Abstract
Background
The relationship between particulate matter <2.5 µm (PM2.5) and large for gestational age (LGA) is unclear, and studies conducted in highly polluted areas are lacking. We aimed to explore the association between PM2.5 and the risk of LGA in China.
Methods
Maternal and neonatal characteristics were collected in the National Prepregnancy Examination Project. The definition of LGA was neonates with a weight over the 90th percentile for gestational age. Logistic regression was used to evaluate the relationship between PM2.5 exposure and the risk of LGA. The dose–response relationship was evaluated using a restricted cubic spline model.
Results
There were 196,243 mother–neonate pairs included, among which the percentage of LGA was 15.3%. The average PM2.5 concentration was 75.29 µg/m3. A 10 µg/m3 increase in PM2.5 during the whole pregnancy was associated with an increased risk of LGA (odds ratio (OR) 1.097, 95% confidence interval (CI) 1.091–1.103). Pregnant women in the high-exposure group had a higher risk of giving birth to an LGA infant (OR 1.37, 95% CI 1.33–1.41). There was a nonlinear relationship between PM2.5 concentration and the risk of LGA, and the risk increased more rapidly at higher PM2.5 levels.
Conclusions
Prenatal exposure to PM2.5 was linked to an increased risk of LGA.
Impact
-
A nation-wide study in a highly polluted country suggested the association between prenatal PM2.5 exposure and LGA.
-
A trimester-specific relationship between PM2.5 exposure and LGA was established.
-
Call for attention on the pregnant women in highly polluted areas who were in high risk of giving birth to LGA.
Similar content being viewed by others
Introduction
The health threats associated with air pollution are a worldwide public health issue. As an increasing number of studies provide evidence on the relationship between air pollution and diseases, such as obesity, hypertension, diabetes mellitus, cancer, etc., the World Health Organization declared air pollution as a fifth risk factor for noncommunicable diseases in 2018.1 Based on the Global Burden of Diseases Study, Cohen et al. estimated that the number of deaths attributed to exposure to particulate matter <2.5 µm (PM2.5) was 4.2 million in 2015, accounting for 7.6% of global deaths. Compared with developed countries, densely populated and less regulated middle- and low-income countries are faced with more severe challenges.2
The association between air pollution and metabolic abnormalities mentioned above has been proved in both adults and children, suggesting the influence of PM2.5 runs through the whole life cycle.3,4,5,6 Since previous studies found that neonates with birth weight exceeding a normal range were more likely to develop obesity, diabetes mellitus, and hypertension.5,6,7,8 It is worth exploring whether PM2.5 plays a role in metabolic diseases partly through increasing birth weight. The supporting evidence came from a study in Lanzhou, China that reported a positive association between PM10 exposure and fetal overgrowth.9 But generally speaking, the evidence on the association between air pollution and birth weight remained to be insufficient. On the one hand, the results from different studies seem to conflict; apart from overgrowth, restricted fetal growth was also observed to be related to air pollution;9,10 on the other hand, researchers tended to take the absolute value of birth weight as an outcome regardless of the impact of gestational age on birth weight. Compared to macrosomia (birth weight ≥4000 g), large for gestational age (LGA), defined as neonates with a weight above a certain percentile for gestational age, is a more appropriate measure of abnormal birth weight. LGA includes a wider range of neonates with intrauterine overgrowth, some of whom might weigh <4000 g and could not be diagnosed as macrosomia due to prematurity, but are also at high risk of perinatal complications and future obesity.8,11
Therefore, we aimed to explore the relationship between prenatal PM2.5 exposure and the incidence of LGA in a nationwide prospective study conducted in China, a country with poor air quality.
