Abstract
Objectives
Several studies demonstrated a positive relationship between hemoglobin level and bone mineral density (BMD). Thus, the association between hemoglobin concentration and osteoporosis in elders with type 2 diabetes mellitus (T2DM) was explored in this study.
Methods
Totally, 573 elders with T2DM were included in the study. BMD was measured by dual-energy X-ray absorptiometry. Hemoglobin levels were tested. The association between the hemoglobin level and osteoporosis was subjected to logistic regression analysis.
Results
For men, the hemoglobin levels were significantly lower in osteoporosis group than that in non-osteoporosis group (135.98 ± 16.20 vs. 142.84 ± 13.78 g/L, P = 0.002). Hemoglobin levels were positively related with BMD of total hip and femoral neck in men (r = 0.170, P = 0.004; r = 0.148, P = 0.012, respectively). After adjusting for age, body mass index (BMI), hemoglobin A1c (HbA1c), estimated glomerular filtration rate (eGFR) and 25-hydroxyvitamin D3 [25(OH) D3], the hemoglobin level was related with a 0.97-fold lower risk of osteoporosis (odds ratio (OR): 0.97; 95% confidence interval (CI): 0.95–0.99; P = 0.004) in men, but no such association was found in women.
Conclusion
Higher levels of hemoglobin play a protective role against osteoporosis in older men with T2DM.
Similar content being viewed by others
Introduction
Osteoporosis has become a major health issue in aging populations, resulting in fractures, disability, hospitalization, and increased mortality [1, 2]. A recent review reported that 37.8% of Chinese diabetic subjects suffered from osteoporosis [3]. In addition, although the bone mineral density (BMD) in patients with type 2 diabetes mellitus (T2DM) is often normal or even elevated [4], diabetic individuals have an increased risk of fracture than those without T2DM [5, 6]. In recent years, osteoporosis associated fracture has been considered an important complication of diabetes [7]. However, osteoporosis is often unrecognized until a fracture occurs. Thus, exploring the predictive factors for osteoporosis is important in aging population, especially in people with T2DM.
Previous studies demonstrated that osteoporosis was related with aging, a low body mass index (BMI), physical inactivity, low vitamin D levels [1], increased proinflammatory cytokine [8], and poor nutritional status [9]. In addition, several studies reported that anemia was related with a higher risk of osteoporosis and fracture [10, 11] Anemia often occurs in older people. A US survey reported that 14.1% of older men and 10.2% of older women were suffered from anemia [12]. Compared to the prevalence of anemia in the general population, T2DM at least doubles the risk [13]. Furthermore, as with osteoporosis, anemia is often overlooked in subjects with T2DM. It has been suggested that hypoxia increased the risk of osteoporosis [14]. Osteoporosis and anemia share common risk factors, including advanced age, nutritional deficiency, and inflammation.
Several studies have concluded that there were significant correlations between hemoglobin levels and BMD in aging populations [15,16,17], but this finding remains inconsistent. A recent study showed that people with reduced hemoglobin levels had a higher risk of osteoporosis [18], while another study showed no such relationship between hemoglobin concentration and BMD [19]. In addition, some studies showed that anemia increased the risk of fractures but the risk differed by gender. A recent study in Korea demonstrated that anemia increased the risk of fractures by 29% in men and 11% in women [10]. However, most studies used nonstandard measurements of BMD, such as CT scans or ultrasound instead of dual-energy X-ray absorptiometry (DEXA), to determine BMD. In addition, previous studies mainly focused on general populations, and the relationship between hemoglobin concentration and osteoporosis in elderly people with T2DM has not yet been investigated.
Therefore, given the current evidence that subjects with T2DM have a higher risk of fracture and anemia, it is essential to understand the effect of hemoglobin level on osteoporosis in the T2DM population. The purpose of the study was to investigate the relationship between hemoglobin levels and osteoporosis in older people with T2DM.
