Abstract
Subregions within insular cortex and medial prefrontal cortex (mPFC) have been implicated in eating disorders; however, the way these brain regions interact to produce dysfunctional eating is poorly understood. The present study explored how two mPFC subregions, the infralimbic (IL) and prelimbic (PRL) cortices, regulate sucrose hyperphagia elicited specifically by a neurochemical manipulation of the agranular/dysgranular region of gustatory insula (AI/DI). Using intra-AI/DI infusion of the mu-opioid receptor (µ-OR) agonist, DAMGO (1 µg), sucrose hyperphagia was generated in ad-libitum-maintained rats, while in the same rat, either the IL or prelimbic (PRL) subregion of mPFC was inactivated bilaterally with muscimol (30 ng). Intra-IL muscimol markedly potentiated AI/DI DAMGO-induced sucrose hyperphagia by increasing eating bout duration and food consumption per bout. In contrast, PRL attenuated intra-AI/DI DAMGO-driven sucrose intake and feeding duration and eliminated the small DAMGO-induced increase in feeding bout initiation. Intra-IL or -PRL muscimol alone (i.e., without intra-AI/DI DAMGO) did not alter feeding behavior, but slightly reduced exploratory-like rearing in both mPFC subregions. These results reveal anatomical heterogeneity in mPFC regulation of the intense feeding-motivational state engendered by µ-OR signaling in the gustatory insula: IL significantly curtails consummatory activity, while PRL modestly contributes to feeding initiation. Results are discussed with regard to potential circuit-based mechanisms that may underlie the observed results.
Similar content being viewed by others
Introduction
The neural control of food motivation has been studied intensively for decades, and considerable progress has been made in identifying neuroanatomical sites and transmitter systems that mediate goal salience, food-reward value, or hedonic experience [1,2,3]; or that energize goal-seeking responses [4]. One neurochemical system that has received considerable attention is the endogenous opioid system, which is thought to play a role in reward-driven, non-homeostatic feeding [5,6,7,8]. Discrete subcortical loci spanning the neural axis have been identified where stimulation of opioid receptors (primarily the µ-opioid receptor (µ-OR) subtype) markedly enhances food intake [9,10,11,12,13,14]. Perhaps the best-studied of these sites is the nucleus accumbens (Acb), where µ-OR stimulation engenders intense hyperphagia, amplifies food-seeking operant behaviors, and augments hedonic taste responses [15,16,17,18].
Relative to the Acb, cortical µ-OR actions have been less intensively studied. Recently, however, studies have revealed cortical loci where μ-OR stimulation produces intense feeding responses, amplifies food-reinforced operant responding, and generates hedonic taste reactions, rivaling effects seen in the Acb [19,20,21,22,23,24]. Infusions of the selective µ-OR agonist, DAMGO, elicit robust hyperphagia in specific subregions of rat medial prefrontal and orbitofrontal cortices, including infralimbic (IL) cortex and medial aspects of the ventral orbitofrontal cortex (OFC) [19, 22, 24]. These effects are anatomically circumscribed; placements 1–2 mm dorsal to IL in prelimbic cortex (PRL), or lateral in OFC, fail to alter food intake [24]. In agranular/dysgranular insular cortex (AI/DI), a cluster of sites beginning at the level of primary gustatory cortex and proceeding 1–2 mm rostrally elicit hyperphagia and, further rostrally, enhance hedonic taste reactions [22, 24]. Finally, DAMGO infusions into IL engenders “impulsive-like” premature responding in a sucrose-reinforced differential reinforcement of low rates (DRL) task of inhibitory control [21, 24]. Hence, cortical mapping using DAMGO has revealed dissociable control of food intake, food-seeking impulsivity, and hedonic taste processing across an interconnected network of sites. Importantly, these effects are not recapitulated by a variety of amino-acid neurotransmitter or monoamine neuromodulator manipulations [19], suggesting that µ-OR agonists represent powerful and specialized tools to reveal insights into the cortical localization of specific motivation functions.
The aim of the present study was to explore how cortical sites interact to modulate appetitive behavior in real time. Two sites of particular interest are IL and AI/DI around the level innervated by the gustatory thalamus. These two areas are linked via both direct and two-stage projections [25,26,27], and electrophysiological studies have revealed responses to chemosensory stimuli in both gustatory insula and prefrontal cortex (mPFC), with temporal characteristics suggesting an AI/DI-to-mPFC follow of information [28]. Previous work suggests that IL can either activate or inhibit food-motivated behaviors via projections to the lateral hypothalamus or Acb, respectively [20]. Hence, IL is positioned to shape the topography of appetitive behavior sequences, and potentially has the capacity to act as either a “driver” or “limiter” of consummatory activity generated elsewhere [29], including in AI/DI.
The present study explored the functional interactions between AI/DI and IL using a dual-site cannulation approach. Intra-AI/DI DAMGO infusions were employed to generate feeding responses, while in the same rat, specific sites in mPFC (the IL and PRL subregions) were inactivated using the GABA-A agonist, muscimol. We chose a 30 ng dose of muscimol based on prior dose-response work with this drug, which showed that this dose produces consistent behavioral effects while avoiding major activity-suppression confounds [30]. This dose also silences mPFC unit activity with a radius of action between 1.5 and 2 mm (C.W. Berridge, personal communication). Hence, the 30 ng muscimol dose is sufficient to achieve local inactivation and to spatially resolve discrete effects in infralimbic vs. prelimbic cortices. The main questions of interest were whether neural activity in mPFC enables or inhibits consummatory activity driven by AI/DI, and whether this modulation is homogeneous through the dorsal-ventral extent of the medial prefrontal cortex.