Methods
Study design
The cohort study was conducted in the frame of the National Free Preconception Health Examination Project, which was a project that provided women preparing for pregnancy with health examinations across mainland China. Two hundred and twenty experimental counties in 31 provinces, municipalities and autonomous regions were selected in the pilot project from 1 January 2010 to 31 December 2012, and all rural and migrant women who planned to get pregnant and agreed to participate in the project were consecutively included. During the project, women received a pregestational baseline examination and were followed up throughout gestation until delivery.12 Apart from routine physical and laboratory examinations, the factors that might influence pregnancy outcomes were also recorded through face-to-face interviews, including sociodemographic characteristics, medical history, family history, and lifestyle habits (smoking, alcohol consumption, etc.). All examinations and information acquisition were conducted by qualified staff following the standard protocol. Women who failed to complete the follow-up examination and interview were excluded, as well as those with stillbirth, multiple births, and missing or extreme data. The study was approved by the National Health Commission of China, and informed consent was obtained from participants.
Outcome assessment
The outcome was LGA birth, defined as a newborn whose birth weight is above the 90th percentile for gestational age.13 The 2015 Chinese reference of birth weight for gestational age was used to construct LGA in our study population.14 The gestational age was measured as the number of weeks between the date of delivery and the self-reported last menstrual period date.
Exposure assessment
Individual PM2.5 exposure levels were evaluated using monthly county-specific PM2.5 concentrations according to women’s registered residences. The PM2.5 concentration in all included counties was estimated based on satellite data using an ensemble machine learning model, which was a combination model of random forest, generalized additive model, and XGBoost. The model was trained using PM2.5 monitoring data obtained from 1593 monitoring stations and satellite retrieved aerosol optic depth from 2013 to 2019. In order to overcome spatial heterogeneity, the training process was conducted in different regions of China separately via a spatial clustering method. The prediction accuracy of the model was validated through by-year cross-validation, and data during the 2008 Beijing Olympic Games were used as external validation as well. A high accuracy of historical PM2.5 concentration prediction was achieved using this prediction model (cross-validation R2 0.76, root mean square error 16 µg/m3). More details about the exposure assessment could be available in the study by Xiao et al.15 The PM2.5 exposure level in each province in mainland China was estimated using the average of PM2.5 concentration in its experimental counties. The average monthly PM2.5 concentration during the first to the third month of gestation, fourth to the sixth month of gestation, and seventh month of gestation to delivery was calculated as the trimester-specific PM2.5 concentration in the first, second, and third trimesters. We excluded women whose birthplaces were different from their registered residences to eliminate inaccurate PM2.5 predictions as a result of moving during pregnancy.
Statistical analysis
Binary logistic regression was conducted to calculate odds ratios (ORs) and 95% confidence intervals (CIs) to assess the association between a 10 µg/m3 increase in prenatal PM2.5 exposure and LGA. Potential confounding factors were adjusted in three different models: (i) model 1 adjusted for maternal age and prepregnancy BMI;16 (ii) model 2 adjusted for multiparity, gestational age, and neonatal sex in addition to the factors adjusted in model 1;17 (iii) model 3 adjusted for prepregnancy hypertension, prepregnancy diabetes mellitus, family history of diabetes mellitus, cigarette use and alcohol consumption during pregnancy, maternal educational level, and season of delivery in addition to the variables adjusted in model 2.18,19,20,21 The relationship between PM2.5 exposure and LGA, defined as birth weight >97th percentile for gestational age, was evaluated in a sensitivity analysis. We also conducted propensity score matching incorporating the same covariates as those included in model 3: maternal age, prepregnancy BMI, multiparity, gestational age, neonatal sex, prepregnancy hypertension and diabetes mellitus, family history of diabetes mellitus, maternal educational level, gestational cigarette use and alcohol consumption, and season of delivery. Patients were divided into a low-dose exposure group and a high-dose exposure group according to their exposure level during their whole pregnancy; the cut-off value was set as 75 μg/m3 based on Ambient Air Quality Standards (GB 3095-2012).22 Pregnancies in the high-exposure group were matched to low-exposure pregnancies at a ratio of 1:1 using a caliper of 0.02, and the standardized differences in baseline characteristics of unmatched and matched cohorts were calculated to evaluate match performance.23 We analyzed outcomes between the high- and low-exposure groups using logistic regression. Restricted cubic spline models fitted with four knots adjusted for covariates in model 3 were used to evaluate the dose–response relationship between PM2.5 concentration and ORs of LGA, which was compared with the chosen reference PM2.5 of 75 μg/m3. Subgroup analyses were performed to explore the subgroup effects of maternal age (<25, 25–29, ≥30 years) and prepregnancy BMI (<18.5, 18.6–23.9, and ≥24 kg/m2).24 Inverse probability weight (IPW) method was used to evaluate the selection bias that resulted from the exclusion of participants lost to follow-up or with missing data. A stepwise logistic regression model was fitted to find the variables associated with the inclusion and exclusion of participants. Unstabilized weights were estimated with the numerator of 1 and denominator computerized using logistic regression with inclusion (yes/no) as the outcome and factors associated with participants inclusion as variables. The ORs adjusted for missing data using IPW were calculated. Statistical analyses were conducted using R 3.6.1. P values <0.05 in the two-sided test and standardized differences >0.2 were considered statistically significant.
