Abstract
Background
Vitamin A and D deficiencies are common in preterm infants. Megalin is an endocytic receptor in the proximal tubule, which reabsorbs retinol-binding protein (RBP) and vitamin D-binding protein (VDBP). Although the proximal tubule is immature in preterm infants, little is known about megalin expression during kidney development. In this study, we establish the abundance of megalin in the developing human kidney and its relationship to the urinary excretion of vitamin carriers in preterm infants.
Methods
We analyzed a postmortem group (20–40 weeks gestation), where we used morphometric means of measuring megalin and its ligands in kidney tissue and a living group of patients (28–40 weeks), where urinary RBP and VDBP were measured.
Results
The presence of megalin, RBP, and VDBP increased in the proximal tubule through gestation. At birth the urinary concentration of RBP and VDBP were higher in the 28–32 week group compared to the 38–40 week group and a significant inverse correlation of tissue megalin and urinary loss of RBP and VDBP existed.
Conclusions
Preterm infants experience vitamin carrier protein losses, which are associated with decreased megalin expression. This developmental expression of megalin in the kidney has clinical implications in the prevention of vitamin deficiencies in preterm babies.
Similar content being viewed by others
Introduction
Vitamin deficiencies, particularly of vitamins A (retinol) and D, are common in preterm infants1,2 and can affect ex utero organogenesis.3 Several theories have been proposed to explain the etiology of vitamin A and D deficiencies in preterm infants, including inadequate stores, suboptimal supplementation, and urinary losses. In fetal life, proteins present in the urine are detectable in the amniotic fluid. However, when a preterm neonate is born, ongoing urinary losses may contribute to the development of deficiencies. The placenta carries out the majority of excretory function in utero, however in the ex utero preterm kidney, urinary losses may result from immaturity of the reabsorptive system. Clinically, preterm infants have variable degrees of proximal tubular immaturity, as evidenced by metabolic acidosis and excretion of higher levels of low molecular weight proteins.4 Our previous work demonstrates the changes in the urinary profiles of 26 neonates stratified into a preterm and term group from birth to 12 months. Some of these proteins had known biological roles such as the insulin like growth factor family including IGFBP1, −6, and IGF II, and others had unknown significance such as Siglec-5. Importantly, none of the differences between the preterm and term group’s urinary profiles existed 6 months after birth.4 The degree and composition of the proteinuria may be particularly relevant for the long term renal health of these infants as proteinuria at the time of discharge from the hospital may be a harbinger of chronic kidney disease in the smallest, most preterm neonates.5
The endocytic receptors megalin and cubilin are responsible for prevention of low molecular weight proteinuria by mediating reabsorption of a variety of low molecular weight proteins at the apical pole of the proximal tubule, and they account for the transport mechanism by which aminoglycosides enter the proximal tubule.6,7,8,9 In animal models, the expression of megalin increases within the kidney through gestation and into the early postnatal period.10 Although data for neonatal kidney megalin expression are lacking, megalin ligands such as cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), liver type fatty acid binding protein (L-FABP), and clusterin have been found in the urine of preterm infants.11,12,13 However, the relationship between megalin expression in the developing human kidney and loss of vitamin carriers has not been established.
To investigate the relationship between proximal tubule megalin expression and urinary megalin ligands in humans, we designed a study examining two separate cohorts of neonates. In cohort #1, megalin and megalin ligand abundance in the proximal tubule during 20–40 weeks gestation were evaluated in the renal cortex of autopsy tissue using a semi-quantitative method. In cohort #2, RBP and VDBP were quantitated in the urine from living preterm infants from 28–40 weeks. Our results demonstrate that megalin expression increases in the developing kidney and that there is a relationship between renal megalin expression and urinary losses of vitamin carriers. This suggests that immature megalin expression may be important with regard to vitamin homeostasis and organogenesis in the preterm neonate.
Methods
Postmortem kidney analysis from fetal and preterm neonates: cohort #1
Archived kidneys were obtained from the University of Virginia, Department of Pathology. Only samples for which there was informed parental consent for autopsy were included in the study. Because of the de-identified nature of the samples, the University of Virginia’s Institutional Review Board determined this project did not meet the criteria for Human Subjects research and therefore was not subject to IRB-HSR review. Samples were collected from 2006 to 2014.
