Abstract
Background
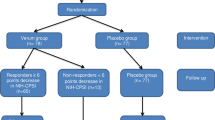
We performed this systematic review and meta-analysis to investigate the efficacy and safety of Li-ESWT combined with or without medications for patients with Chronic prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS).
Methods
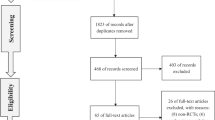
A comprehensive search was conducted of PUBMED, Cochrane Library, and Web of Science databases from inception to February 2022 for randomized controlled trials (RCTs) assessing the efficacy and safety of Li-ESWT with or without the combination of medications compared with the control group. The National Institutes of Health Chronic Prostatitis Symptom Index (NIH‐CPSI), Visual Analogue Scale/Score (VAS), International Index of Erectile Function (IIEF), and International prostate symptom score (IPSS) were used to assess the improvements of symptoms in CP/CPPS patients.
Results
651 patients from 12 randomized controlled studies were included in this study. The total NIH-CPSI scores, pain domain scores, and quality of life (QOL) scores were significantly lower in the Li-ESWT group than those in the control group at the termination of treatment, and 1, 4, 12, and 24 weeks after treatment. And these scores were significantly reduced in the Li-ESWT group than in baselines. In the subgroup analysis, reductions of these scores lasted longer and were greater in Li-ESWT combined with medications than in Li-ESWT alone. In the Li-ESWT group, the VAS score; IIEF score; and IPSS score were significant improvements than those in control group at the termination of treatment, and 1, 4, and 12 weeks after treatment; 4, 12, and 24 weeks after treatment; and 1, 4, and 12 weeks after treatment, respectively.
Conclusions
Li-ESWT is a safe, non-invasive, and effective option for patients with CP/CPPS, whether combined with medications or not, should be recommended for widespread use in clinical practice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the results and supplementary materials of this article.
References
Polackwich AS, Shoskes DA. Chronic prostatitis/chronic pelvic pain syndrome: a review of evaluation and therapy. Prostate Cancer Prostatic Dis. 2016;19:132–8.
Krieger JN, Lee SW, Jeon J, Cheah PY, Liong ML, Riley DE. Epidemiology of prostatitis. Int J Antimicrob Agents. 2008;31:S85–90.
Liang CZ, Li HJ, Wang ZP, Xing JP, Hu WL, Zhang TF, et al. The prevalence of prostatitis-like symptoms in China. J Urol. 2009;182:558–63.
Murphy SF, Schaeffer AJ, Thumbikat P. Immune mediators of chronic pelvic pain syndrome. Nat Rev Urol. 2014;11:259–69.
Krieger JN, Nyberg L Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–7.
Passavanti MB, Pota V, Sansone P, Aurilio C, De Nardis L, Pace MC. Chronic pelvic pain: assessment, evaluation, and objectivation. Pain Res Treat. 2017;2017:9472925.
Clemens JQ, Markossian T, Calhoun EA. Comparison of economic impact of chronic prostatitis/chronic pelvic pain syndrome and interstitial cystitis/painful bladder syndrome. Urology 2009;73:743–6.
Walz J, Perrotte P, Hutterer G, Suardi N, Jeldres C, Benard F, et al. Impact of chronic prostatitis-like symptoms on the quality of life in a large group of men. BJU Int. 2007;100:1307–11.
Rees J, Abrahams M, Doble A, Cooper A.Prostatitis Expert Reference Group (PERG) Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: a consensus guideline. BJU Int. 2015;116:509–25.
Magistro G, Wagenlehner FM, Grabe M, Weidner W, Stief CG, Nickel JC. Contemporary management of chronic prostatitis/chronic pelvic pain syndrome. Eur Urol. 2016;69:286–97.
Thakkinstian A, Attia J, Anothaisintawee T, Nickel JC. α-blockers, antibiotics and anti-inflammatories have a role in the management of chronic prostatitis/chronic pelvic pain syndrome. BJU Int. 2012;110:1014–22.
Bates SM, Hill VA, Anderson JB, Chapple CR, Spence R, Ryan C, et al. A prospective, randomized, double-blind trial to evaluate the role of a short reducing course of oral corticosteroid therapy in the treatment of chronic prostatitis/chronic pelvic pain syndrome. BJU Int. 2007;99:355–9.
Nickel JC, Downey J, Clark J, Casey RW, Pommerville PJ, Barkin J, et al. Levofloxacin for chronic prostatitis/chronic pelvic pain syndrome in men: a randomized placebo-controlled multicenter trial. Urology 2003;62:614–7.
Nickel JC, Krieger JN, McNaughton-Collins M, Anderson RU, Pontari M, Shoskes DA, et al. Chronic prostatitis collaborative research network. Alfuzosin and symptoms of chronic prostatitis-chronic pelvic pain syndrome. N. Engl J Med. 2008;359:2663–73.
Franco JV, Turk T, Jung JH, Xiao YT, Iakhno S, Garrote V, et al. Non-pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome. Cochrane Database Syst Rev. 2018;5:CD012551.