Results
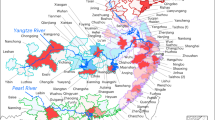
Of 248,501 women in 220 counties who were pregnant during our study period, we excluded 31,539 participants who were lost to follow-up, 4961 who delivered somewhere other than their residence region, 1417 who gave birth to more than one child, 1103 who had a stillbirth, and 13,238 who had extreme or undocumented baseline characteristics (Supplementary Fig. S1). The final study population included the remaining 196,243 births, which consisted of 166,289 (84.7%) non-LGA and 29,954 (15.3%) LGA births. Table 1 summarizes the baseline characteristics of the included population. The distribution of PM2.5 around China and the average PM2.5 level in each stage of pregnancy are shown in Fig. 1. The mean PM2.5 concentration was 79.2 ± 22.8 and 74.6 ± 22.5 µg/m3 during the whole pregnancy in the LGA and non-LGA groups, respectively. The LGA group was exposed to a significantly higher level of PM2.5 than the non-LGA group (P < 0.01).
a Distribution of exposure to PM2.5 over the entire pregnancy in each province in mainland China based on data from experimental counties. The gray color indicates missing data. b PM2.5 concentration during the entire pregnancy in each province. Boxplots represent the medians, interquartile range, and minimum and maximum interval. c Average PM2.5 concentration during each stage of pregnancy. *P < 0.01.
As shown in Table 2, we found a significant positive association between PM2.5 exposure and LGA outcomes during each trimester of pregnancy. In a fully-adjusted model, the ORs (95% CI) of LGA for every 10 µg/m3 increase of PM2.5 in the first trimester, second trimester, third trimester, and whole pregnancy were 1.055 (1.051, 1.060), 1.078 (1.073, 1.084), 1.042 (1.038, 1.046), and 1.097 (1.091, 1.103), respectively. When we defined LGA as birth weight above the 97th percentile for gestational age, the result was compatible with the main finding (Table 3).
After 1:1 propensity score matching of 90,320 low-exposed pregnancies with high-exposed pregnancies, 170,376 pregnancies with balanced covariates were included in a matched cohort (Supplementary Table S1). The incidence of LGA was 13.1% and 17.1% in the low- and high-exposure groups, respectively. Propensity-score-matching analysis showed results similar to those of the above logistic regression models, with an OR of 1.37 (95% CI 1.33–1.41).
Figure 2 shows the dose–response relationship between the PM2.5 exposure level and the risk of LGA. The risks of LGA increased as the PM2.5 concentration increased with a nonlinear relationship. The shape of the dose–response curve during the whole pregnancy (Fig. 2d) indicated that the risk of LGA increased more rapidly at high levels of PM2.5 exposure than at low levels of exposure.
In subgroup analyses, we found no significant difference in the risk of LGA among BMI and maternal age subgroups (Table 4).
In IPW analysis, 191,155 births included in the final analysis and 33,897 births excluded from the final analysis with complete records of variables used as potential predictors of inclusion/exclusion were included. Supplementary Table S2 shows the results of the logistic regression model used to predict the probability of being included. Table 5 shows that the ORs are adjusted for missing data using IPW, which did not differ significantly from the results of the main analysis.