Inclusion and exclusion criteria
Kidney samples were collected from fetuses and neonates from 20–40 weeks gestation. Any neonates that survived greater than 7 days, had a diagnosis of congenital anomaly of the kidney or urinary tract, or showed histologic evidence of renal disease were excluded from analysis. Kidney tissue with evidence of significant autolysis was also excluded.
Population characteristics
The gestational age, sex, cause of death, kidney, and body weight for each subject was recorded (Table 1). Subjects were stratified into the following groups: fetal or preterm, and gestational age categories (Group #1: 20–23 6/7, #2: 24–28 6/7, #3: 29–32 6/7, #4: 33–35 6/7, and #5: > 37 weeks). Subjects who died in utero were classified as “fetal”. Those that survived resuscitation, but died prior to 7 days were deemed “preterm”.
Histologic preparation of kidneys
At autopsy, each kidney was removed, weighed, formalin-fixed, and cut into portions containing both cortex and medulla. The kidney sample was dehydrated, embedded in paraffin using standard techniques, and sectioned at 5 microns. Lotus tetragonolobus lectin (Vector Laboratories) staining, which identifies mature human proximal tubule cells, was identified in the following manner: paraffin embedded kidney sections were treated with proteinase K enzymatic digestion prior to contact with biotinylated Lotus lectin (1:50 dilution) followed by the ABC-DAB reaction.14 Megalin and the megalin ligands RBP and VDBP were detected in a similar fashion. Kidney tissue sections were pre-treated in order to quench and neutralize both endogenous peroxidase (H2O2 in methanol) and biotin (avidin-biotin (ABC) blocking kit, Vector Laboratories, Burlingame, CA) with a citrate antigen retrieval step. Details of the antigen targets and antibodies are provided in Supplemental Table S1.
Quantitation
Quantitation of proximal tubules, megalin, RBP, and VDBP abundance was accomplished by analyzing the DAB reaction within each image (ImagePro Plus 5.1, Media Cybernetics, Silver Springs, MD). Renal cortical volume fraction of proximal tubules and antigens of interest were measured using a stereologic approach as described in previous publications,14 in which 10 fields were photographed at ×20 magnification in the subcapsular region (Fig. 1a) and the DAB reaction product was expressed as a percent area value (volume fraction [Vv]). By low magnification, Fig. 1b demonstrates the signal intensity of the expression of Lotus, megalin, RBP, and VDBP. A positive control (a healthy wildtype mouse kidney) was stained in each batch of human tissues to validate the staining procedure and ensure that methodological variability was detected.
a Diagram of proximal tubule quantification by microscopy and morphometric evaluation. Example of methodology for collection and quantification of cortical proximal tubule fraction (proximal tubule epithelium is identified by Lotus lectin staining in upper micrograph); similar staining was used to identify megalin, RBP, and VDBP. Ten subcapsular areas (red squares) were photographed and analyzed for the four parameters by highlighting DAB staining (highlighted in red in lower version of the micrograph) and calculating the fractional area contribution to generate volume fraction. b Representative images of Lotus, megalin and megalin ligands: RBP and VDBP from the same subject, 23 week fetus. The sections stained for Lotus (a) and megalin (b) are serial sections and demonstrate Lotus + /megalin− proximal tubules in the subcapsular region. The RBP and VDBP panel demonstrate the expression in different proximal tubules with a lack of ligand expression in the subcapsular region
Assessment of RBP and VDBP in preterm neonates: cohort #2
Subject selection
The University of Virginia Institutional Review Board approved the urine collection and informed parental consent was obtained for each subject. The subjects were recruited from the newborn nursery and Neonatal Intensive Care Unit. Infants were enrolled in two preterm groups ( < 33 weeks and 33–35 weeks) and a term group ( > 38 weeks). A physical exam, including estimation of gestational age by Ballard exam, demographics, and prenatal history were performed or collected from the chart. Subjects were excluded if a congenital renal abnormality was detected. A summary of subject characteristics is provided in Table 2.