Methods
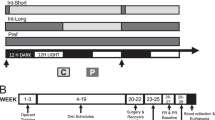
Subjects
Subjects were Sprague-Dawley rats (Envigo; Madison, WI) consisting of age-matched males and females, males weighing 300–325 g and females 275–300 g upon arrival. Rats were housed in temperature-controlled, vivarium rooms with a 12:12 h light-dark cycle (lights on at 0700 h). All animals had food and water available ad libitum, except for 2 h food deprivation prior to habituation or testing. Animals were habituated to the experimenter in daily handling sessions and acclimated to the testing environment and procedures to decrease stress during experimental testing. Testing occurred between 1200 and 1600 h, during the light-cycle when feeding baselines are relatively low, allowing for sensitive detection of drug-induced feeding effects. All experimental procedures and facilities were in accordance with NIH Guidelines and approved by the Institutional Animal Care and Use Committee of the University of Wisconsin–Madison.
Surgical procedures
Rats underwent stereotaxic surgery under isofluorane anesthesia following procedures previously described [31]. Two pairs of stainless-steel guide cannulae were surgically implanted in each rat, aimed bilaterally at either IL and AI/DI, or PRL and AI/DI. Cannulae were implanted according to the coordinates shown in Table 1, with small differences between males and females to adjust for their slightly different body sizes. These small adjustments yielded placements in the same anatomical sites in both sexes. AI/DI cannulae were implanted straight (0° from vertical), while those targeting IL and PRL were implanted at an angle (19° and 23°, respectively, from vertical) to minimize damage to the medial cortical wall. For IL and AI/DI, cannulae were implanted 2.5 mm above injection site and, for PRL, 1 mm above injection site due to the more dorsal location of this site.
Microinfusion procedures and drugs
Intracerebral microinfusion procedures were conducted as described previously [31]. Rats were received injections of 0.5 µL at a rate of 0.32 µL/min with an extra minute allowed post-infusion to insure diffusion of the injectate into the tissue. Rats received infusions of either 0.9% saline or 30 ng/µL muscimol (Sigma-Aldrich) dissolved in 0.9% saline into either the IL or PRL. Simultaneously, they received injections into AI /DI of either 0.9% saline or 1.0 µg/µL DAMGO ([D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin; Bachem) dissolved in saline. All infusions were counterbalanced according to Latin square designs.
Behavior-observation procedure
Each rat was assigned a clear polycarbonate testing cage (9.5 in width x 17 in length x 8 in height) identical to their home cages, but with the bottoms replaced by wire-grid floors for food-spillage collection. Glass jars were affixed to the cage floors and filled with pre-weighed amounts of 45 mg sucrose pellets (BioServ). Water was available from overhead bottles. Males and females were never tested on the same day; all surfaces and cages were wiped with ethanol 24 h before testing took place. Rats were habituated to this testing environment in two 90 min sessions after mild (2 h) food deprivation with no infusions. Next, again under 2 h food deprivation, animals were acclimated to microinfusion procedures with sham injections (i.e., injectors lowered through the guide cannulae but nothing infused), then saline infusions on the following day. Both sham and saline injections were given 15 min prior to placement into the testing cages for a 90 min session.
On drug testing days, rats received drug or vehicle infusions into AI/DI, and also either IL or PRL, and were placed into the testing cages 15 min post-infusion for 90 min testing sessions. All testing sessions were separated from one another by one testing-free interim day.
Behavior was recorded with a digital camcorder during the 90 min sessions. An experimenter blind to treatment reviewed the digital files off-line and recorded number and length of bouts of spontaneous motor activity (cage crossings, rears), eating, and drinking, using an event recorder interfaced to a PC-based desktop computer. Several measures were derived from these data: mean eating or drinking bout duration (total # of bouts/total duration), global eating or drinking rate (total intake/total duration), and global eating or drinking efficiency (total intake/total # of bouts). To record the duration of a particular behavioral event, a timer (specific for that behavior) on the event recorder was started at the initiation of the behavior. The timer was switched off when the behavior was interrupted by a different behavior.
Histology
At the end of each experiment, rats were deeply anesthetized and perfused with 10% formalin in phosphate buffer. Brains were removed and stored in 10% formalin. Coronal sections (60 µm) were cut through the infusion site on a cryostat microtome, collected on slides, stained with Cresyl Violet, and subsequently reviewed to verify injection placements. Subjects with incorrect placements (4 out of 19) were not included in the study. Final group sizes were as follows: IL-AI/DI: males, N = 6; females, N = 4; PRL-AI/DI: males, N = 6; females, N = 3.
Statistical analyses
Data were analyzed in two-way ANOVAs (DAMGO x Muscimol). Note that the “DAMGO” factor represents the AI/DI treatment, and the “Muscimol” factor, the medial prefrontal treatment (either IL or PRL). Following significance in the ANOVAs, comparisons among individual means were conducted using Tukey’s HSD test. The level of statistical significance level was set at P < 0.05 for all experiments.
Results
Histological analyses
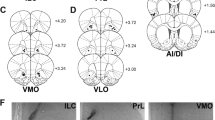
Chartings in Fig. 1A denote injector tip placements in mPFC (either IL or PRL, accordingly) and AI/DI (Fig. 1B) for all male and female rats included in this study. In Fig. 1C, representative photos are shown of injector tip views in Nissl (Cresyl violet)-stained tissue used for anatomical placement charting for each of the targeted sites: IL, PRL, and AI/DI. The inclusion of rats in the study was contingent on the absence of atypical or excessive damage and good tissue integrity (i.e., intact cells noted when viewed under high power) at the vicinity of the injector tip.