Discussion
Our study showed that a 10 µg/m3 increase in prenatal PM2.5 concentration was associated with a 9.7% increase in the risk of LGA. To the best of our knowledge, this is the first nationwide study evaluating the relationship between maternal PM2.5 exposure during pregnancy and neonatal birth weight in the context of LGA in areas with high levels of pollution. It is of significance to use LGA as an indicator of birth weight because birth weights are highly dependent on gestational age. For example, some premature neonates might not meet the diagnostic standard of macrosomia due to prematurity but may weigh more than most other neonates of the same gestational age and could be diagnosed with LGA. These neonates are confronted with similar risks of perinatal complications and future obesity as those with macrosomia.8,11 The severity of air pollution in the study areas also matters. Contradictory to observations in low-pollution countries, a study conducted in highly polluted areas found that prenatal exposure to high levels of PM10 would increase the risk of fetal overgrowth.9
Our results were different from those of most previous studies. Sun et al. summarized a total of 32 studies and reported a statistically significant association between low birth weight and PM2.5 exposure (per 10 µg/m3) (OR 1.090, 95% CI 1.032, 1.150).25 The difference could be explained by two aspects. First, there was a large gap between the PM2.5 level in our study and those in previous studies, in which the mean monitored PM2.5 concentration ranged from 9.4 to 22.1 µg/m3.25 It is interesting that both our study and the study by Zhang et al., which reported a positive association between air pollution and fetal overgrowth, were conducted in highly polluted areas.9 It is possible that maternal behavior patterns might be influenced by serious air pollution (e.g., less outdoor activities), and thus impact birth weights. Second, we adjusted for confounding factors related to demography, anthropometric measurement, medical history, family history, and personal history through logistic regression and propensity score matching. Compared with previous studies mainly adjusting for demographic factors, we adjusted for a more comprehensive set of confounders.26,27 The full adjustment was attributed to a large sample size and the detailed data collection during enrollment and follow-up and might reduce possible confounding bias.
The positive association between trimester-specific PM2.5 concentration and risk of LGA was consistent in all three trimesters. It is generally accepted that genetic factors play a major role in the early stage of pregnancy,28 while nutrition and metabolic factors have more influence as a pregnancy enters its later stage.29 So the consistent relationship through three trimesters suggested both genetic factors and intrauterine environmental factors are involved in the overgrowth associated with LGA. Air pollution could influence fetal growth through epigenetic modification. A study enrolling 527 mother–infant pairs in Zhengzhou, China revealed that prenatal air pollution could influence birth size via DNA methylation.30 Also, a significant relationship between prenatal PM2.5 exposure and cord blood DNA methylation of genes related to fetal growth was observed.31 Air pollution might also play a role in the development of LGA by altering maternal metabolism. Animal studies indicated that exposure to unfiltered polluted air increased insulin resistance and oxidative stress in pregnant rats. Compared to those exposed to filtered air, polluted air-exposed mice had more gestational weight gain,32 which is a recognized risk factor for LGA.33 Therefore, based on our findings, the impact of PM2.5 on LGA lasted for the whole pregnancy. The possible mechanisms involve epigenetic modification and metabolic regulation.
The dose–response relationship between PM2.5 concentration and odds ratios of LGA showed that at high levels of exposure, the risk of LGA increased faster as PM2.5 concentration increased. This result confirmed the importance of air pollution control.