Urine collection and analysis
Urine was collected within 48 hours of birth using a U-bag collection device. In the < 33 week group urine was collected again 4 and 8 weeks after birth. The 33–35 week had urine collected at birth and again 4 weeks after birth. This provided birth samples and urine samples at the time the neonate was 40 weeks postmenstrual age, or full term. The urine was centrifuged, aliquoted, and stored at −80 °C for analysis of protein, creatinine, RBP, and VDBP. RBP was quantified using an ELISA kit, the DetectX RBP Immunoassay Kit (Arbor Assays, Ann Arbor, MI). VDBP was quantified using the Human Vitamin DBP Quantikine ELISA Kit (R&D systems, Minneapolis, MN).
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 7.00 (GraphPad Software; La Jolla, California). Two-way ANOVA was used to compare the expression of the target of interest within the gestational age categories and further analyzed using Dunnett’s multiple comparisons test. The urine RBP and VDBP data was analyzed using Kruskal Wallis test and further analyzed using Dunn’s multiple comparison test. Spearman’s correlation was used to compare tissue targets of interest and tissue megalin to urinary biomarkers. A two-tailed significance of 0.05 was used for all tests.
Results
Tissue content of megalin, RBP, and VDBP
The mature proximal tubular fraction, measured by Lotus expression, was significantly lower in the 20–23, 24–28, and 33–35 week groups as compared to 38–40 week group, but no significant changes were observed from 20 to 35 weeks (Fig. 2). Megalin expression in the proximal tubule increased over gestation, indicating that the Lotus positive tubules increasingly express megalin as part of their maturation (Fig. 1b highlights the Lotus + /megalin− tubules in a serial section). The abundance of both RBP and VDBP in the proximal tubule similarly increased across the gestational ages. Tissue abundance of RBP was lower in the kidneys of those from 20–35 weeks gestation compared to the 38–40 week group. Although the VDBP tissue levels were significantly lower in the 20–23 and 24–28 week groups, by 29–32 weeks there was no difference in VDBP tissue abundance compared to the 38–40 week group. The fractional expression of megalin within the proximal tubule was compared to the megalin ligands, RBP, and VDBP. The significant correlation between megalin expression and both RBP and VDBP is demonstrated in Fig. 3 (Spearman correlation; RBP: r = 0.99, p = 0.008; VDBP: r = 0.9, p = 0.04).
a Proximal tubular assessment. This graph shows the percentage of proximal tubules positive for Lotus lectin, megalin, RBP, and VDBP in the 5 groups of fetal and premature neonates. The percentage of Lotus, megalin, and RBP-positive proximal tubules was significantly lower at all gestational ages than at term, and VDBP positive tubules were significantly lower in the 3 earliest gestation ages as compared to term positive proximal tubules. Statistically significant difference of each group as compared to term levels (38–40) is indicated by the symbols **p < 0.001, *p < 0.01. b Proximal tubular histology: Each row represents a different subject, A–D are from a 21 week fetus and E–H are from a 40 week fetus. These images are a representative sample of the expression of Lotus, megalin, RBP, and VDBP. Images are not serial sections but are from the same kidney
Correlation of megalin expression and tissue content of RBP and VDBP. A significant correlation exists between megalin expression and the tissue content of the megalin ligands RBP (R = 1.0; p = 0.008) and VDBP (R = 0.9; p = 0.04), thus supporting the increasing endocytic role of megalin with gestation age
Urinary excretion of RBP and VDBP
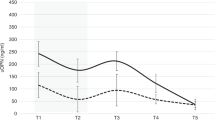
In cohort #2, urinary RBP and VDBP were found in higher concentrations in the 28–32 week group as compared to the 38–40 week group at birth (RBP: mean rank difference: 29.0, p = 0.0001; VDBP: mean rank difference: 17.9, p = 0.049). Urinary RBP, but not VDBP, decreased over time in the 28–32 week group. Urinary RBP was higher at birth compared to that at 2 months after birth (corrected full term) (mean rank difference: 37.5, p = 0.005) (Fig. 4). When the 28–32 and 33–35 week groups were corrected to full-term gestational age and compared to the 38–40 week group, there was no difference in the urine RBP or VDBP concentration (28–32 wk: RBP: mean rank: −8.5, p > 0.99; VDBP: mean rank: 0.25, p > 0.99 and 33–35 wk: RBP: mean rank: 14.1, p = 0.52; VDBP: and mean rank: 3.1, p > 0.99).