As indicated in the key, different symbols distinguish among IL males (N = 6), IL females (N = 4), PRL males (N = 7), and PRL females (N = 3). A Medial prefrontal cortex placements for all rats. B Agranular/dysgranular insula (AI/DI) placements for all rats. C Photomicrographs displaying injector tip placements. Abbreviations: AC Anterior commissure; AI/DI Agranular/dysgranular region of gustatory insula; CC Corpus callosum; E/OV Subependymal zone/olfactory ventricle; IL Infralimbic cortex; LV Lateral ventricle; Pir Piriform cortex; PRL Prelimbic cortex.
Intra-IL (but not intra-PRL) muscimol potentiated DAMGO-induced sucrose-pellet hyperphagia elicited from AI/DI
Statistical analysis of sucrose intake data (Fig. 2) yielded a main effect of DAMGO (IL-AI/DI experiment: F(1,9) = 57.22, P < 0.0001; PRL-AI/DI experiment: F(1,8) = 29.27, P = 0.0006) and, in the IL-AI/DI but not PRL-AI/DI experiment, both a muscimol main effect (IL: F(1,9) = 31.41, P = 0.0003) and a DAMGO x muscimol interaction (IL: F(1,9) = 14.11, P = 0.0045). Post-hoc comparisons among means revealed a robust increase in sucrose-pellet intake in rats given intra-AI/DI DAMGO plus intra-IL muscimol compared to either intra-AI/DI DAMGO or to any other treatment combination. Hence, there was a marked potentiation of intra-AI/DI DAMGO-driven sucrose intake by concomitant muscimol-induced inactivation of IL, but not PRL. There was no significant effect Sex or Sex interactions with the other factors for either the IL-AI/DI or PRL-AI/DI experiments (Fs = 0.052–0.71, NS). Correcting for male-female body weight differences did not alter these conclusions. Corrected sucrose intake data are shown in Supplementary Fig. S1.
Bar graphs depict sucrose intake after infusions of muscimol into either IL (open bars) or PRL (shaded bars) with concomitant DAMGO infusions into AI/DI. Small circles and squares denote individual data points for male and female rats, respectively, as indicated in the key. Insets show treatments schematically illustrated on line drawings of coronal rat brain sections. *P < 0.05, different than vehicle-vehicle control; #P < 0.05, different than muscimol-vehicle; ‡P < 0.05, different from all other treatments. Error bars depict ±1 SEM. Abbreviations: DAM DAMGO; IL Infralimbic cortex; PRL Prelimbic cortex.
IL inactivation enhanced specific elements of the feeding response elicited by intra-AI/DI DAMGO
Microanalysis of feeding behavior revealed potentiation of various intra-AI/DI-DAMGO-driven feeding metrics by simultaneous inactivation of IL. In the IL-AI/DI group, ANOVA revealed a statistically significant interaction between IL muscimol and AI/DI-DAMGO on total eating duration (F(1,9) = 5.99, P = 0.037; Fig. 3A), mean eating bout duration (F(1,9) = 6.77, P = .029; Fig. 3C), and global eating efficiency (average amount of sucrose eaten per bout; (F(1,9) = 11.02, P = 0.0089, Fig. 3D). For each of those measures, post-hoc comparisons among means indicated that means associated with the DAMGO + muscimol groups were significantly different from those associated with DAMGO alone or with any other treatment combination. Interestingly, there were no significant effects on total feeding bouts in the IL-AI/DI experiment. Hence, the overall effect of IL inactivation was to prolong the feeding bouts engendered by intra-AI/DI DAMGO rather than to alter the number of bouts initiated.
Bar graphs depict eating duration (A), eating bouts (B), mean eating bout duration (C), and sucrose intake per bout (D), after infusions of muscimol into either IL (open bars) or PRL (shaded bars) with concomitant DAMGO infusions into AI/DI. Small circles and squares denote individual data points for male and female rats, respectively, as indicated in the key. Insets show treatments schematically illustrated on line drawings of coronal rat brain sections. *P < 0.05, different than vehicle-vehicle control; #P < 0.05, different than muscimol-vehicle; ‡P < 0.05, different from all other treatments. Error bars depict ±1 SEM. Abbreviations: DAM DAMGO; IL Infralimbic cortex; PRL Prelimbic cortex.
For the PRL group, ANOVA revealed a main effect of DAMGO on total eating duration (F(1,8) = 13.20, P = 0.0067, Fig. 3A) with post-hoc analysis confirming the mean associated with intra-AI/DI DAMGO, but not that associated with DAMGO + muscimol, to be significantly higher than the saline + saline and saline + muscimol conditions. In the PRL-AI/DI experiment, there were no other significant effects, and no DAMGO x muscimol interactions, for either mean eating bout duration (Fig. 3C) or global eating efficiency (Fig. 3D). Stimulation of µ-ORs in the PRL-AI/DI experiment yielded a slight increase in feeding duration and number of eating bouts initiated (Fig. 3B), as indicated by a significant main effect of DAMGO (F(1,8) = 7.43, P = 0.026). Post-hoc means comparisons did not resolve differences between the DAMGO + saline and DAMGO + muscimol means; however, DAMGO + saline but not the DAMGO + muscimol mean differed from the saline+saline and saline+muscimol means.
Importantly, intra-IL or intra-PRL muscimol alone (i.e., without concomitant intra-AI/DI DAMGO) never differed from vehicle control for any measure. There were no statistically significant effects of sex for any measure in the microanalysis of feeding (Fs = 0–0.42, NS).
