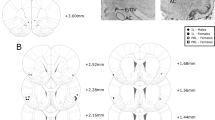
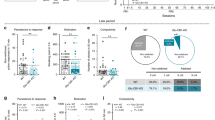
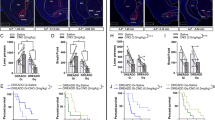
Abstract
Excessive consumption of palatable foods that are rich in fats and sugars has contributed to the increasing prevalence of obesity worldwide. Similar to addictive drugs, such foods activate the brain’s reward circuit, involving mesolimbic dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and the prefrontal cortex. Neuroadaptations occurring in this circuit are hypothesized to contribute to uncontrolled consumption of such foods, a common feature of most of eating disorders and obesity. The rostromedial tegmental nucleus (RMTg), also named tail of the VTA (tVTA), is an inhibitory structure projecting to the VTA and the lateral hypothalamus (LH), two key brain regions in food intake regulation. Prior research has demonstrated that the RMTg responds to addictive drugs and influences their impact on mesolimbic activity and reward-related behaviors. However, the role of the RMTg in food intake regulation remains largely unexplored. The present study aimed to investigate the role of the RMTg and its projections to the VTA and the LH in regulating food intake in rats. To do so, we examined eating patterns of rats with either bilateral excitotoxic lesions of the RMTg or specific lesions of RMTg-VTA and RMTg-LH pathways. Rats were exposed to a 6-week ‘free choice high-fat and high-sugar’ diet, followed by a 4-week palatable food forced abstinence and a 24 h re-access period. Our results indicate that an RMTg-VTA pathway lesion increases fat consumption following 6 weeks of diet and at time of re-access. The RMTg-LH pathway lesion produces a milder effect with a decrease in global calorie intake. These findings suggest that the RMTg influences palatable food consumption and relapse through its projections to the VTA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data supporting the findings of this study can be provided upon request from the corresponding author.
References
Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–4.
Demeke S, Rohde K, Chollet-Hinton L, Sutton C, Kong KL, Fazzino TL. Change in hyper-palatable food availability in the US food system over 30 years: 1988-2018. Public Health Nutr. 2023;26:182–9.
Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–79.
Ahn BH, Kim M, Kim SY. Brain circuits for promoting homeostatic and non-homeostatic appetites. Exp Mol Med. 2022;54:349–57.
Berridge KC, Ho CY, Richard JM, Difeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64.
Ferrario CR, Labouebe G, Liu S, Nieh EH, Routh VH, Xu S, et al. Homeostasis meets motivation in the battle to control food intake. J Neurosci. 2016;36:11469–81.
Bijoch Ł, Klos J, Pawłowska M, Wiśniewska J, Legutko D, Szachowicz U, et al. Whole-brain tracking of cocaine and sugar rewards processing. Transl Psychiatry. 2023;13:20.
Bijoch Ł, Klos J, Pękała M, Fiołna K, Kaczmarek L, Beroun A. Diverse processing of pharmacological and natural rewards by the central amygdala. Cell Rep. 2023;42:113036.
Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, et al. Decoding neural circuits that control compulsive sucrose seeking. Cell. 2015;160:528–41.
Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341:1517–21.
Rossi MA, Basiri ML, McHenry JA, Kosyk O, Otis JM, Van Den Munkhof HE, et al. Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science. 2019;364:1271–4.
Bourdy R, Barrot M. A new control center for dopaminergic systems: pulling the VTA by the tail. Trends Neurosci. 2012;35:681–90.
Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–96.
Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier M-J, Barrot M. gamma-Aminobutyric acid cells with cocaine-induced DeltaFosB in the ventral tegmental area innervate mesolimbic neurons. Biol Psychiatry. 2010;67:88–92.
Taylor NE, Long H, Pei JZ, Kukutla P, Phero A, Hadaegh F, et al. The rostromedial tegmental nucleus: A key modulator of pain and opioid analgesia. Pain. 2019;160:2524–34.
Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral Habenula to Dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31:11457–71.
Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800.
Sánchez-Catalán M-J, Barrot M. Fos response of the tail of the ventral tegmental area to food restriction entails a prediction error processing. Behav Brain Res. 2022;425:113826.
Godfrey N, Qiao M, Borgland SL. Activation of LH GABAergic inputs counteracts fasting-induced changes in tVTA/RMTG neurons. J Physiol. 2022;600:2203–24.