Our study enrolled more than 190,000 pregnant women throughout mainland China, which made our results highly representative. Although there were no monitoring networks in China during our study period, we managed to accurately estimate the PM2.5 concentration on the basis of satellite data using a machine learning method. We first found the PM2.5 exposure level in the LGA group was significantly higher than that in the non-LGA group, and then we robust the relationship between PM2.5 and the risk of LGA using a logistic regression model adjusted for different combinations of confounding factors and propensity-score-matching analysis. The dose–response relationship further suggested that the higher the exposure, the higher the risk of LGA. There were also some limitations of our study. First, we failed to adjust for gestational diabetes mellitus and gestational weight gain in our analysis due to the lack of records. The LGAs accounted for 15.3% of the total population in our study. Theoretically, the LGA proportion in a nationwide study should be around 10% according to the LGA definition. The difference might be partly due to different provinces and target populations selected. Compared with the study plotting the Chinese growth chart, more provinces were included in our study, and rural and migrant women were our target population.14 The living habits and diets vary across China, thus there might be different nutrient supply and gestational weight gain in different samples and then result in different fetal growth and prevalence of LGAs. It is necessary to consider the impact of gestational weight gain on birth weight in the future study. Second, the exposure was estimated at a county level, which means that the participants in the same county were assumed to have the same exposure on the same day. In reality, the PM2.5 distribution in different areas within a county varies. The exposure level of a participant was dependent on the specific residence of the participant, influenced by multiple factors including topography, traffic, greenery coverage, etc. However, we thought the rough estimates ignoring individual differences could still reflect the average exposure of the participants since the internal diversity of PM2.5 level within a county was relatively small, and the main conclusion was credible though less precise. Third, for the lacking of statistical data, we failed to adjust for socioeconomic level of each county in our analysis, which might result in confounding bias. Besides, the program management in different counties might affect participant’s motivation. In some counties with poor management, there might be fewer women willing to participate in the program and result in selection bias. Fourth, we excluded women who delivered in a region different from their registered residence to minimize estimation errors resulting from moving, but there might still have been undetected moves during pregnancy.
Conclusions
In conclusion, our study supported that prenatal exposure to PM2.5 increased the risk of LGA, and the effect was consistent in different exposure windows. Prenatal and perinatal care in highly polluted areas should be reinforced to reduce the likelihood of LGA and relevant complications. The underlying mechanisms of our findings need to be studied further.
References
Linou, N., Beagley, J., Huikuri, S. & Renshaw, N. Air pollution moves up the global health agenda. BMJ 363, k4933 (2018).
Cohen, A. J. et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389, 1907–1918 (2017).
Seo, M. Y., Kim, S. H. & Park, M. J. Air pollution and childhood obesity. Clin. Exp. Pediatr. 63, 382–388 (2020).
Deschenes, O., Wang, H., Wang, S. & Zhang, P. The effect of air pollution on body weight and obesity: evidence from China. J. Dev. Econ. 145, 102461 (2020).
Zhang, M. et al. Maternal exposure to ambient particulate matter ≤2.5 μm during pregnancy and the risk for high blood pressure in childhood. Hypertension 72, 194–201 (2018).
Moody, E. C. et al. Association of prenatal and perinatal exposures to particulate matter with changes in hemoglobin A1c levels in children aged 4 to 6 years. JAMA Netw. Open 2, e1917643 (2019).
Lei, X. et al. Childhood health outcomes in term, large-for-gestational-age babies with different postnatal growth patterns. Am. J. Epidemiol. 187, 507–514 (2018).
Boney, C. M., Verma, A., Tucker, R. & Vohr, B. R. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115, e290–296 (2005).
Zhao, N. et al. Effects of prenatal exposure to ambient air pollutant PM10 on ultrasound-measured fetal growth. Int. J. Epidemiol. 47, 1072–1081 (2018).
Cao, Z. et al. Maternal exposure to ambient fine particulate matter and fetal growth in Shanghai, China. Environ. Health 18, 49 (2019).
Chauhan, S. P. et al. Neonatal morbidity of small- and large-for-gestational-age neonates born at term in uncomplicated pregnancies. Obstet. Gynecol. 130, 511–519 (2017).
Zhang, S., Wang, Q. & Shen, H. Design of the National Free Preconception Health Examination Project in China [Chinese]. Natl Med. J. China 95, 162–165 (2015).
Xu, H., Simonet, F. & Luo, Z. C. Optimal birth weight percentile cut-offs in defining small- or large-for-gestational-age. Acta Paediatr. 99, 550–555 (2010).
Zhu, L. et al. Chinese neonatal birth weight curve for different gestational age [Chinese]. J. Pediatr. 53, 97–103 (2015).