Muscimol infusions reduced non-food-directed motor activity equivalently in IL and PRL
Two measures of general activity were analyzed: rearing (Fig. 4A), and cage crosses (Fig. 4B). Analysis of rearing in both IL-AI/DI and PRL-AI/DI experiments revealed only a main effect of muscimol (F(1,9) = 16.63, P = 0.0028; F(1,8) = 9.14, P = 0.017, respectively). Post-hoc comparison of means revealed that intra-IL muscimol reduced rearing similarly under intra-AI/DI saline and DAMGO conditions. In the PRL-AI/DI experiment, there was an overall lower level of rearing relative to the IL-AI/DI experiment; nevertheless, muscimol still mildly diminished rearing in the PRL experiment (see Fig. 4 for detailed means comparisons). Similarly, muscimol mildly suppressed cage crosses, an effect that manifested as a diminished response in the DAMGO + muscimol condition relative to the DAMGO + saline condition (DAMGO x Muscimol interactions: for IL-AI/DI, F(1,9) = 13.09, P = 0.0056; for PRL-AI/DI, F(1,8) = 11.69, P = 0.0091). Post-hoc comparisons are detailed in Fig. 4. There were no statistically significant effects of sex on non-food-directed activity (Fs = 0.17–2.29, NS).
Bar graphs depict effects on two measures of motor activity, rears (A) and locomotion (cage crosses (B)) after infusions of muscimol into either IL (open bars) or PRL (shaded bars) with concomitant DAMGO infusions into AI/DI. Small circles and squares denote individual data points for male and female rats, respectively, as indicated in the key. Insets show treatments schematically illustrated on line drawings of coronal rat brain sections. *different than vehicle-vehicle control, †different than vehicle-DAMGO. Error bars depict ±1 SEM. Abbreviations: DAM DAMGO; IL Infralimbic cortex; PRL Prelimbic cortex.
Effects of mPFC inactivation on drinking behavior in males vs. females
Although this study was not specifically designed or powered to identify sex differences, both male and female rats were included as per NIH requirements. This was done to identify possible male/female dissociations that could inform future investigations beyond the scope of the present study. Of all the measures in this study, we only noted male/female separation in measures of drinking. DAMGO-induced water intake, drinking bouts, and total drinking duration were increased to a greater degree in females than males (data shown in Supplementary Table S1). On an exploratory basis, 3-factor ANOVAs (DAMGO X Muscimol X Sex) were carried out. These analyses revealed a three-way interaction of DAMGO x Muscimol x Sex (drinking bouts: F(1,8) = 9.01, P = 0.017; total drinking duration: F(1,8) = 7.36, P = 0.027) in the IL/AI/DI experiment. In the PRL-AI/DI experiment, a main effect of Sex was found for water intake (PRL-AI/DI: F(1,7) = 7.61, P = .028), drinking bouts (F(1,7) = 7.79, P = 0.027), and total drinking duration (F(1,7) = 11.20, P = 0.012), but there was no interaction of Sex with either the DAMGO or Muscimol factors. These results seem to indicate a possible intrinsic sex difference in cortical µ-opioid regulation of drinking behavior that could be explored in future studies.
Discussion
The present results revealed contrasting roles of IL and PRL in modulating a strong feeding drive produced by µ-OR stimulation in the gustatory insula. Intra-AI/DI infusions of the µ-OR-selective agonist, DAMGO, engendered sucrose hyperphagia characterized mainly by long, sustained eating bouts [24]. Inactivation of IL with muscimol (which silences electrophysiological activity in the vicinity of the infusion [32, 33]) markedly potentiated sucrose feeding duration, leading to a robust increase in sucrose consumption beyond the already-heightened levels elicited by DAMGO. In contrast, intra-PRL muscimol infusions neither significantly potentiated nor antagonized intra-AI/DI DAMGO-driven feeding measures but attenuated them to the extent that they no longer significantly differed from vehicle baseline. In both IL and PRL, muscimol modestly suppressed general activity (ambulation, rearing) as previously observed [30]. However, these effects were dissociable from alterations in feeding behavior; different feeding metrics were affected in IL and PRL, and in different directions, even though rearing and ambulation changes were similar in both sites. Muscimol alone (i.e., without intra-AI/DI DAMGO) did not alter baseline sucrose intake in either IL or PRL, indicating that the present results were due to modulation of activity associated with the AI/DI stimulus, rather than representing the simple summation of independent effects. Together, these findings demonstrate that neural activity in IL constrains, while PRL activity modestly enables, consummatory responses elicited directly from the gustatory insula. Possible behavioral and anatomical mechanisms underlying these findings are discussed below.
The present results reveal significant functional heterogeneity along the dorsal-ventral axis of mPFC in the regulation of eating elicited from the gustatory insula. The main effect of IL inactivation was to amplify AI/DI-driven behaviors occurring during commerce with the sucrose goal. These effects indicate that, when unperturbed, IL inhibits consummatory responses driven by the gustatory insula, a result that was not necessarily predictable from prior findings. The rostrocaudal level of AI/DI targeted in this study comprises the primary gustatory cortex (between + 1.56 mm and +2.52 mm anterior to bregma) where multimodal taste information and palatability are encoded [28, 34,35,36]. This insular site has reciprocal cortico-cortical connections with IL both directly and indirectly via orbitofrontal cortex [25,26,27]; electrophysiological recordings in AI/DI and mPFC (including IL) show neuronal responses to tastants in AI/DI and, with a slight lag, in mPFC [28], suggesting an AI/DI-to-mPFC flow of information [28]. These responses highlight the relevance of AI/DI projections, as IL is not directly innervated by any other node of the gustatory pathway [28]. Hence, IL could conceivably function as a motor effector of palatability computations arising in the gustatory insula. Accordingly, prior studies employing local µ-OR stimulation (which is hypothesized to indiscriminately disinhibit IL output [29]) indicate that IL is capable of generating a “fragmented” feeding response consisting of numerous, brief feeding bouts interrupted by intense bursts of non-food-directed hyperactivity [19, 24]. These divergent responses are subserved by separate terminal fields: feeding elicited from IL depends on intact glutamate transmission in LH, while hyperactivity is modulated by glutamate transmission in the nucleus accumbens shell (AcbSh) [20, 29]. IL projects strongly to both sites [37,38,39,40,41]; in fact, an extensive analysis of mPFC efferent circuitry indicated that ventral striatum and LH represent by far the densest terminal fields of IL-layer 5 [37]. Hence, IL can function to either drive or limit feeding behavior based on the proportionate recruitment of terminal fields in the AcbSh or lateral hypothalamus (LH), respectively. The present results suggest that, in the context of feeding responses elicited from gustatory insula, the “limiter” function of IL predominates.