Urinary excretion of RBP (panel a) and VDBP (panel b) over time. The graphs show the urinary ratio of RBP/creatinine and VDBP/creatinine, respectively, at birth until corrected full term. In the 28–32 week gestation group (2 months preterm or 2 months prior to full term), urinary RBP decreased significantly from birth until term, which was not significant in the 33–35 week group (1 month preterm or 1 month prior to full term) and not for VDBP in either group
The urinary concentration of RBP was significantly higher than VDBP in the 28–32 week group at birth (mean diff; 257.0, p < 0.001) (Fig. 5). There was no difference between the RBP and VDBP concentrations at birth in either the 33–35 or 38–40 week groups.
Urinary excretion of RBP and VDBP at birth stratified into gestation age cohorts. The urinary RBP and VDBP levels were all normalized to urine creatinine concentration in these premature neonates at birth. The urinary loss of RBP and VDBP was higher in the gestation group 28–32 weeks as compared to infants born at term, whereas the loss in the gestation group 33–35 did not differ significantly from the term group (shown by horizontal lines, RBP: p < 0.001 and VDBP: p = 0.04). Additionally, urine content of RBP was higher than VDBP content in the 28–32 week group (p < 0.01)
Correlation between tissue megalin and urinary ligands
As demonstrated in Fig. 6, there is a strong inverse correlation between megalin content assessed in cohort #1 and urinary excretion of RBP and VDBP in cohort #2 (RBP: r = −0.79, −0.89 to −0.61, p = <0.001; VDBP: r = −0.65 −0.83 to −0.34, p = 0.003).
Correlation between tissue megalin and urinary RBP and VDBP. Each patient’s urine sample was compared to the average tissue megalin fraction corresponding to that week of gestation. There is a strong inverse correlation between tissue megalin and urinary RBP represented by stars (r = −0.79, p < 0.001). The relationship between tissue megalin and urinary VDBP is moderate with an r = −0.65, but with a level of significance of p = 0.003
Discussion
This study demonstrates a progressive increase in megalin expression in human renal tissue during the gestational ages of 20 to 40 weeks, emphasizing a relative state of megalin deficiency in the immature preterm kidney. Proximal tubule immaturity is clinically notable in the preterm neonate, characterized by the variable degrees of electrolyte wasting, metabolic acidosis and low molecular weight proteinuria, including the megalin ligands β-2-microglobulin, cystatin C, and NGAL, which can now be directly attributed to the developmental expression of megalin shown in this study.11.Although there are a multitude of megalin ligands, we chose to focus on RBP and VDBP,15,16 as both have biologic relevance to preterm infants. We additionally recognize the critical role that cubilin may play in the developing kidney, however restricted this study to examining only megalin, since the uptake of cubilin-ligands is dependent on megalin driven endocytosis in the kidney.8,17 Here, we show a concurrent increase of the endocytosed megalin ligands, RBP, and VDBP in human renal tissue during gestational ages 20–40 weeks. The increased expression of megalin in human renal tissue of preterm infants through the second half of gestation appears is inversely correlated with urinary excretion of RBP and VDBP.
Megalin is an endocytic receptor encoded by the large LRP2 gene and is located within the proximal tubule of the kidney, however it is also expressed in other absorptive human tissues including the placenta, yolk sack, ciliary epithelium, and the choroid plexus.10 Many megalin ligands are carrier proteins for vitamin transportation. Vitamin A and its derivative retinoids are bound and transported in the in human body by RBP.18 Direct measurement of vitamin A is challenging, while measurement of RBP has been shown to be a good surrogate of vitamin A status.19 In this study, we used a semi-quantitative method to detect an increasing amount of RBP in the proximal tubules of preterm neonates from 20–38 weeks gestation and a similar decrease in RBP excretion in the urine of living infants from 29 weeks gestation. There was no difference in the urinary loss of RBP in premature infants when they reached 38 weeks corrected gestational age as compared to the term babies at birth. The calculated urinary losses in the 28–32 week group amount to ~84 μg retinol/mmol creatinine. Although it is intriguing to note that the preterm urinary loss of retinol are in the same range as observed in full blown Fanconi syndrome adult patients (132 μg retinol/mmol creatinine),7 care must be taken to not misinterpret our results as serum retinol was not measured in the present study and the degree of urinary creatinine excretion can vary in preterm neonates.