Xiao, Q., Chang, H. H., Geng, G. & Liu, Y. An ensemble machine-learning model to predict historical PM2.5 concentrations in China from satellite data. Environ. Sci. Technol. 52, 13260–13269 (2018).
Spada, E., Chiossi, G., Coscia, A., Monari, F. & Facchinetti, F. Effect of maternal age, height, BMI and ethnicity on birth weight: an Italian multicenter study. J. Perinat. Med. 46, 1016–1021 (2018).
Olbertz, D. M. et al. Somatic development at birth as influenced by maternal characteristics—an analysis of the German Perinatal Survey. J. Perinat. Med. 46, 889–892 (2018).
Ladfors, L. et al. Fetal overgrowth in women with type 1 and type 2 diabetes mellitus. PLoS ONE 12, e0187917 (2017).
Segal, P. et al. Maternal obesity and familial history of diabetes have opposing effects on infant birth weight in women with mild glucose intolerance in pregnancy. J. Matern. Fetal Neonatal Med. 21, 73–79 (2008).
Pastorino, S. et al. Associations between maternal physical activity in early and late pregnancy and offspring birth size: remote federated individual level meta-analysis from eight cohort studies. Int. J. Obstet. Gynaecol. 126, 459–470 (2019).
Wang, Q. et al. Seasonal analyses of the association between prenatal ambient air pollution exposure and birth weight for gestational age in Guangzhou, China. Sci. Total Environ. 649, 526–534 (2019).
Wang, Y. GB3095-2012 Air Quality Standard would be implemented by stages [Chinese]. China Standards Rev. 4, 4–5 (2012).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 28, 3083–3107 (2009).
Force, C. O. T. Guideline for the prevention and management of overweight and obesity in chinese adults [Chinese]. Acta Nutr. Sin. 1, 1–4 (2004).
Sun, X. et al. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: a meta-analysis. Environ. Pollut. 211, 38–47 (2016).
Trasande, L., Wong, K., Roy, A., Savitz, D. A. & Thurston, G. Exploring prenatal outdoor air pollution, birth outcomes and neonatal health care utilization in a nationally representative sample. J. Expos. Sci. Environ. Epidemiol. 23, 315–321 (2013).
Xiao, Q. et al. Associations between birth outcomes and maternal PM2.5 exposure in Shanghai: a comparison of three exposure assessment approaches. Environ. Int. 117, 226–236 (2018).
Workalemahu, T. et al. Genetic and environmental influences on fetal growth vary during sensitive periods in pregnancy. Sci. Rep. 8, 7274–7274 (2018).
Tanaka, K. et al. Influence of maternal obesity on fetal growth at different periods of pregnancies with normal glucose tolerance. J. Obstet. Gynaecol. Res. 44, 691–696 (2018).
He, T. et al. Ambient air pollution, H19/DMR methylation in cord blood and newborn size: a pilot study in Zhengzhou City, China. Chemosphere 212, 863–871 (2018).
Wang, C. et al. DNA methylation of insulin-like growth factor 2 and H19 cluster in cord blood and prenatal air pollution exposure to fine particulate matter. Environ. Health. 19, 129–129 (2020).
Wei, Y. et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: findings from a natural experiment in Beijing. FASEB J. 30, 2115–2122 (2016).
Goldstein, R. F. et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA 317, 2207–2225 (2017).
Funding
This work was supported by the National Natural Science Foundation of China (81673184), Beijing Municipal Natural Science Foundation (7192153), and CAMS Initiative for Innovative Medicine (2016-I2M-1-008).
Author information
Authors and Affiliations
Contributions
SC: conception and drafting the data; SW: analysis and interpretation of data and drafting the data; TL: acquisition of data and article revision; HZ: article revision; YZ: analysis and interpretation of data; SL: acquisition of data; YY: acquisition of data; HP: conception and final approval of the version to be published; XS: conception and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Category of Study: Population study article (cohort study)
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, S., Wang, S., Li, T. et al. Association between prenatal PM2.5 exposure and the risk of large for gestational age. Pediatr Res 92, 1773–1779 (2022). https://doi.org/10.1038/s41390-021-01889-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01889-6