The question then arises as to the specific behavioral process under inhibitory control. For example, IL activity could constrain taste/palatability computations occurring in AI/DI. This could occur either through a reciprocal projection back to AI/DI [25] or to a downstream terminal field that converges with AI/DI output [26, 38, 40]. Interestingly, units in mPFC are biased toward responding more strongly to aversive tastants [28], and catecholamine depletion or excitotoxic lesions in the vicinity of IL augment hedonic taste reactions and eliminate the few aversive reactions elicited by sucrose [42]. Hence, inactivating IL could have removed a countervailing influence on palatability encoding in AI/DI, resulting in the feeding enhancement seen here. On the other hand, intra-IL muscimol alone (i.e., combined with intra-AI/DI vehicle infusions) failed to alter any measure of sucrose ingestion, and we have previously shown that muscimol-inactivation of IL does not alter absolute intake of either a highly palatable chocolate shake or of standard chow in hungry rats [30]. Thus, if IL inactivation indirectly enhances gustatory reward sufficiently to increase intake, the effect must be limited to specific eliciting conditions, such as µ-OR signaling in AI/DI. Interestingly, however, a recent DAMGO mapping study did not detect hedonic taste enhancement after µ-OR stimulation in that insular region [22].
Alternatively, IL may perform a “supervisory” motoric or action-selection function over feeding behaviors elicited from AI/DI, such as monitoring ongoing consummatory bouts and enforcing a limit upon their duration, and/or arbitrating the expression of competing consummatory and non-consummatory repertoires. Our prior work demonstrated that IL inactivation increased mean feeding bout duration in both hunger- and palatability-driven feeding (but without increasing intake) [30], in agreement with the present observation that the main effect of IL inactivation was to prolong feeding bouts engendered by intra-AI/DI DAMGO. Hence, feeding bout prolongation after IL inactivation appears to be a generalized phenomenon. Under the unusually strong feeding drive seen with µ-OR stimulation of AI/DI, removing the inhibitory control of IL over consummatory bouts could translate into increased sucrose intake, as seen here. Importantly, the “palatability modulation” vs. “action monitoring” accounts described above are not necessarily mutually exclusive; it is possible that a combination of both mechanisms contributed to the results seen here. More work needs to be done to resolve this question. Nevertheless, the present findings clearly identify a net inhibitory influence of IL over feeding responses elicited from the gustatory insula.
In contrast to IL, inactivation of PRL mildly blunted the increases in feeding bout initiation, sucrose intake, and feeding duration produced by intra-AI/DI DAMGO. These results suggest that PRL (at least the level studied here) may play a supporting role in generating bout initiation and maintaining ingestive bouts driven by the gustatory insula, in contrast to the robust negative modulation of consummatory responses mediated by IL. A series of detailed studies examined cortical control of sustained sucrose licking responses in an incentive-contrast task [43,44,45]; these studies identified loci in PRL where muscimol-inactivation or optogenetic inhibition impaired sustained licking to a high-incentive sucrose solution [43] and where sustained spiking and theta-band phase-locking were strongly associated with bouts of consummatory licking [43, 45]. Interestingly, these sites were found in the anterior PRL and rostral cortical pole but not more caudally [43], suggesting a longitudinal gradient of feeding control along the anteroposterior extent of PRL. Integrating the findings from intra-IL and intra-PRL manipulations, it could be hypothesized that there are longitudinally oriented dorsal and ventral “columns” in mPFC, both having access to palatability encoding in the gustatory insula [28]. Each column uses this information in different ways to regulate sustained consummatory activity: either to maintain it (rostral pole and anterior PRL; [43]), or to curtail/interrupt it with competing motor repertoires (IL; [20, 24, 29, 46]).
Presently, the anatomical basis for the contrasting roles of IL vs. PRL in modulating AI/DI-driven feeding responses is unclear. One obvious route of control would be through direct projections to AI/DI. Both IL and PRL both receive and reciprocate projections to AI/DI (with some evidence for slightly denser connections with anterior PRL) [25, 27, 38, 47], and, as discussed above, both mPFC subregions display neuronal responses associated with palatability-related AI/DI activity [28]. It is unclear how these similar direct cortico-cortical projections to AI/DI would translate into the contrasting modes of functional regulation seen here. Further downstream, however, PRL and IL terminal fields diverge in feeding-regulatory zones of the Acb [6, 39, 48,49,50]. Both IL and PRL project to the Acb, but with distinct topographic distributions [37,38,39,40, 51]. A highly circumscribed region of the anteromedial AcbSh plays a unique role feeding control [52, 53]; this AcbSh site is innervated by IL and strongly modulates a feeding-regulatory zone of the lateral hypothalamus (LH) [54, 55]. Stimulating this AcbSh subregion pharmacologically or electrically “arrests” feeding [56, 57], while inhibiting this subregion generates sustained consummatory bouts via activation of the LH [52, 53, 58]. Our prior work showed that functional interactions between IL and AcbSh delimit feeding and sucrose-reinforced operant responding [20]. Relatedly, chemogenetic stimulation of IL-AcbSh projections decreased bingeing on palatable foods [59], and optogenetic activation of mPFC glutamatergic synapses in AcbSh produced transient cessation in sucrose licking in mice [43]. Hence, it could be hypothesized that the “limiter” function of IL is mediated by glutamatergic signaling to anteromedial AcbSh, which negatively modulates feeding via control of the LH.