The urinary loss of retinol is particularly important to preterm neonates who continue organogenesis after birth, including a significant portion of renal development, vitamin A was first implicated in renal hypoplasia in the early 1950s when Wilson et al. found that maternal vitamin A deficiency resulted in renal hypoplasia.20 Since that time, several studies have reported the effects of retinoic acid, a vitamin A metabolite, on kidney embryogenesis. Retinoic acid has been found to act on mesenchymal cells, stimulating them to release key branching morphogens, induce kidney tubulogenesis, and promote branching nephrogenesis in animal studies, tissue culture and in vitro, respectively.21,22,23,24 Animal studies revealed that a 50% decrease in maternal vitamin A concentrations is associated with reduction of fetal nephron number by as much as 20%.2 Notably, supplementation with retinoic acid or vitamin A restored nephron endowment in the offspring of rats subjected to maternal protein restriction.25 Beyond the kidney, the loss of RBP may have clinical impact on other important organs in the preterm infant. For example, human studies have shown that preterm infants with low plasma and tissue concentrations of vitamin A are more likely to have chronic lung disease.26 In a multicenter randomized trial, supplementation of Vitamin A to preterm infants reduced the risk of chronic lung disease.27 Interestingly, there were no signs of vitamin A toxicity in this study. However, urinary RBP was not measured. Lack of vitamin A toxicity in this study raises an interesting question: could increased urinary excretion of RBP in preterm infants confer protection from vitamin A toxicity?
The second megalin ligand examined in this study was VDBP, to which the majority of vitamin D in the circulation is bound. We demonstrate less VDBP in the tissues of the most preterm infants, less VDBP in the urine than RBP in the 28–32 week group at birth by nearly 100-fold, and more VDBP in the urine of the 28–32 week group than the 38–40 week group. However, there was a high degree of variability in the fractional volume of VDBP measured within the human kidneys possibly due to biologic variability secondary to maternal exposures or neonatal conditions. Additionally, there was no increase in the tissue VDBP levels from the 33–35 to the 38–40 weeks groups simultaneously with the decrease in urinary VDBP levels mentioned above. It is possible that equilibrium has been reached between uptake and lysosomal degradation of VDBP. Thus, at some point the tissue level of e.g., VDBP will not increase, even though more VDBP is taken up, because it is degraded in the lysosomes at the same rate as the system matures. Both the vitamin and its carrier, VDBP, are biologically important in the fetus and developing neonate. VDBP is a carrier of vitamin D, a second fat-soluble vitamin which exerts effects on various organs, VDBP may also have anti-inflammatory and immunomodulatory functions independent of vitamin D carriage.28 The role of vitamin D and VDBP in the renal health of preterm infants has not been explored; however, vitamin D deficiency has been associated with worse outcomes in many preterm conditions. Up to 55% of preterm infants born weighing < 1000 g have vitamin D deficiency which is associated with metabolic bone disease of prematurity, respiratory distress syndrome, sepsis and retinopathy of prematurity.29,30,31
Premature birth is, as we have shown in this study, accompanied by an immature reabsorptive system in the proximal tubule. Despite a modest increase in Lotus positive proximal tubules at 38–40 weeks, there was no difference in volume fraction of total proximal tubules from 20–35 weeks and furthermore there were areas, most prominently at the cortical surface of the kidney, which were Lotus positive and megalin negative (Fig. 1b), highlighting that the machinery for reabsorption is immature in developing proximal tubules. At the same time, we observed an increase in megalin during gestation, suggesting that the maturation of proximal tubules includes expression of megalin. This is supported by a study by Lima et al. showing a positive relationship between differentiation and megalin expression.32 In utero a robust proximal tubule reabsorptive capacity is not essential, as the urinary excretion of vital substances will be preserved in the amniotic fluid, from which the fetus potentially can reclaim them by gastrointestinal absorption. Gastrointestinal