Methods
Study participants
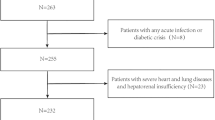
This was a cross-sectional analysis of elderly diabetic patients to explore the relationship between hemoglobin levels and osteoporosis. The participants were consecutively recruited by Xuanwu Hospital from July 2016 to May 2018. Detailed information on the assessment of related factors for osteoporosis was reported in our previous study [9]. The Research Ethics Boards at Xuanwu Hospital approved this study (approval number: CTR-IPR-2019002). All participants provided informed consent for participation in this study.
In total, 830 individuals ≥60 years old and met the WHO diagnosis criteria for T2DM were recruited [20]. The exclusion criteria were hematological disorders, acute inflammatory diseases, thyroid disease, Cushing syndrome, malignant tumor, severe kidney, and hepatic disease. Subjects taking glucocorticoid drugs, thiazolidinedione, bisphosphonates, or calcitonin drugs were excluded. Finally, 573 older adults were included in the analysis (Fig. 1).
Hemoglobin concentration
Hemoglobin levels were measured in venous blood samples by the hematology analyzer CELL DYN 3200 (Abbott Laboratories, USA). Anemia was defined according to the hemoglobin concentration: <13 g/dl for men, <12 g/dl for women [21].
Definition of osteoporosis
The BMD (g/cm2) for the lumbar spine (L2-L4), right femoral neck and total hip were determined by DEXA (LUNAR iDXA, USA) using methods that had been previously reported [9]. According to the criteria recommended by the WHO in 1994, participants with a T-score ≤ −2.5 SD at any of the lumbar spine, femoral neck and hip sites were classified as osteoporosis [22].
Covariates
The following potential risk factors for osteoporosis were assessed in our study: age, sex, BMI, duration of diabetes, serum vitamin D concentration, proinflammatory cytokines, blood glucose control, insulin resistance, and kidney function.
Demographic variables (age, sex), diabetic duration, height (cm) and body weight (kg) were collected. BMI was calculated as the body weight divided by height in square meters. The complications of T2DM were also assessed.
A 10 h fasting blood sample was collected form every subject. Fasting blood glucose (FBG), creatinine (CRE), fasting insulin (FINS), and fasting C peptide (FCP) were determined by using an automatic biochemical analyzer (BioTek Instrument, Inc., Beijing, China). Hemoglobin A1c (HbA1c), 25-hydroxyvitamin D3 [25(OH)D3], interleukin (IL) − 6 and high-sensitivity C-reactive protein (hs-CRP) were measured as previously described [9]. The insulin resistance (IR) index (HOMA-IR) was calculated as FINS (μIU/mL) × FBG (mmol/L)/22.5. The estimated glomerular filtration rate (eGFR) was got using the Modification of Diet in Renal Disease (MDRD) equation [23].
Statistical analysis
The normality of the data was assessed by using the Kolmogorov–Smirnov test. The data are reported as the mean ± SD or medians (interquartile ranges) based on the distribution of the data. Unpaired Student t-test or the Mann–Whitney U test was performed for the comparisons between groups. Differences in proportions were explored using chi-square tests. Pearson correlation analysis was performed to investigate the correlations between hemoglobin levels and BMD values. Binary logistic regression models were performed to analyze whether hemoglobin levels were independently related with osteoporosis. Mixed-effects models adjusted for (1) hemoglobin, age, and BMI; and (2) hemoglobin, age, BMI, 25(OH) D3, HbA1c, and eGFR were used to investigate the relationship between hemoglobin and osteoporosis. Statistical analysis was carried out using SPSS 19.0, and a two- tailed P < 0.05 was considered statistically significant.
Results
Characteristics of the study population according to sex and osteoporosis status
Table 1 showed the characteristics of the elderly based on sex and osteoporosis status. The mean age was 67.52 ± 6.90 years, and 286 (49.91%) subjects were male. The overall prevalence of osteoporosis was 30.89% (177/573). The prevalence of osteoporosis was 19.23% (55/286) in men and 42.51% (122/287) in women. Individuals with osteoporosis had lower BMI in both men and women (P < 0.05-P < 0.001). For men, the hemoglobin levels were significantly lower in osteoporosis group than that in non-osteoporosis group (135.98 ± 16.20 vs. 142.84 ± 13.78 g/L, P = 0.002). However, no obvious differences in hemoglobin levels were observed in women according to osteoporosis status. For women, persons with osteoporosis were older and had a lower eGFR level (P < 0.001).