Conclusions and future directions
The present results suggest that the strong feeding-motivation state generated by µ-OR signaling in AI/DI actuates distinct pathways that drive and limit ingestive behaviors, in a manner reminiscent of a system in which output is constrained by a governor mechanism. The schematic in Fig. 5 incorporates the pathways discussed above to illustrate potential forebrain circuitry that could actualize such a control-systems function. This model posits that µ-OR signaling in AI/DI engages feeding “drivers”, including projections to PRL, that ultimately impinge on feeding generators in the LH. A copy of the intra-AI/DI µ-OR signaling event travels along parallel circuitry that engages a feeding “limiter” pathway through IL to the medial AcbSh and finally the LH [49, 55,56,57, 59, 60]. This IL→AcbSh→LH pathway subserves a “system-governor” function, and can be engaged by feed-forward AI/DI projections to IL, either directly [25] or via a relay in the anterior insula (aI)) [26]). Additional routes of control are possible. For example, certain midline thalamic nuclei (particularly the paraventricular thalamic nucleus (PVT)) are well-positioned to serve as an interface between the LH and telencephalon [61,62,63,64,65,66]. The PVT robustly innervates both IL and AcbSh [27, 67, 68]. Functional studies have demonstrated that PVT plays a complex role in feeding control, mediating the interplay among feeding, stress, arousal, and circadian states [61,62,63, 66, 69]; and arbitrating trade-offs in food approach/avoidance conflicts [62]. Additional evidence suggests that the PVT exerts tonic restraint over feeding that can be amplified in conflict situations [70, 71], and that inhibition of the PVT results in binge-like eating [71, 72]. Hence, the PVT is a strong candidate to participate in a feedback loop that monitors ongoing activity in LH and conveys it to IL and AcbSh. Regardless of the precise circuitry, however, the general concept of a dual-pathway feeding “driver/limiter” system accounts for both the amplification of AI/DI-driven feeding by IL inactivation and the lack of intra-IL muscimol effects absent an initiating neurochemical event in AI/DI. In other words, the “governor” function of IL may represent an adaptive control mechanism that is engaged in the context of strong feeding drives and intense consummatory activity, as putatively mediated by a strong µ-OR signal in gustatory insula. There is considerable evidence from human neuroimaging studies for insular involvement in eating disorders [73,74,75,76,77], and, interestingly, for µ-OR changes in insular cortex in both bulimia nervosa and obesity [78, 79]. The present model would predict that µ-OR signaling events in the insula, coupled with deficient IL activity, could tip already-intense feeding drives into pathological bingeing behavior. Conversely, supernormal activity in the IL “limiter circuit” could override normal feeding drives, potentially resulting in anorexia. Hence, the present results may help provide a context for understanding the clinical correlates of localized cortical abnormalities in anorexia nervosa, bulimia, and other psychiatric disorders characterized by dysfunctional appetitive behavior.
The model proposes that an intra-AI/DI µ-OR signaling event activates both “feeding driver” pathways (green arrows), and a “limiter” or “governor” pathway (red arrows). These pathways ultimately integrate with feeding generators in the lateral hypothalamus (LH). Potential feeding “drivers” emanating from AI/DI include the basolateral complex (BLA) and central (CeA) divisions of the amygdala that could serve as a relay between AI/DI and the LH [26, 47, 80,81,82,83], and direct projections from AI/DI to the LH [40, 41, 47, 82]. Also included is the direct projection from AI/DI to PRL and IL [25, 47], which could reach LH feeding generators [38, 41] as well as nucleus accumbens (Acb)-based systems for appetitive/anticipatory behavior [39, 84]. The putative “limiter/governor” pathway involves IL-mediated activation of the medial nucleus accumbens shell (mAcbSh), which in turn projects to the LH and inhibits feeding [55,56,57, 60]. The AI/DI can recruit this IL → mAcbSh → LH pathway via a direct AI/DI → IL projection [25, 47]; a relay in the anterior insula (aI) [26]; or a feedback loop linking the LH to IL through the midline thalamus [61, 66, 68]. See further explanation in the Discussion.
References
Malvaez M, Shieh C, Murphy MD, Greenfield VY, Wassum KM. Distinct cortical-amygdala projections drive reward value encoding and retrieval. Nat Neurosci. 2019;22:762–69.
Mahler SV, Berridge KC. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci. 2009;29:6500–13.
Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86:646–64.
Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacol. 2007;191:461–82.
Caref K, Nicola SM. Endogenous opioids in the nucleus accumbens promote approach to high-fat food in the absence of caloric need. Elife. 2018;7:e34955.
Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–95.
Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–77.
Olszewski PK, Alsio J, Schioth HB, Levine AS. Opioids as facilitators of feeding: can any food be rewarding? Physiol Behav. 2011;104:105–10.