Hu M, Wazir J, Ullah R, Wang W, Cui X, Tang M, et al. Phytotherapy and physical therapy in the management of chronic prostatitis-chronic pelvic pain syndrome. Int Urol Nephrol. 2019;51:1081–8.
Zimmermann R, Cumpanas A, Hoeltl L, Janetschek G, Stenzl A, Miclea F. Extracorporeal shock-wave therapy for treating chronic pelvic pain syndrome: a feasibility study and the first clinical results. BJU Int. 2008;102:976–80.
Zimmermann R, Cumpanas A, Miclea F, Janetschek G. Extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome in males: a randomised, double-blind, placebo-controlled study. Eur Urol. 2009;56:418–24.
Zeng XY, Liang C, Ye ZQ. Extracorporeal shock wave treatment for non-inflammatory chronic pelvic pain syndrome: a prospective, randomized and sham-controlled study. Chin Med J (Engl). 2012;125:114–8.
Vahdatpour B, Alizadeh F, Moayednia A, Emadi M, Khorami MH, Haghdani S. Efficacy of extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome: a randomized, controlled trial. ISRN Urol. 2013;2013:972601–6.
Mykoniatis I, Pyrgidis N, Sokolakis I, Sountoulides P, Hatzichristodoulou G, Apostolidis A, et al. Low-intensity shockwave therapy for the management of chronic prostatitis/chronic pelvic pain syndrome: a systematic review and meta-analysis. BJU Int. 2021;128:144–52.
Yuan P, Ma D, Zhang Y, Gao X, Liu Z, Li R, et al. Efficacy of low-intensity extracorporeal shock wave therapy for the treatment of chronic prostatitis/chronic pelvic pain syndrome: A systematic review and meta-analysis. Neurourol Urodyn. 2019;38:1457–66.
Moayednia A, Haghdani S, Khosrawi S, Yousefi E, Vahdatpour B. Long-term effect of extracorporeal shock wave therapy on the treatment of chronic pelvic pain syndrome due to non bacterial prostatitis. J Res Med Sci. 2014;19:293–6.
Pajovic B, Radojevic N, Dimitrovski A, Vukovic M. Comparison of the efficiency of combined extracorporeal shock-wave therapy and triple therapy versus triple therapy itself in Category III B chronic pelvic pain syndrome (CPPS). Aging Male. 2016;19:202–7.
Salama AB, Abouelnaga WA. Effect of radial shock wave on chronic pelvic pain syndrome/chronic prostatitis. J Phys Ther Sci. 2018;30:1145–9.
Rayegani SM, Razzaghi MR, Raeissadat SA, Allameh F, Eliaspour D, Abedi AR, et al. Extracorporeal shockwave therapy combined with drug therapy in chronic pelvic pain syndrome: a randomized clinical trial. Urol J 2020;17:185–91.
Mykoniatis I, Kalyvianakis D, Zilotis F, Kapoteli P, Fournaraki A, Poulios E, et al. Evaluation of a low-intensity shockwave therapy for chronic prostatitis type IIIb/chronic pelvic pain syndrome: a double-blind randomized sham-controlled clinical trial. Prostate Cancer Prostatic Dis. 2021;24:370–9.
Kim KS, Choi YS, Bae WJ, Cho HJ, Ha US, Hong SH, et al. Clinical efficacy of multi-focal low-intensity extracorporeal shockwave therapy in the treatment of chronic prostatitis/chronic pelvic pain syndrome: prospective-randomized, double blind, placebo-controlled study. World J Mens Health. 2021;39:e42.
Trishch VI, Matskevych VM, Mysak AI, Zhulkevych IV. Evaluation of efficacy of extracorporeal shock wave therapy in complex treatment of patients with chronic non-bacterial prostatitis / chronic pelvic pain syndrome. Wiad Lek. 2021;74:1834–8.
Kim KS, Choi YS, Bae WJ, Cho HJ, Ha US, Hong SH, et al. Efficacy of low-intensity extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome iiib: a prospective-randomized, double-blind, placebo-controlled study. World J Mens Health. 2022;40:473–80.
Sakr AM, Fawzi AM, Kamel M, Ali MM. Outcomes and clinical predictors of extracorporeal shock wave therapy in the treatment of chronic prostatitis/chronic pelvic pain syndrome: a prospective randomized double-blind placebo-controlled clinical trial. Prostate Cancer Prostatic Dis. 2021;25:93–9.
Pontari M, Giusto L. New developments in the diagnosis and treatment of chronic prostatitis/chronic pelvic pain syndrome. Curr Opin Urol. 2013;23:565–9.
Chen KH, Yang CH, Wallace CG, Lin CR, Liu CK, Yin TC, et al. Combination therapy with extracorporeal shock wave and melatonin markedly attenuated neuropathic pain in rat. Am J Transl Res. 2017;9:4593–606.
Song Z, Jin C, Bian Z, Liang C. Extracorporeal shock wave therapy decreases the number of total and degranulated mast cells and alleviates pelvic pain in a rat model of prostatitis. Mol Cell Biochem. 2021;476:1905–13.
Done JD, Rudick CN, Quick ML, Schaeffer AJ, Thumbikat P. Role of mast cells in male chronic pelvic pain. J Urol. 2012;187:1473–82.