Comparison of BMD according to different osteoporosis or anemia statuses
Compared with individuals without osteoporosis, participants with osteoporosis had significantly lower BMD at each site for both sexes (Table 2). For men, the femoral neck BMD and hip BMD were lower in participants with anemia compared with individuals without anemia (0.89 ± 0.16 vs. 0.96 ± 0.14 g/cm2, P = 0.001; 0.96 ± 0.16 vs. 1.02 ± 0.15 g/cm2, P = 0.005, respectively). However, there was no significant difference in lumbar spine BMD between the two groups among men. No difference in BMD at each site was found in women according to anemia status (Fig. 2).
Correlations between hemoglobin levels and BMD
There were significant correlations between hemoglobin levels and femoral neck BMD and total hip BMD values in men (r = 0.170, P = 0.004; r = 0.148, P = 0.012, respectively). No significant correlation was found between hemoglobin concentration and lumbar spine BMD in men. For women, there was no correlation between hemoglobin concentration and BMD at each site (Fig. 3).
Risk of osteoporosis associated with hemoglobin level
Association between hemoglobin concentration and osteoporosis was assessed using logistic regression models (Table 3A, B). Two predictive logistic models were developed. Hemoglobin, age, BMI were adjusted in Model 1, and HbA1c, eGFR, and 25(OH)D3 were added to Model 2. We found that the hemoglobin level (OR: 0.97, 95% CI: 0.95–0.99, P = 0.004) was significantly related with a decreased risk of osteoporosis in men, but no such association was found in women. A higher BMI played a protective role on osteoporosis in both sexes. Older age was related with a higher risk for osteoporosis in women (OR: 1.06, 95% CI: 1.01–1.11, P = 0.011).
Discussion
Our study revealed a negative association between hemoglobin level and osteoporosis in elder men with diabetes after adjusting for BMI and other potential confounders (age, HbA1c, eGFR, 25(OH) D3, and eGFR). However, no such association was found in women.
Anemia is a highly prevalent condition in individuals with T2DM, and the reported prevalence ranges from 20% to 44% in various populations [24, 25]. Subjects with diabetes may be especially vulnerable to hypoxia-induced organ damage [26]. Previously, several studies have concluded that hemoglobin concentration is positively related with BMD [15,16,17], while some studies have reported inconsistent findings [19, 27]. Pan et al. [11] reported that iron deficiency anemia (IDA) was associated with an increased risk of osteoporosis. However, Oh et al. [19] demonstrated that hemoglobin concentration was positively related with BMD in male participants but negatively related with BMD in female participants. Onal et al. [28] thought that anemia was an epiphenomenon of osteoporosis rather than the cause. Differences in the results of these studies may be relate to participant characteristics and methodological limitations, including cross-sectional design and lack of information on the etiology of anemia. However, few studies have investigated the association between hemoglobin and osteoporosis in people with T2DM. Therefore, our study was carried out among 573 individuals with T2DM to investigate the relationship between hemoglobin and BMD, which was assessed using DEXA. The findings of our study are in consistent with the results of studies in the general population [16, 29]. After adjusting for several covariates, hemoglobin remained significantly related with osteoporosis in male participants but not in women. A possible explanation could be that sex hormones mediate the effect of hemoglobin on bone [10]. Low testosterone levels have been supposed to be related with anemia and decreased BMD in men [30, 31].
A possible mechanism underlying the link between hemoglobin and osteoporosis may be hypoxemia, which has been reported to mediate the risk of osteoporosis [14, 32]. An experimental study showed that hypoxia resulted in a three-fold increase in osteoclast formation and a 10-fold stimulation of resorption pit formation [33]. Bone formation and remodeling may be affected by chronic hypoxia. Karadag et al. [32] demonstrated that the severity of the illness was independently related with BMD in individuals suffered from chronic obstructive pulmonary disease.