reabsorption of megalin ligands is supported by rodent data. In 2014, Vazquez-Carretero et al. published their findings, showing that megalin was present in the jejunum and ileum of suckling rats (5 days after birth) and that both mRNA and protein expression of megalin decreased as the pups aged.33 Furthermore, the expression of megalin was maintained if the pups were continued on a commercially available milk diet as opposed to moving to a solid diet of rat chow. Our group has preliminary data suggesting the human fetal intestine expresses megalin in early gestation, and that this expression may be lost or attenuated after birth, similar to the situation in rodents (unpublished data). However significant autolysis of the human intestine after intrauterine death precluded the inclusion of the fetal intestinal megalin in the present study. Thus, in contrast to the “closed” in utero system where fetal urine contributes to amniotic fluid and reabsorbed by the gastrointestinal tract, it could be hypothesized that the neonate born preterm loses this ability to conserve what is lost in the urine.
A recognized limitation to this study is the different subject cohorts for the renal tissue and urinary biomarker assessments. It is possible that the renal tissue evaluation was confounded by factors that lead to death and at most this study highlights correlations between tissue expression. However, there is no ethical means to directly correlate urine biomarkers such as RBP and VDBP to histologic findings. Despite this limitation, our results are in alignment with published work that demonstrates the urinary excretion of many megalin ligands such as β-2-microglobulin, cystatin C and NGAL, decrease with increasing gestational age.11 The urinary excretion of megalin ligands, particularly urinary NGAL, has been shown to be an excellent noninvasive biomarker for AKI in preterm infants (AUC = 0.91).13 This increase of many megalin ligands in the urine is supported by the observation in animal models of AKI demonstrating downregulation of megalin in the proximal tubule after AKI was induced with resultant increases of urinary megalin ligands.34,35,36,37 Megalin deficiency, or immaturity in the proximal tubule of preterm neonates, may not be the only reason for urinary losses of megalin ligands. Further work is necessary to determine the significance of other apical receptors in the proximal tubule (including but not limited to cubilin, villin, and amnionless) of preterm infants and what factors may influence their maturity. Notwithstanding, our results demonstrate an underlying mechanism for the urinary presence of these megalin ligands, namely decreased megalin levels in the proximal tubule both in development and during AKI.
In conclusion, our study shows that megalin expression increases in the proximal tubule throughout gestation, and therefore that preterm babies have sub-normal megalin levels at birth. This is correlated with a decreased rescue of RBP and VDBP from urinary losses, as evidenced by decreased endocytosis and supported by increased urinary RBP excretion, both of which decrease with age in preterm infants. Although further validation is necessary, urinary RBP and VDBP may serve as biomarkers for proximal tubule megalin expression. The developmental expression of the megalin receptor has several important clinical consequences for the preterm population, most importantly the conservation of vitamins A and D with potential impact on nephrogenesis. Furthermore, the developmental expression of megalin in preterm neonates may have broader implications including the discovery of other biological important urinary losses and the development of rapid assessments of the urine in order to replace clinically relevant factors with greater precision.
References
Dawodu, A. & Nath, R. High prevalence of moderately severe vitamin D deficiency in preterm infants. Pediatr. Int 53, 207–210 (2011).
Lelievre-Pegorier, M., Vilar, J. & Ferrier, M. L. et al. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int 54, 1455–1462 (1998).
Mactier, H. & Weaver, L. T. Vitamin A and preterm infants: what we know, what we don’t know, and what we need to know. Arch. Dis. Child Fetal Neonatal Ed. 90, F103–8 (2005).
Charlton, J. R., Norwood, V. F., Kiley, S. C., Gurka, M. J. & Chevalier, R. L. Evolution of the urinary proteome during human renal development and maturation: variations with gestational and postnatal age. Pediatr. Res 72, 179–185 (2012).
