Abstract
Background
Currently, there is no efficacious treatment method for chronic prostatitis type IIIb/chronic pelvic pain syndrome (CP/CPPS). Aim of the study was to investigate and compare the efficacy and safety of low-intensity shockwave therapy (LiST) vs. sham treatment in CP/CPPS patients.
Methods
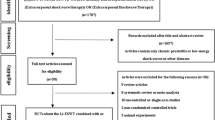
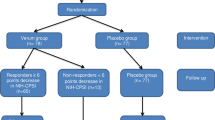
Patients with CP/CPPS diagnosis were randomized in this prospective, sham-controlled, double-blind study either to the active groups (Group B, C) who received 5000 shockwaves per session with energy flux density 0.1 mJ/mm2 or to the sham group (Group A) who received 5000 shockwaves from a visually identical sham probe. All groups underwent six sessions (once/week). LiST effects on pain, micturition, quality of life (QoL), and erectile function were evaluated at 4, 12, and 24 weeks after treatment. The parameters were investigated using validated questionnaires. Uroflowmetry and post void residual calculation were performed at baseline and at 4- and 12-week FU visit. Prostate mpMRI and PSA measurement were performed at baseline and 12-week FU visit.
Results
Overall, 45 men were randomized to the active (n = 30) and sham groups (n = 15). Regarding impact of LiST in National Institutes of Health-Chronic Prostatitis Symptom Index (NIH-CPSI) total, pain, and QoL subdomains scores a clear and persistent in all FU timepoints improvement was found compared to sham treatment. NIH-CPSI urinary subdomain, International Prostate Symptom Score [IPSS], PSA, and mpMRI-PIRADS scores did not differ between the two groups. The mean difference between the LiST and sham group in the change of the NIH-CPSI pain-domain score (Q1–4) from baseline to 12 weeks after final treatment which was 3.3 (95% CI, 1.8, 4.7). Perineal LiST was easy and safe to perform without anesthesia or any side-effects.
Conclusions
LiST seems to be a safe and effective treatment option for CP/CPPS, considerably improving pain and quality of life. Lack of any side-effects, and the potential for repetition make LiST a promising treatment choice for CP/CPPS patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schaeffer AJ, Datta NS, Fowler JE, Krieger JN, Litwin MS, Nadler RB, et al. Overview summary statement. Diagnosis and management of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). Urology 2002;60:1–4.
Franco JV, Turk T, Jung JH, Xiao Y-T, Iakhno S, Garrote V, et al. Non-pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome. Cochrane Database Syst Rev. 2018;1:CD012551.
Franco JV, Turk T, Jung JH, Xiao Y-T, Iakhno S, Tirapegui FI, et al. Pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome. Cochrane Database Syst Rev. 2019;10:CD012552.
Magistro G, Wagenlehner FME, Grabe M, Weidner W, Stief CG, Nickel JC. Contemporary management of chronic prostatitis/chronic pelvic pain syndrome. Eur Urol. 2016;69:286–97.
Doiron RC, Shoskes DA, Nickel JC. Male CP/CPPS: where do we stand? World J Urol. 2019;37:1015–22.
Zimmermann R, Cumpanas A, Hoeltl L, Janetschek G, Stenzl A, Miclea F. Extracorporeal shock-wave therapy for treating chronic pelvic pain syndrome: a feasibility study and the first clinical results. BJU Int. 2008;102:976–80.
Zimmermann R, Cumpanas A, Miclea F, Janetschek G. Extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome in males: a randomised, double-blind, placebo-controlled study. Eur Urol. 2009;56:418–24.
Vahdatpour B, Alizadeh F, Moayednia A, Emadi M, Khorami MH, Haghdani S. Efficacy of extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome: a randomized, controlled trial. ISRN Urol. 2013. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3755541/.
Zeng X-Y, Liang C, Ye Z-Q. Extracorporeal shock wave treatment for non-inflammatory chronic pelvic pain syndrome: a prospective, randomized and sham-controlled study. Chin Med J. 2012;125:114–8.
Yuan P, Ma D, Zhang Y, Gao X, Liu Z, Li R, et al. Efficacy of low-intensity extracorporeal shock wave therapy for the treatment of chronic prostatitis/chronic pelvic pain syndrome: a systematic review and meta-analysis. Neurourol Urodyn. 2019;38:1457–66.
Engeler D, Baranowski PA, Berghmans B, Borovicka J, Cottrell AM, Dinis-Oliveira P, et al. EAU Guidelines 2018. EAU Guidelines Office, Arnhem, The Netherlands. 2018.