Inflammation may also mediate the association between anemia and osteoporosis. It has been showed that proinflammatory cytokines affected hematopoiesis [34]. IL-6 has been reported to promote osteoclast differentiation and activation [35]. In addition, hs-CRP levels have been associated with BMD in several studies [8]. However, our study failed to find a relationship between proinflammatory cytokines and hemoglobin or BMD. These results were different from those of previous studies [35]. The discrepancies may be due to the different populations.
Another possible explanation for the findings could be that erythropoietin (EPO) involves in the physiology of skeletal remodeling. Shiozawa et al. [36] demonstrated that EPO might have anabolic and catabolic effects on bone. EPO accelerates bone loss through promoting osteoclastogenesis. The risk of anemia in individuals with diabetes may be due to inadequate responsiveness to EPO, which can be caused by decreased EPO concentration, EPO functional defect and/or EPO resistance [37]. Diabetic nephropathy is related with a higher risk of anemia in diabetic individuals. Furthermore, EPO decreases in an inverse relationship to decreasing GFR [38]. However, the present study did not allow us to determine whether EPO has an effect on the link between hemoglobin and osteoporosis due to a lack of EPO measurements. Indeed, the pathophysiological mechanism underlying the association between hemoglobin and bone health require further investigation.
Our study suggested that finding low hemoglobin levels might add to the currently known risk factors to help identify subjects at high risk of osteoporosis in the T2DM population. Furthermore, low hemoglobin can be easily found by a blood test. Low hemoglobin in most older adults is caused by modifiable causes which can be corrected by treatment [39]. Therefore, considering that correcting low hemoglobin may help reduce the risk of osteoporosis, health professionals should watch for low hemoglobin in older men with T2DM.
To our knowledge, studies that have explored the link between hemoglobin and osteoporosis in diabetic individuals are sparse. Osteoporosis was diagnosed by DEXA to ensure the accuracy of the BMD values in our study rather than by nonstandard measurements of BMD, such as ultrasound or CT scans, as used in other studies. As the fracture risk in individuals with T2DM is increased and is often overlooked in clinical practice, early recognition and initiation of optimal management for osteoporosis might improve patient outcomes and reduce the increased risk of fracture in subjects with diabetes.
Some limitations should be pointed out in this study. First, we were unable to assess the cause-effect relationship between hemoglobin and osteoporosis due to the cross-sectional design. Second, our analysis was restricted to older people with T2DM selected only from Xuanwu Hospital. Third, although we adjusted for many demographic and biochemical parameters in our analysis, medications that may affect BMD were not adjusted. Some osteoporosis medications were also not considered in the study due to their very low rate of use in this study. Finally, we could not determine the etiology of anemia in these subjects due to the lack of measurement of serum levels of EPO, iron, vitamin B12 or folic acid.
Conclusions
Our study found that higher levels of hemoglobin play a protective role during osteoporosis in elderly men with T2DM. Considering the increased risk of osteoporosis in individuals with low hemoglobin concentration, it is important that clinicians should be alert to the presence of low hemoglobin. Further well-designed studies are warranted to explore the association between hemoglobin and osteoporosis in patients living with T2DM.
Data availability
The data reported in this study are available from the corresponding author upon reasonable request.
References
Paschou SA, Dede AD, Anagnostis PG, Vryonidou A, Morganstein D, Goulis DG. Type 2 diabetes and osteoporosis: a guide to optimal management. J Clin Endocrinol Metab. 2017;102:3621–34.
Leslie WD, Morin SN. New developments in fracture risk assessment for current osteoporosis reports. Curr Osteoporos Rep. 2020;18:115–29.
Si Y, Wang C, Guo Y, Yin H, Ma Y. Prevalence of osteoporosis in patients with type 2 diabetes mellitus in the Chinese mainland: A protocol of systematic review and meta-analysis. Medicine. 2020;99:e19762.
Holloway-Kew KL, Marijanovic N, De Abreu LLF, Sajjad MA, Pasco JA, Kotowicz MA. Bone mineral density in diabetes and impaired fasting glucose. Osteoporos Int. 2019;30:1799–806.
Liu JM, Zhu DL, Mu YM, Xia WB, Chinese Society of Osteoporosis and Bone Mineral Research, the Chinese Society of Endocrinology, Chinese Diabetes Society, Chinese Medical Association; Chinese Endocrinologist Association, Chinese Medical Doctor Association. Management of fracture risk in patients with diabetes-Chinese Expert Consensus. J Diabetes. 2019;11:906–19.