Abitbol, C. L., Bauer, C. R., Montane, B., Chandar, J., Duara, S. & Zilleruelo, G. Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatr. Nephrol. 18, 887–893 (2003).
Moestrup, S. K., Cui, S. & Vorum, H. et al. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J. Clin. Invest 96, 1404–1413 (1995).
Leheste, J. R., Rolinski, B. & Vorum, H. et al. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am. J. Pathol. 155, 1361–1370 (1999).
Storm, T., Tranebjaerg, L. & Frykholm, C. et al. Renal phenotypic investigations of megalin-deficient patients: novel insights into tubular proteinuria and albumin filtration. Nephrol. Dial. Transplant. 28, 585–591 (2013).
Kantarci, S., Al-Gazali, L. & Hill, R. S. et al. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat. Genet 39, 957–959 (2007).
Ziak, M. & Roth, J. Expression of oligo/polyalpha2,8-linked deaminoneuraminic acid and megalin during kidney development and maturation: mutually exclusive distribution withpolyalpha2,8-linked N-acetylneuraminic acid of N-CAM. Histochem Cell Biol. 112, 169–178 (1999).
DeFreitas, M. J., Seeherunvong, W. & Katsoufis, C. P. et al. Longitudinal patterns of urine biomarkers in infants across gestational ages. Pediatr. Nephrol. 31, 1179–1188 (2016).
Askenazi, D. J., Koralkar, R., Patil, N., Halloran, B., Ambalavanan, N. & Griffin, R. Acute kidney injury urine biomarkers in very low-birth-weight infants. Clin. J. Am. Soc. Nephrol. 11, 1527–1535 (2016).
Hanna, M., Brophy, P. D., Giannone, P. J., Joshi, M. S., Bauer, J. A. & RamachandraRao, S. Early urinary biomarkers of acute kidney injury in preterm infants. Pediatr. Res 80, 218–223 (2016).
Forbes, M. S., Thornhill, B. A., Minor, J. J., Gordon, K. A., Galarreta, C. I. & Chevalier, R. L. Fight-or-flight: murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis. Am. J. Physiol. Ren. Physiol. 303, F120–9 (2012).
Christensen, E. I., Moskaug, J. O. & Vorum, H. et al. Evidence for an essential role of megalin in transepithelial transport of retinol. J. Am. Soc. Nephrol. 10, 685–695 (1999).
Nykjaer, A., Dragun, D. & Walther, D. et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96, 507–515 (1999).
Amsellem, S., Gburek, J. & Hamard, G. et al. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J. Am. Soc. Nephrol. 21, 1859–1867 (2010).
Kono, N. & Arai, H. Intracellular transport of fat-soluble vitamins A and E. Traffic 16, 19–34 (2015).
Furr, H. C., Green, M. H. & Haskell, M. et al. Stable isotope dilution techniques for assessing vitamin A status and bioefficacy of provitamin A carotenoids in humans. Public Health Nutr. 8, 596–607 (2005).
Wilson, J. G., Roth, C. B. & Warkany, J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am. J. Anat. 92, 189–217 (1953).
Batourina, E., Gim, S. & Bello, N. et al. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat. Genet 27, 74–78 (2001).
Humes, H. D. & Cieslinski, D. A. Interaction between growth factors and retinoic acid in the induction of kidney tubulogenesis in tissue culture. Exp. Cell Res 201, 8–15 (1992).
Vilar, J., Gilbert, T., Moreau, E. & Merlet-Benichou, C. Metanephros organogenesis is highly stimulated by vitamin A derivatives in organ culture. Kidney Int 49, 1478–1487 (1996).
Rosselot, C., Spraggon, L. & Chia, I. et al. Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137, 283–292 (2010).
Makrakis, J., Zimanyi, M. A. & Black, M. J. Retinoic acid enhances nephron endowment in rats exposed to maternal protein restriction. Pediatr. Nephrol. 22, 1861–1867 (2007).
Kennedy, K. A. Epidemiology of acute and chronic lung injury. Semin Perinatol. 17, 247–252 (1993).
