Abstract
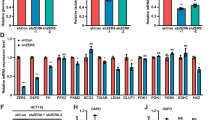
Although ΔNp63 is known to promote cancer cell proliferation, the underlying mechanism behind its oncogenic function remains elusive. We report here a functional interplay between ΔNp63 and Δ133p53. These two proteins are co-overexpressed in a subset of human cancers and cooperate to promote cell proliferation. Mechanistically, Δ133p53 binds to ΔNp63 and utilizes its transactivation domain to upregulate GLUT1, GLUT4, and PGM expression driving glycolysis. While increased glycolysis provides cancer cells with anabolic metabolism critical for proliferation and survival, it can be harnessed for selective cancer cell killing. Indeed, we show that tumors overexpressing both ΔNp63 and Δ133p53 exhibit heightened sensitivity to vitamin C that accumulate to a lethal level due to accelerated uptake via overexpressed GLUT1. These observations offer a new therapeutic avenue that could be exploited for clinical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol. 2015;16:393–405.
Shen L, Sun X, Fu Z, Yang G, Li J, Yao L. The fundamental role of the p53 pathway in tumor metabolism and its implication in tumor therapy. Clin Cancer Res. 2012;18:1561–7.
Green DR, Chipuk JE. p53 and metabolism: inside the TIGAR. Cell. 2006;126:30–2.
Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–20.
Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004;64:2627–33.
Mathupala SP, Heese C, Pedersen PL. Glucose catabolism in cancer cells. The type II hexokinase promoter contains functionally active response elements for the tumor suppressor p53. J Biol Chem. 1997;272:22776–80.
Liu J, Zhang C, Hu W, Feng Z. Tumor suppressor p53 and its mutants in cancer metabolism. Cancer Lett. 2015;356:197–203.
Khoury MP, Bourdon JC. p53 isoforms: an intracellular microprocessor? Genes Cancer. 2011;2:453–65.
Joruiz SM, Bourdon JC. p53 Isoforms: key regulators of the cell fate decision. Cold Spring Harbor Perspect Med. 2016;6. https://doi.org/10.1101/cshperspect.a02
Ji W, Zhang N, Zhang H, Ma J, Zhong H, Jiao J, et al. Expression of p53beta and Delta133p53 isoforms in different gastric tissues. Int J Clin Exp Pathol. 2015;8:10468–74.
Moore HC, Jordan LB, Bray SE, Baker L, Quinlan PR, Purdie CA, et al. The RNA helicase p68 modulates expression and function of the Delta133 isoform(s) of p53, and is inversely associated with Delta133p53 expression in breast cancer. Oncogene. 2010;29:6475–84.
Aoubala M, Murray-Zmijewski F, Khoury MP, Fernandes K, Perrier S, Bernard H, et al. p53 directly transactivates Delta133p53alpha, regulating cell fate outcome in response to DNA damage. Cell Death Differ. 2011;18:248–58.
Marcel V, Vijayakumar V, Fernandez-Cuesta L, Hafsi H, Sagne C, Hautefeuille A, et al. p53 regulates the transcription of its Delta133p53 isoform through specific response elements contained within the TP53 P2 internal promoter. Oncogene. 2010;29:2691–700.
Chen J, Ng SM, Chang C, Zhang Z, Bourdon JC, Lane DP, et al. p53 isoform delta113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev. 2009;23:278–90.
Gong L, Pan X, Yuan ZM, Peng J, Chen J. p53 coordinates with Delta133p53 isoform to promote cell survival under low-level oxidative stress. J Mol Cell Biol. 2016;8:88–90.
Gong L, Gong H, Pan X, Chang C, Ou Z, Ye S, et al. p53 isoform Delta113p53/Delta133p53 promotes DNA double-strand break repair to protect cell from death and senescence in response to DNA damage. Cell Res. 2015;25:351–69.
Gong L, Pan X, Chen H, Rao L, Zeng Y, Hang H, et al. p53 isoform Delta133p53 promotes efficiency of induced pluripotent stem cells and ensures genomic integrity during reprogramming. Sci Rep. 2016;6:37281.
Gong L, Chen J. Delta113p53/Delta133p53 converts P53 from a repressor to a promoter of DNA double-stand break repair. Mol Cell Oncol. 2016;3:e1033587.
Candi E, Dinsdale D, Rufini A, Salomoni P, Knight RA, Mueller M, et al. TAp63 and DeltaNp63 in cancer and epidermal development. Cell Cycle. 2007;6:274–85.