Lee TC, Lee YL, Chen JC, Chen CH, Ho PS. Impact of type 2 diabetes on postoperative outcome after hip fracture: nationwide population-based study in Taiwan. BMJ Open Diabetes Res Care. 2020;8:e000843.
Kurra S, Fink DA, Siris ES. Osteoporosis-associated fracture and diabetes. Endocrinol Metab Clin North Am. 2014;43:233–43.
Wu ZJ, He JL, Wei RQ, Liu B, Lin X, Guan J, et al. C-reactive protein and risk of fracture: a systematic review and dose-response meta-analysis of prospective cohort studies. Osteoporos Int. 2015;26:49–57.
Xiu S, Chhetri JK, Sun L, Mu Z, Wang L. Association of serum prealbumin with risk of osteoporosis in older adults with type 2 diabetes mellitus: a cross-sectional study. Ther Adv Chronic Dis. 2019;10:2040622319857361.
Lee EA, Shin DW, Yoo JH, Ko HY, Jeong SM. Anemia and risk of fractures in older korean adults: a nationwide population-based study. J Bone Min Res. 2019;34:1049–57.
Pan ML, Chen LR, Tsao HM, Chen KH. Iron deficiency anemia as a risk factor for osteoporosis in Taiwan: a nationwide population-based study. Nutrients. 2017;9:616.
Seitz AE, Eberhardt MS, Lukacs SL. Anemia prevalence and trends in adults aged 65 and older: U.S. national health and nutrition examination survey: 2001-2004 to 2013-2016. J Am Geriatr Soc. 2018;66:2431–2.
Gauci R, Hunter M, Bruce DG, Davis WA, Davis TME. Anemia complicating type 2 diabetes: Prevalence, risk factors and prognosis. J Diabetes Complications. 2017;31:1169–74.
Fujimoto H, Fujimoto K, Ueda A, Ohata M. Hypoxemia is a risk factor for bone mass loss. J Bone Min Metab. 1999;17:211–6.
Cesari M, Pahor M, Lauretani F, Penninx BW, Bartali B, Russo R, et al. Bone density and hemoglobin levels in older persons: results from the InCHIANTI study. Osteoporos Int. 2005;16:691–9.
Laudisio A, Marzetti E, Pagano F, Bernabei R, Zuccalà G. Haemoglobin levels are associated with bone mineral density in the elderly: a population-based study. Clin Rheumatol. 2009;28:145–51.
Korkmaz U, Korkmaz N, Yazici S, Erkan M, Baki AE, Yazici M, et al. Anemia as a risk factor for low bone mineral density in postmenopausal Turkish women. Eur J Intern Med. 2012;23:154–8.
Kim SY, Yoo DM, Min C, Choi HG. Association between osteoporosis and low hemoglobin levels: a nested case-control study using a national health screening cohort. Int J Environ Res Public Health. 2021;18:8598.
Oh YH, Moon JH, Cho B. Association between hemoglobin level and bone mineral density in korean adults. J Bone Metab. 2017;24:161–73.
World Health Organization (1999) Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. World Health Organization, Geneva.
WHO Scientific Group. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep. Ser. 1968;405:5–37.
Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368–81.
Eckardt KU, Berns JS, Rocco MV, Kasiske BL. Definition and classification of CKD: the debate should be about patient prognosis—a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53:915–20.
Thomas MC, Tsalamandris C, MacIsaac RJ, Jerums G. The epidemiology of hemoglobin levels in patients with type 2 diabetes. Am J Kidney Dis. 2006;48:537–45.
Ezenwaka CE, Jones-Lecointe A, Nwagbara E, Seales D, Okali F. Anaemia and kidney dysfunction in Caribbean type 2 diabetic patients. Cardiovasc Diabetol. 2008;7:25.
Angelousi A, Larger E. Anaemia, a common but often unrecognized risk in diabetic patients: a review. Diabetes Metab. 2015;41:18–27.
