2008;89:531–7.
Ravensbergen HJC, de Groot S, Post MWM, Slootman HJ, van der Woude LHV, Claydon VE. Cardiovascular function after spinal cord injury: prevalence and progression of dysfunction during inpatient rehabilitation and 5 years following discharge. Neurorehab Neural Repair. 2013;28:219–29.
de Groot S, Post MW, Hoekstra T, Valent LJ, Faber WX, van der Woude LH. Trajectories in the course of body mass index after spinal cord injury. Arch Phys Med Rehabil. 2014;95:1083–92.
New PW, Sundararajan V. Incidence of non-traumatic spinal cord injury in Victoria, Australia: a population-based study and literature review. Spinal Cord. 2008;46:406–11.
Gupta A, Taly AB, Srivastava A, Murali T. Non-traumatic spinal cord lesions: epidemiology, complications, neurological and functional outcome of rehabilitation. Spinal Cord. 2009;47:307–11.
Gedde MH, Lilleberg HS, Aßmus J, Gilhus NE, Rekand T. Traumatic vs non-traumatic spinal cord injury: A comparison of primary rehabilitation outcomes and complications during hospitalization. J Spinal Cord Med. 2019;42:695–701.
Fekete C, Gurtner B, Kunz S, Gemperli A, Gmünder HP, Hund-Georgiadis M, et al. Inception cohort of the Swiss Spinal Cord Injury Cohort Study (SwiSCI): Design, participant characteristics, response rates and non-response. J Rehab Med. 2021;53:jrm00159.
Post MW, Brinkhof MW, von Elm E, Boldt C, Brach M, Fekete C, et al. Design of the swiss spinal cord injury cohort study. Am J Phys Med Rehabil. 2011;90:S5–16.
Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26:S50–6.
Nash MS, Groah SL, Gater DR, Dyson-Hudson TA, Lieberman JA, Myers J, et al. Identification and management of cardiometabolic risk after spinal cord injury: clinical practice guideline for health care providers. Topics Spinal Cord Injury Rehab. 2018;24:379–423.
D’Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53.
DeVivo MJ, Biering-Sorensen F, New P, Chen Y. International Spinal Cord Injury Data S. Standardization of data analysis and reporting of results from the International Spinal Cord Injury Core Data Set. Spinal Cord. 2011;49:596–9.
Apstein MD, George BC. Serum lipids during the first year following acute spinal cord injury. Metabolism. 1998;47:367–70.
Guilcher SJT, Hogan ME, Calzavara A, Hitzig SL, Patel T, Packer T, et al. Prescription drug claims following a traumatic spinal cord injury for older adults: a retrospective population-based study in Ontario, Canada. Spinal Cord. 2018;56:1059–68.
Woller SA, Hook MA. Opioid administration following spinal cord injury: implications for pain and locomotor recovery. Exp Neurol. 2013;247:328–41.
Guan Q, Hogan ME, Calzavara A, McCormack D, Lofters AK, Patel T, et al. Prevalence of prescribed opioid claims among persons with nontraumatic spinal cord dysfunction in Ontario, Canada: a population-based retrospective cohort study. Spinal Cord. 2021;59:512–9.
Laughton GE, Buchholz AC, Martin Ginis KA, Goy RE, Group SSR. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47:757–62.
Gill S, Sumrell RM, Sima A, Cifu DX, Gorgey AS. Waist circumference cutoff identifying risks of obesity, metabolic syndrome, and cardiovascular disease in men with spinal cord injury. PLoS One. 2020;15:e0236752.
Sumrell RM, Nightingale TE, McCauley LS, Gorgey AS. Anthropometric cutoffs and associations with visceral adiposity and metabolic biomarkers after spinal cord injury. PLoS One. 2018;13:e0203049.
Barton TJ, Low DA, Bakker EA, Janssen T, de Groot S, van der Woude L, et al. Traditional cardiovascular risk factors strongly underestimate the 5-year occurrence of cardiovascular morbidity and mortality in spinal cord injured individuals. Arch Phys Med Rehabil. 2021;102:27–34.
McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–25.
Acknowledgements
We thank the SwiSCI Study Group with its members Xavier Jordan and Fabienne Reynard (Clinique Romande de Réadaptation, Sion); Michael Baumberger, Luca Jelmoni (Swiss Paraplegic Center, Nottwil); Armin Curt (University Clinic Balgrist, Zürich); Margret Hund-Georgiadis (REHAB Basel, Basel); Laurent Prince (Swiss Paraplegic Association, Nottwil); Heidi Hanselmann (Swiss Paraplegic Foundation, Nottwil); Daniel Joggi (Representative of persons with SCI); NN (Parahelp, Nottwil); and Carla Sabariego (SwiSCI Coordination Group at Swiss Paraplegic Research, Nottwil).
Funding
PFR and OAI have received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 801076, through the SSPH+ Global Ph.D. Fellowship Programme in Public Health Sciences (GlobalP3HS) of the Swiss School of Public Health. Open access funding provided by University of Bern.
Author information
Authors and Affiliations
Contributions
Study concept and design: MG. Statistical analyses and preparation of first draft of the manuscript: PFR & OAI. Interpretation of data and drafting of the manuscript: PFR, OAI, IE-H, MB, GS, TM, JS, MG. Study supervision: MG. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare competing interests.
Ethical approval
SwiSCI study is compliant with the Swiss Human Research Act (810.30 Federal Act of September 30, 2011, on Research involving Human Beings) and Federal Regulations on Data Protection (235.1 Federal Act of June 19, 1992, on Data Protection). Different regional ethical committees cleared the study protocol before enrolling participants [Ethics Committee northwest/central Switzerland (EKNZ): PB-2016-00183, Ethics Committee Vaud (CEVD): 032/13 (CEVS), Ethics Committee Zurich (KEKZH): 2013-0249]. All study participants provided informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raguindin, P.F., Itodo, O.A., Eriks-Hoogland, I. et al. Does cardiometabolic risk profile differ among individuals with traumatic and non-traumatic spinal cord injury (SCI): the evidence from the multicenter SCI cohort in Switzerland (SwiSCI). Spinal Cord (2024). https://doi.org/10.1038/s41393-024-00996-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41393-024-00996-5