Jeon SH, Zhu GQ, Kwon EB, Lee KW, Cho HJ, Ha US, et al. Extracorporeal shock wave therapy decreases COX-2 by inhibiting TLR4-NFκB pathway in a prostatitis rat model. Prostate 2019;79:1498–504.
Feng B, Dong Z, Wang Y, Yan G, Yang E, Cheng H, et al. Li-ESWT treatment reduces inflammation, oxidative stress, and pain via the PI3K/AKT/FOXO1 pathway in autoimmune prostatitis rat models. Andrology. 2021;9:1593–602.
Wu WL, Bamodu OA, Wang YH, Hu SW, Tzou KY, Yeh CT, et al. Extracorporeal shockwave therapy (ESWT) alleviates pain, enhances erectile function and improves quality of life in patients with chronic prostatitis/chronic pelvic pain syndrome. J Clin Med. 2021;10:3602.
Guu SJ, Geng JH, Chao IT, Lin HT, Lee YC, Juan YS, et al. Efficacy of low-intensity extracorporeal shock wave therapy on men with Chronic Pelvic Pain Syndrome refractory to 3-As therapy. Am J Mens Health. 2018;12:441–52.
Guu SJ, Liu CC, Juan YS, Li CC, Tsai CC. The 12-month follow-up of the low-intensity extracorporeal shockwave therapy in the treatment of patients with chronic pelvic pain syndrome refractory to 3-As medications. Aging Male. 2020;23:793–800.
Kim JH, Kim JY, Choi CM, Lee JK, Kee HS, Jung KI, et al. The dose-related effects of extracorporeal shock wave therapy for knee osteoarthritis. Ann Rehabil Med. 2015;39:616–23.
Mykoniatis I, Pyrgidis N, Kalyvianakis D, Zilotis F, Kapoteli P, Fournaraki A, et al. Comparing two different low-intensity shockwave therapy frequency protocols for nonbacterial chronic prostatitis/chronic pelvic pain syndrome: A two-arm, parallel-group randomized controlled trial. Prostate 2021;81:499–507.
Chang KV, Chen SY, Chen WS, Tu YK, Chien KL. Comparative effectiveness of focused shock wave therapy of different intensity levels and radial shock wave therapy for treating plantar fasciitis: a systematic review and network meta-analysis. Arch Phys Med Rehabil. 2012;93:1259–68.
Van der Worp H, van den Akker-Scheek I, van Schie H, Zwerver J. ESWT for tendinopathy: technology and clinical implications. Knee Surg Sports Traumatol Arthrosc. 2013;21:1451–8.
Maier M, Schmitz C. Shock wave therapy: what really matters. Ultrasound Med Biol. 2008;34:1869–70. 1868-9; author reply.
Ghahhari J, De Nunzio C, Lombardo R, Ferrari R, Gatti L, Ghidini N, et al. Shockwave therapy for erectile dysfunction: which gives the best results? a retrospective national, multi-institutional comparative study of different shockwave technologies. Surg Technol Int. 2022;40:213–8.
Funding
This study was supported by the “Cuiying Science and Technology Innovation” Program (2020QN-15) of the Second Hospital of Lanzhou University, the Innovation Fund Project (2021B-042) of the Gansu Provincial Education Department, National Natural Science Foundation of China (PRO133011) and the Fundamental Research Funds for the Central Universities of Lanzhou University (lzujbky-2021-kb29).
Author information
Authors and Affiliations
Contributions
Conceptualization: XBK, WWH, ZLD, ZPW, Data curation: XBK, WWH, ZLD, Formal analysis: XBK, WWH, ZLD, JQT, Funding acquisition: XBK, WWH, ZLD, ZPW, Investigation: XBK, WWH, ZLD, YHW, Methodology: XBK, WWH, ZLD, Project administration: ZPW, CCL, ZYH, Supervision: ZPW, JQT, CCL, ZYH, Validation: CJ, ZPW, CCL, ZYH, Writing: XBK.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41391_2022_571_MOESM4_ESM.docx
Table S3 Mean differences of Li-ESWT group vs baseline at the termination of treatment, and 1, 4, 12, and 24 weeks after treatment.
41391_2022_571_MOESM5_ESM.docx
Table S4 Mean differences of control group vs baseline, at the termination of treatment, 1, 4, 12, and 24 weeks after treatment.
Rights and permissions
About this article
Cite this article
Kong, X., Hu, W., Dong, Z. et al. The efficacy and safety of low-intensity extracorporeal shock wave treatment combined with or without medications in Chronic prostatitis/chronic pelvic pain syndrome: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 26, 483–494 (2023). https://doi.org/10.1038/s41391-022-00571-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-022-00571-0
This article is cited by
-
The diagnostic yield of the Meares & Stamey test can be significantly improved by symptom-based patient selection and the experience of the test performer
Prostate Cancer and Prostatic Diseases (2024)
-
The methodological quality assessment of systematic reviews/meta-analyses of chronic prostatitis/chronic pelvic pain syndrome using AMSTAR2
BMC Medical Research Methodology (2023)