Wu LY, Yang TC, Kuo SW, Hsiao CF, Hung YJ, Hsieh CH, et al. Correlation between bone mineral density and plasma lipids in Taiwan. Endocr Res. 2003;29:317–25.
Onal ED, Usluogullar A. Anemia and osteoporosis: Causal association or epiphenomenon? Eur J Intern Med. 2012;23:e117.
Wang M, Wang X, Cui W, Zhu G, Liang Y, Chen X, et al. The association between hemoglobin level and osteoporosis in a Chinese population with environmental lead and cadmium exposure. Environ Geochem Health. 2021. https://doi.org/10.1007/s10653-021-01129-0.
Lewerin C, Nilsson-Ehle H, Jacobsson S, Johansson H, Sundh V, Karlsson MK, et al. Serum estradiol associates with blood hemoglobin in elderly men: the MrOS Sweden study. J Clin Endocrinol Metab. 2014;99:2549–56.
Kenny AM, Prestwood KM, Marcello KM, Raisz LG. Determinants of bone density in healthy older men with low testosterone levels. J Gerontol A Biol Sci Med Sci. 2000;55:M492–M497.
Karadag F, Cildag O, Yurekli Y, Gurgey O. Should COPD patients be routinely evaluated for bone mineral density? J Bone Min Metab. 2003;21:242–6.
Utting JC, Flanagan AM, Brandao-Burch A, Orriss IR, Arnett TR. Hypoxia stimulates osteoclast formation from human peripheral blood. Cell Biochem Funct. 2010;28:374–80.
Baraldi-Junkins CA, Beck AC, Rothstein G. Hematopoieis and cytokines. Relevance Cancer Aging Hematol Oncol Clin North Am. 2000;14:45–61.
Shahen VA, Gerbaix M, Koeppenkastrop S, Lim SF, McFarlane KE, Nguyen ANL, et al. Brennan-Speranza TC. Multifactorial effects of hyperglycaemia, hyperinsulinemia and inflammation on bone remodelling in type 2 diabetes mellitus. Cytokine Growth Factor Rev. 2020;55:109–18.
Shiozawa Y, Taichman RS. Bone: Elucidating which cell erythropoietin targets in bone. Nat Rev Endocrinol. 2015;11:263–4.
Singh DK, Winocour P, Farrington K. Erythropoietic stress and anemia in diabetes mellitus. Nat Rev Endocrinol. 2009;5:204–10.
Mojiminiyi OA, Abdella NA, Zaki MY, El Gebely SA, Mohamedi HM, Aldhahi WA. Prevalence and associations of low plasma erythropoietin in patients with Type 2 diabetes mellitus. Diabet Med. 2006;23:839–44.
Lanier JB, Park JJ, Callahan RC. Anemia in older adults. Am Fam Physician. 2018;98:437–42.
Acknowledgements
The authors thank all the staff and the subjects who participated in this study.
Funding
This work was supported by Beijing Municipal Administration of Hospitals Incubating Program (PX2020034), the pilot project for public welfare development and reform of Beijing-affiliated Medical Research Institutes (Beijing Medical Research 2021-8) and the Open Fund of Beijing Key Laboratory of Diabetes Prevention and Control Research (10025220102).
Author information
Authors and Affiliations
Contributions
SX contributed to study design and manuscript preparation. JF did data analysis and interpretation. LS, ZM, and LZ recruited the participants and collected the data. All authors reviewed the manuscript and provided approval of the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiu, S., Mu, Z., Sun, L. et al. Hemoglobin level and osteoporosis in Chinese elders with type 2 diabetes mellitus. Nutr. Diabetes 12, 19 (2022). https://doi.org/10.1038/s41387-022-00198-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-022-00198-z
This article is cited by
-
The impact of diabetes, anemia, and renal function in the relationship between osteoporosis and fasting blood glucose among Taiwanese women: a cross-sectional study
BMC Women's Health (2024)
-
Association between admission hemoglobin level and prognosis in sepsis patients based on a critical care database
Scientific Reports (2024)
-
Association between triglyceride-glucose index and bone mineral density in US adults: a cross sectional study
Journal of Orthopaedic Surgery and Research (2023)