Tyson, J. E., Wright, L. L. & Oh, W. et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N. Engl. J. Med 340, 1962–1968 (1999).
Chishimba, L., Thickett, D. R., Stockley, R. A. & Wood, A. M. The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax 65, 456–462 (2010).
Kolodziejczyk, A., Borszewska-Kornacka, M. K. & Seliga-Siwecka, J. MOnitored supplementation of VItamin D in preterm infants (MOSVID trial): study protocol for a randomised controlled trial. Trials 18, 424-017-2141-y (2017).
Fort, P., Salas, A. A., Nicola, T., Craig, C. M., Carlo, W. A. & Ambalavanan, N. A comparison of 3 vitamin D dosing regimens in extremely preterm infants: a randomized controlled trial. J. Pediatr. 174, 132–8.e1 (2016).
Boskabadi, H., Mamoori, G., Khatami, S. F. & Faramarzi, R. Serum level of vitamin D in preterm infants and its association with premature-related respiratory complications: a case-control study. Electron Physician 10, 6208–6214 (2018).
Lima, W. R., Parreira, K. S. & Devuyst, O. et al. ZONAB promotes proliferation and represses differentiation of proximal tubule epithelial cells. J. Am. Soc. Nephrol. 21, 478–488 (2010).
Vazquez-Carretero, M. D., Palomo, M. & Garcia-Miranda, P. et al. Dab2, megalin, cubilin and amnionless receptor complex might mediate intestinal endocytosis in the suckling rat. J. Cell Biochem 115, 510–522 (2014).
Lebeau, C., Arlt, V. M. & Schmeiser, H. H. et al. Aristolochic acid impedes endocytosis and induces DNA adducts in proximal tubule cells. Kidney Int 60, 1332–1342 (2001).
Lebeau, C., Debelle, F. D. & Arlt, V. M. et al. Early proximal tubule injury in experimental aristolochic acid nephropathy: functional and histological studies. Nephrol. Dial. Transplant. 20, 2321–2332 (2005).
Schreiber, A., Theilig, F., Schweda, F. & Hocherl, K. Acute endotoxemia in mice induces downregulation of megalin and cubilin in the kidney. Kidney Int 82, 53–59 (2012).
Vinuesa, E., Sola, A., Jung, M., Alfaro, V. & Hotter, G. Lipocalin-2-induced renal regeneration depends on cytokines. Am. J. Physiol. Ren. Physiol. 295, F1554–F1562 (2008).
Acknowledgments
We would like to acknowledge the technical assistance of Mrs. Valeria Pearl and Drs. Sheri Johnson and Robin LeGallo in conducting this study. We would also like to acknowledge the critical advice and mentorship of Dr. Robert Chevalier.
Funding
J.R.C. received funding from the following sources for this project: The National Kidney Foundation, Joseph M. Krainin Memorial Young Investigator Award, The Little Giraffe Foundation, and the University of Virginia Children’s Hospital Faculty Grant in Aid. J.R.C. is funded by R01DK110622 and R01DK111861.
Author information
Authors and Affiliations
Contributions
J.R.C. designed the study, obtained consent from participants, collected samples, analyzed data, prepared the initial draft, and critically reviewed the manuscript and has approved the final version of this manuscript. MWH participated in data collection, organization, analysis, and critically reviewed the manuscript and has approved the final version of this manuscript. C.S. participated in data collection, organization, and critically reviewed the manuscript and has approved the final version of this manuscript. R.N. contributed to the design and development of the project, interpretation of data, drafting the manuscript, and critically reviewed the manuscript and has approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Charlton, J.R., Harer, M.W., Swan, C. et al. Immature megalin expression in the preterm neonatal kidney is associated with urinary loss of vitamin carrier proteins. Pediatr Res 85, 405–411 (2019). https://doi.org/10.1038/s41390-018-0261-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0261-z
This article is cited by
-
Early postnatal nutrition and renal consequences in preterm infants
Pediatric Research (2024)
-
Advances in pediatric acute kidney injury pharmacology and nutrition: a report from the 26th Acute Disease Quality Initiative (ADQI) consensus conference
Pediatric Nephrology (2024)