Gressner O, Schilling T, Lorenz K, Schulze Schleithoff E, Koch A, Schulze-Bergkamen H, et al. TAp63alpha induces apoptosis by activating signaling via death receptors and mitochondria. EMBO J. 2005;24:2458–71.
Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP, et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19:2122–37.
Tannapfel A, Schmelzer S, Benicke M, Klimpfinger M, Kohlhaw K, Mossner J, et al. Expression of the p53 homologues p63 and p73 in multiple simultaneous gastric cancer. J Pathol. 2001;195:163–70.
Barbareschi M, Pecciarini L, Cangi MG, Macri E, Rizzo A, Viale G, et al. p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol. 2001;25:1054–60.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308.
Hainaut P, Pfeifer GP. Somatic TP53 mutations in the era of genome sequencing. Cold Spring Harbor Perspect Med. 2016;6. https://doi.org/10.1101/cshperspect.a026
Turnquist C, Horikawa I, Foran E, Major EO, Vojtesek B, Lane DP, et al. p53 isoforms regulate astrocyte-mediated neuroprotection and neurodegeneration. Cell Death Differ. 2016;23:1515–28.
Mondal AM, Horikawa I, Pine SR, Fujita K, Morgan KM, Vera E, et al. p53 isoforms regulate aging- and tumor-associated replicative senescence in T lymphocytes. J Clin Invest. 2013;123:5247–57.
Horikawa I, Park KY, Isogaya K, Hiyoshi Y, Li H, Anami K, et al. Delta133p53 represses p53-inducible senescence genes and enhances the generation of human induced pluripotent stem cells. Cell Death Differ. 2017;24:1017–28.
Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350:1391–6.
Fujita K, Mondal AM, Horikawa I, Nguyen GH, Kumamoto K, Sohn JJ, et al. p53 isoforms Delta133p53 and p53beta are endogenous regulators of replicative cellular senescence. Nat Cell Biol. 2009;11:1135–42.
Roth I, Campbell H, Rubio C, Vennin C, Wilson M, Wiles A, et al. The Delta133p53 isoform and its mouse analogue Delta122p53 promote invasion and metastasis involving pro-inflammatory molecules interleukin-6 and CCL2. Oncogene. 2016;35:4981–9.
Bernard H, Garmy-Susini B, Ainaoui N, Van Den Berghe L, Peurichard A, Javerzat S, et al. The p53 isoform, Delta133p53alpha, stimulates angiogenesis and tumour progression. Oncogene. 2013;32:2150–60.
Arsic N, Gadea G, Lagerqvist EL, Busson M, Cahuzac N, Brock C, et al. The p53 isoform Delta133p53beta promotes cancer stem cell potential. Stem Cell Rep. 2015;4:531–40.
Hofstetter G, Berger A, Schuster E, Wolf A, Hager G, Vergote I, et al. Delta133p53 is an independent prognostic marker in p53 mutant advanced serous ovarian cancer. Br J Cancer. 2011;105:1593–9.
Yang X, Lu H, Yan B, Romano RA, Bian Y, Friedman J, et al. DeltaNp63 versatilely regulates a broad NF-kappaB gene program and promotes squamous epithelial proliferation, migration, and inflammation. Cancer Res. 2011;71:3688–700.
Ibrahim YH, Garcia-Garcia C, Serra V, He L, Torres-Lockhart K, Prat A, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2:1036–47.
Acknowledgements
This work was supported in part by the Morningside Foundation, the Zhu Fund and grants from the National Cancer Institute at the National Institute of Health (R01CA85679, R01CA167814, and R01CA125144).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Gong, L., Pan, X., Lim, CB. et al. A functional interplay between Δ133p53 and ΔNp63 in promoting glycolytic metabolism to fuel cancer cell proliferation. Oncogene 37, 2150–2164 (2018). https://doi.org/10.1038/s41388-017-0117-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-017-0117-8
This article is cited by
-
p63: a crucial player in epithelial stemness regulation
Oncogene (2023)
-
Δ133p53β isoform pro-invasive activity is regulated through an aggregation-dependent mechanism in cancer cells
Nature Communications (2021)
-
Functional interplay between p53 and Δ133p53 in adaptive stress response
Cell Death & Differentiation (2020)
-
K120R mutation inactivates p53 by creating an aberrant splice site leading to nonsense-mediated mRNA decay
Oncogene (2019)
-
The Δ133p53β isoform promotes an immunosuppressive environment leading to aggressive prostate cancer
Cell Death & Disease (2019)