Bourdy R, Hertz A, Filliol D, Andry V, Goumon Y, Mendoza J, et al. The endocannabinoid system is modulated in reward and homeostatic brain regions following diet-induced obesity in rats: a cluster analysis approach. Eur J Nutr. 2021;60:4621–33.
Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: Open source software for digital pathology image analysis. Sci Rep. 2017;7:16878.
Ferreira JGP, Del-Fava F, Hasue RH, Shammah-Lagnado SJ. Organization of ventral tegmental area projections to the ventral tegmental area-nigral complex in the rat. Neuroscience. 2008;153:196–213.
Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–64.
Fu R, Chen X, Zuo W, Li J, Kang S, Zhou L-H, et al. Ablation of μ opioid receptor-expressing GABA neurons in rostromedial tegmental nucleus increases ethanol consumption and regulates ethanol-related behaviors. Neuropharmacology. 2016;107:58–67.
Brown PL, Palacorolla H, Brady D, Riegger K, Elmer GI, Shepard PD. Habenula-induced inhibition of midbrain Dopamine neurons is diminished by lesions of the Rostromedial Tegmental nucleus. J Neurosci. 2017;37:217–25.
Smith RJ, Vento PJ, Chao YS, Good CH, Jhou TC. Gene expression and neurochemical characterization of the rostromedial tegmental nucleus (RMTg) in rats and mice. Brain Struct Funct. 2019;224:219–38.
Dela Cruz JAD, Coke T, Karagiorgis T, Sampson C, Icaza-Cukali D, Kest K, et al. c-Fos induction in mesotelencephalic dopamine pathway projection targets and dorsal striatum following oral intake of sugars and fats in rats. Brain Res Bull. 2015;111:9–19.
Chang G-Q, Karatayev O, Lukatskaya O, Leibowitz SF. Prenatal fat exposure and hypothalamic PPAR β/δ: Possible relationship to increased neurogenesis of orexigenic peptide neurons. Peptides. 2016;79:16–26.
True C, Arik A, Lindsley S, Kirigiti M, Sullivan E, Kievit P. Early high-fat diet exposure causes dysregulation of the Orexin and Dopamine neuronal populations in nonhuman primates. Front Endocrinol. 2018;9:508.
Wang S, Tan Y, Zhang JE, Luo M. Pharmacogenetic activation of midbrain dopaminergic neurons induces hyperactivity. Neurosci Bull. 2013;29:517–24.
Boekhoudt L, Omrani A, Luijendijk MCM, Wolterink-Donselaar IG, Wijbrans EC, van der Plasse G, et al. Chemogenetic activation of dopamine neurons in the ventral tegmental area, but not substantia nigra, induces hyperactivity in rats. Eur Neuropsychopharmacol. 2016;26:1784–93.
Costall B, Domeney AM, Naylor RJ. Long-term consequences of antagonism by neuroleptics of behavioural events occurring during mesolimbic dopamine infusion. Neuropharmacology. 1984;23:287–94.
Bourdy R, Sánchez-Catalán MJ, Kaufling J, Balcita-Pedicino JJ, Freund-Mercier MJ, Veinante P, et al. Control of the nigrostriatal dopamine neuron activity and motor function by the tail of the ventral tegmental area. Neuropsychopharmacology. 2014;39:2788–98.
Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TMK, et al. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14:620–6.
Morel C, Montgomery SE, Li L, Durand-de Cuttoli R, Teichman EM, Juarez B, et al. Midbrain projection to the basolateral amygdala encodes anxiety-like but not depression-like behaviors. Nat Commun. 2022;13:1532.
Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98.
Sharma S, Fernandes MF, Fulton S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int J Obes. 2013;37:1183–91.
Liu S, Globa AK, Mills F, Naef L, Qiao M, Bamji SX, et al. Consumption of palatable food primes food approach behavior by rapidly increasing synaptic density in the VTA. Proc Natl Acad Sci USA. 2016;113:2520–5.
Nieh EH, Vander Weele CM, Matthews GA, Presbrey KN, Wichmann R, Leppla CA, et al. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron. 2016;90:1286–98.
Carlin J, Hill-Smith TE, Lucki I, Reyes TM. Reversal of dopamine system dysfunction in response to high-fat diet. Obesity. 2013;21:2513–21.
Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53.
Espitia-Bautista E, Escobar C. Fat rather than sugar diet leads to binge-type eating, anticipation, effort behavior and activation of the corticolimbic system. Nutr Neurosci. 2021;24:508–19.
Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, et al. Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci USA. 2011;108:16446–50.
Gakare SG, Ugale RR. Pharmacological evaluation of lateral habenula and rostromedial tegmental nucleus in the expression of ethanol-induced place preference. Behav Pharm. 2023;34:225–35.
Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61:1021–9.
Lavezzi HN, Parsley KP, Zahm DS. Modulation of locomotor activation by the rostromedial tegmental nucleus. Neuropsychopharmacology. 2015;40:676–87.
Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res Rev. 1998;28:309–69.
Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35:1124–43.
Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–22.
Kalivas PW, Widerlöv E, Stanley D, Breese G, Prange AJ. Enkephalin action on the mesolimbic system: a dopamine-dependent and a dopamine-independent increase in locomotor activity. J Pharmacol Exp Ther. 1983;227:229–37.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91.
Blum K, Liu Y, Shriner R, Gold MS. Reward circuitry dopaminergic activation regulates food and drug craving behavior. Curr Pharm Des. 2011;17:1158–67.
Rossi MA. Control of energy homeostasis by the lateral hypothalamic area. Trends Neurosci. 2023;46:738–49.
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85.
Barson JR, Leibowitz SF. Orexin/Hypocretin system: role in food and drug overconsumption. Int Rev Neurobiol. 2017;136:199–237.
Yoshida K, McCormack S, España RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–61.
Stanley BG, Urstadt KR, Charles JR, Kee T. Glutamate and GABA in lateral hypothalamic mechanisms controlling food intake. Physiol Behav. 2011;104:40–46.
Lee J, Raycraft L, Johnson AW. The dynamic regulation of appetitive behavior through lateral hypothalamic orexin and melanin concentrating hormone expressing cells. Physiol Behav. 2021;229:113234.
Subramanian KS, Lauer LT, Hayes AMR, Décarie-Spain L, McBurnett K, Nourbash AC, et al. Hypothalamic melanin-concentrating hormone neurons integrate food-motivated appetitive and consummatory processes in rats. Nat Commun. 2023;14:1755.
Guerdjikova AI, Mori N, Casuto LS, McElroy SL. Update on binge eating disorder. Med Clin North Am. 2019;103:669–80.
Freeman LR, Bentzley BS, James MH, Aston-Jones G. Sex differences in demand for highly palatable foods: role of the Orexin system. Int J Neuropsychopharmacol. 2021;24:54–63.
Buczek L, Migliaccio J, Petrovich GD. Hedonic eating: sex differences and characterization of Orexin activation and signaling. Neuroscience. 2020;436:34–45.
Spierling SR, Kreisler AD, Williams CA, Fang SY, Pucci SN, Kines KT, et al. Intermittent, extended access to preferred food leads to escalated food reinforcement and cyclic whole-body metabolism in rats: Sex differences and individual vulnerability. Physiol Behav. 2018;192:3–16.
Melis M, De Felice M, Lecca S, Fattore L, Pistis M. Sex-specific tonic 2-arachidonoylglycerol signaling at inhibitory inputs onto dopamine neurons of Lister Hooded rats. Front Integr Neurosci. 2013;7:93.
Acknowledgements
We thank Marion Neurdin and Adeline Coursan for their help with neuroanatomical studies, Romain Goutagny for providing retro Cre EGFP virus, Jorge Mendoza for providing the OX antibody, and Victor Mathis and Mary C. Olmstead for manuscript proofreading and fruitful discussions. We thank the PIV (Plateforme d’Imagerie in Vitro, INCI, Strasbourg) and the LNCA animal facility staff.
Funding
This project was supported by the Université de Strasbourg, the Centre National de la Recherche Scientifique (CNRS). Florian Schoukroun received a PhD fellowship from the French Ministère de l’Enseignement Supérieur et de la Recherche.
Author information
Authors and Affiliations
Contributions
KB and RB designed the research. FS and RB performed the experiments and analyzed the data. FS, KB and RB wrote the manuscript. KB and RB prepared the revised version of the manuscript. All authors gave final approval to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schoukroun, F., Befort, K. & Bourdy, R. The rostromedial tegmental nucleus gates fat overconsumption through ventral tegmental area output in male rats. Neuropsychopharmacol. (2024). https://doi.org/10.1038/s41386-024-01855-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41386-024-01855-w