Capogrosso P, Frey A, Jensen CFS, Rastrelli G, Russo GI, Torremade J, et al. Low-intensity shock wave therapy in sexual medicine-clinical recommendations from the European Society of Sexual Medicine (ESSM). J Sex Med. 2019;16:1490–505.
Schulz KF, Altman DG, Moher D. CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332.
Nickel JC, Shoskes D, Wang Y, Alexander RB, Fowler JE, Zeitlin S, et al. How does the pre-massage and post-massage 2-glass test compare to the Meares-Stamey 4-glass test in men with chronic prostatitis/chronic pelvic pain syndrome? J Urol. 2006;176:119–24.
Mariotto S, Cavalieri E, Amelio E, Ciampa AR, de Prati AC, Marlinghaus E, et al. Extracorporeal shock waves: from lithotripsy to anti-inflammatory action by NO production. Nitric Oxide. 2005;12:89–96.
Wess OJ. A neural model for chronic pain and pain relief by extracorporeal shock wave treatment. Urol Res. 2008;36:327–34.
Marszalek M, Berger I, Madersbacher S. Low-energy extracorporeal shock wave therapy for chronic pelvic pain syndrome: finally, the magic bullet? Eur Urol. 2009;56:425–6.
Moayednia A, Haghdani S, Khosrawi S, Yousefi E, Vahdatpour B. Long-term effect of extracorporeal shock wave therapy on the treatment of chronic pelvic pain syndrome due to non bacterial prostatitis. J Res Med Sci J Isfahan Univ Med Sci. 2014;19:293–6.
Guu S-J, Geng J-H, Chao I-T, Lin H-T, Lee Y-C, Juan Y-S, et al. Efficacy of low-intensity extracorporeal shock wave therapy on men with chronic pelvic pain syndrome refractory to 3-as therapy. Am J Mens Health. 2018;12:441–52.
Al Edwan GM, Muheilan MM, Atta ONM. Long term efficacy of extracorporeal shock wave therapy [ESWT] for treatment of refractory chronic abacterial prostatitis. Ann Med Surg. 2017;14:12–17.
Pajovic B, Radojevic N, Dimitrovski A, Vukovic M. Comparison of the efficiency of combined extracorporeal shock-wave therapy and triple therapy versus triple therapy itself in Category III B chronic pelvic pain syndrome (CPPS). Aging Male J Int Soc Study Aging Male. 2016;19:202–7.
Salama AB, Abouelnaga WA. Effect of radial shock wave on chronic pelvic pain syndrome/chronic prostatitis. J Phys Ther Sci. 2018;30:1145–9.
Tripp DA, Curtis Nickel J, Landis JR, Wang YL, Knauss JS. CPCRN Study Group. Predictors of quality of life and pain in chronic prostatitis/chronic pelvic pain syndrome: findings from the National Institutes of Health Chronic Prostatitis Cohort Study. BJU Int. 2004;94:1279–82.
Nickel JC. Chronic prostatitis/chronic pelvic pain syndrome: it is time to change our management and research strategy. BJU Int. 2020;125:479–80.
Rayegani SM, Razzaghi MR, Raeissadat SA, Allameh F, Eliaspour D, Abedi AR, et al. Extracorporeal shockwave therapy combined with drug therapy in chronic pelvic pain syndrome: a randomized clinical trial. Urol J. 2020;17:185–91.
Kalyvianakis D, Memmos E, Mykoniatis I, Kapoteli P, Memmos D, Hatzichristou D. Low-intensity shockwave therapy for erectile dysfunction: a randomized clinical trial comparing 2 treatment protocols and the impact of repeating treatment. J Sex Med. 2018;15:334–45.
Funding
LIST device and the three probes were provided by Dornier MedTech GmbH, Wessling, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mykoniatis, I., Kalyvianakis, D., Zilotis, F. et al. Evaluation of a low-intensity shockwave therapy for chronic prostatitis type IIIb/chronic pelvic pain syndrome: a double-blind randomized sham-controlled clinical trial. Prostate Cancer Prostatic Dis 24, 370–379 (2021). https://doi.org/10.1038/s41391-020-00284-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-00284-2
This article is cited by
-
The efficacy and safety of low-intensity extracorporeal shock wave treatment combined with or without medications in Chronic prostatitis/chronic pelvic pain syndrome: a systematic review and meta-analysis
Prostate Cancer and Prostatic Diseases (2023)
-
Treatment of prostatitis with low-intensity extracorporeal shockwave therapy (LI-ESWT)
International Urology and Nephrology (2023)
-
Outcomes and clinical predictors of extracorporeal shock wave therapy in the treatment of chronic prostatitis/chronic pelvic pain syndrome: a prospective randomized double-blind placebo-controlled clinical trial
Prostate Cancer and Prostatic Diseases (2022)