Mucha RF, Iversen SD. Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res. 1986;397:214–24.
Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharm Exp Ther. 1993;265:1253–60.
Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ. An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1055–65.
Kotz CM, Billington CJ, Levine AS. Opioids in the nucleus of the solitary tract are involved in feeding in the rat. Am J Physiol. 1997;272:R1028–32.
Kim EM, Quinn JG, Levine AS, O’Hare E. A bi-directional mu-opioid-opioid connection between the nucleus of the accumbens shell and the central nucleus of the amygdala in the rat. Brain Res. 2004;1029:135–9.
Woods JS, Leibowitz SF. Hypothalamic sites sensitive to morphine and naloxone: effects on feeding behavior. Pharm Biochem Behav. 1985;23:431–8.
Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–77.
Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–11.
Stratford TR, Wirtshafter D. Effects of muscimol, amphetamine, and DAMGO injected into the nucleus accumbens shell on food-reinforced lever pressing by undeprived rats. Pharm Biochem Behav. 2012;101:499–503.
Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–86.
Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by mu-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci. 2011;31:3249–60.
Mena JD, Selleck RA, Baldo BA. Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci. 2013;33:18540–52.
Selleck RA, Lake C, Estrada V, Riederer J, Andrzejewski M, Sadeghian K, et al. Endogenous opioid signaling in the medial prefrontal cortex is required for the expression of hunger-induced impulsive action. Neuropsychopharmacology. 2015;40:2464–74.
Castro DC, Berridge KC. Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc Natl Acad Sci USA. 2017;114:E9125–E34.
Selleck RA, Giacomini J, Buchholtz BD, Lake C, Sadeghian K, Baldo BA. Modulation of appetitive motivation by prefrontal cortical mu-opioid receptors is dependent upon local dopamine D1 receptor signaling. Neuropharmacology. 2018;140:302–09.
Giacomini JL, Geiduschek E, Selleck RA, Sadeghian K, Baldo BA. Dissociable control of mu-opioid-driven hyperphagia vs. food impulsivity across subregions of medial prefrontal, orbitofrontal, and insular cortex. Neuropsychopharmacology. 2021;46:1981–9.
Gabbott PL, Warner TA, Jays PR, Bacon SJ. Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res. 2003;993:59–71.
Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399:440–68.
Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79.
Jezzini A, Mazzucato L, La Camera G, Fontanini A. Processing of hedonic and chemosensory features of taste in medial prefrontal and insular networks. J Neurosci. 2013;33:18966–78.
Baldo BA. Prefrontal cortical opioids and dysregulated motivation: a network hypothesis. Trends Neurosci. 2016;39:366–77.
Baldo BA, Spencer RC, Sadeghian K, Mena JD. GABA-mediated inactivation of medial prefrontal and agranular insular cortex in the rat: contrasting effects on hunger- and palatability-driven feeding. Neuropsychopharmacology. 2016;41:960–70.
Newman S, Pascal L, Sadeghian K, Baldo BA. Sweetened-fat intake sensitizes gamma-aminobutyric acid-mediated feeding responses elicited from the nucleus accumbens shell. Biol Psychiatry. 2013;73:843–50.
Pezze M, McGarrity S, Mason R, Fone KC, Bast T. Too little and too much: hypoactivation and disinhibition of medial prefrontal cortex cause attentional deficits. J Neurosci. 2014;34:7931–46.
van Duuren E, van der Plasse G, van der Blom R, Joosten RN, Mulder AB, Pennartz CM, et al. Pharmacological manipulation of neuronal ensemble activity by reverse microdialysis in freely moving rats: a comparative study of the effects of tetrodotoxin, lidocaine, and muscimol. J Pharm Exp Ther. 2007;323:61–9.
Katz DB, Simon SA, Nicolelis MA. Taste-specific neuronal ensembles in the gustatory cortex of awake rats. J Neurosci. 2002;22:1850–7.
Sadacca BF, Mukherjee N, Vladusich T, Li JX, Katz DB, Miller P. The behavioral relevance of cortical neural ensemble responses emerges suddenly. J Neurosci. 2016;36:655–69.
Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. II. Thalamocortical projections. Brain Res. 1986;379:342–52.
Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–77.
Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58.
Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–47.
Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–28.
Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Brain Res Rev. 1997;24:197–254.
Berta B, Kertes E, Peczely L, Ollmann T, Laszlo K, Galosi R, et al. Ventromedial prefrontal cortex is involved in preference and hedonic evaluation of tastes. Behav Brain Res. 2019;367:149–57.
Parent MA, Amarante LM, Liu B, Weikum D, Laubach M. The medial prefrontal cortex is crucial for the maintenance of persistent licking and the expression of incentive contrast. Front Integr Neurosci. 2015;9:23.
Amarante LM, Caetano MS, Laubach M. Medial frontal theta is entrained to rewarded actions. J Neurosci. 2017;37:10757–69.
Horst NK, Laubach M. Reward-related activity in the medial prefrontal cortex is driven by consumption. Front Neurosci. 2013;7:56.
Selleck RA, Baldo BA. Feeding-modulatory effects of mu-opioids in the medial prefrontal cortex: a review of recent findings and comparison to opioid actions in the nucleus accumbens. Psychopharmacol. 2017;234:1439–49.
Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–74.
Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–88.
Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–8.
Duva MA, Tomkins EM, Moranda LM, Kaplan R, Sukhaseum A, Bernardo JP, et al. Regional differences in feeding and other behaviors elicited by N-methyl-D-aspartic acid in the rodent hypothalamus: a reverse microdialysis mapping study. Brain Res. 2002;925:141–7.
Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN. Circuit-based corticostriatal homologies between rat and primate. Biol Psychiatry. 2016;80:509–21.
Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–36.
Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–70.
Zahm DS, Parsley KP, Schwartz ZM, Cheng AY. On lateral septum-like characteristics of outputs from the accumbal hedonic “hotspot” of Pecina and Berridge with commentary on the transitional nature of basal forebrain “boundaries”. J Comp Neurol. 2013;521:50–68.
Thompson RH, Swanson LW. Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci USA. 2010;107:15235–9.
Stratford TR, Swanson CJ, Kelley A. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res. 1998;93:43–50.
Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci. 2010;30:4746–56.
Urstadt KR, Coop SH, Banuelos BD, Stanley BG. Behaviorally specific versus non-specific suppression of accumbens shell-mediated feeding by ipsilateral versus bilateral inhibition of the lateral hypothalamus. Behav Brain Res. 2013;257:230–41.
Anastasio NC, Stutz SJ, Price AE, Davis-Reyes BD, Sholler DJ, Ferguson SM, et al. Convergent neural connectivity in motor impulsivity and high-fat food binge-like eating in male Sprague-Dawley rats. Neuropsychopharmacology. 2019;44:1752–61.
O’Connor EC, Kremer Y, Lefort S, Harada M, Pascoli V, Rohner C, et al. Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron. 2015;88:553–64.
Hsu DT, Kirouac GJ, Zubieta JK, Bhatnagar S. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front Behav Neurosci. 2014;8:73.
Kirouac GJ. The paraventricular nucleus of the thalamus as an integrating and relay node in the brain anxiety network. Front Behav Neurosci. 2021;15:627633.
Iglesias AG, Flagel SB. The paraventricular thalamus as a critical node of motivated behavior via the hypothalamic-thalamic-striatal circuit. Front Integr Neurosci. 2021;15:706713.
McNally GP. Motivational competition and the paraventricular thalamus. Neurosci Biobehav Rev. 2021;125:193–207.
Petrovich GD. The function of paraventricular thalamic circuitry in adaptive control of feeding behavior. Front Behav Neurosci. 2021;15:671096.
Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85.
Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508:212–37.
Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102.
Mendoza J, Angeles-Castellanos M, Escobar C. A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur J Neurosci. 2005;22:2855–62.
Engelke DS, Zhang XO, O’Malley JJ, Fernandez-Leon JA, Li S, Kirouac GJ, et al. A hypothalamic-thalamostriatal circuit that controls approach-avoidance conflict in rats. Nat Commun. 2021;12:2517.
Stratford TR, Wirtshafter D. Injections of muscimol into the paraventricular thalamic nucleus, but not mediodorsal thalamic nuclei, induce feeding in rats. Brain Res. 2013;1490:128–33.
Zhang X, van den Pol AN. Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science. 2017;356:853–59.
Alfano V, Mele G, Cotugno A, Longarzo M. Multimodal neuroimaging in anorexia nervosa. J Neurosci Res. 2020;98:2178–207.
Donnelly B, Touyz S, Hay P, Burton A, Russell J, Caterson I. Neuroimaging in bulimia nervosa and binge eating disorder: a systematic review. J Eat Disord. 2018;6:3.
Kaye WH, Wagner A, Fudge JL, Paulus M. Neurocircuity of eating disorders. Curr Top Behav Neurosci. 2011;6:37–57.
Frank GK, Shott ME, Hagman JO, Mittal VA. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. 2013;170:1152–60.
Shott ME, Pryor TL, Yang TT, Frank GK. Greater insula white matter fiber connectivity in women recovered from anorexia nervosa. Neuropsychopharmacology. 2016;41:498–507.
Bencherif B, Guarda AS, Colantuoni C, Ravert HT, Dannals RF, Frost JJ. Regional mu-opioid receptor binding in insular cortex is decreased in bulimia nervosa and correlates inversely with fasting behavior. J Nucl Med. 2005;46:1349–51.
Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, et al. Obesity is associated with decreased mu-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. 2015;35:3959–65.
Schiff HC, Bouhuis AL, Yu K, Penzo MA, Li H, He M, et al. An insula-central amygdala circuit for guiding tastant-reinforced choice behavior. J Neurosci. 2018;38:1418–29.
Ponserre M, Peters C, Fermani F, Conzelmann KK, Klein R. The insula cortex contacts distinct output streams of the central amygdala. J Neurosci. 2020;40:8870–82.
Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311:1–16.
Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1996;374:387–420.
Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacol. 2007;191:439–59.
Acknowledgements
The authors thank Julio Diaz, Alexius Lampkin, and Kate Dunaway for assistance with general laboratory tasks. This work was supported by NIH grant R01 MH074723 from the National Institute for Mental Health.
Author information
Authors and Affiliations
Contributions
JLG: writing: original draft, investigation, formal analysis, data curation, visualization; KS: investigation; BAB: conceptualization, supervision & project administration, writing: review & editing, formal analysis, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Giacomini, J.L., Sadeghian, K. & Baldo, B.A. Eating driven by the gustatory insula: contrasting regulation by infralimbic vs. prelimbic cortices. Neuropsychopharmacol. 47, 1358–1366 (2022). https://doi.org/10.1038/s41386-022-01276-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01276-7
This article is cited by
-
Recruitment of hippocampal and thalamic pathways to the central amygdala in the control of feeding behavior under novelty
Brain Structure and Function (2024)
-
Delayed estrogen actions diminish food consumption without changing food approach, motor activity, or hypothalamic activation elicited by corticostriatal µ-opioid signaling
Neuropsychopharmacology (2023)
-
Sex differences in activation of extra-hypothalamic forebrain areas during hedonic eating
Brain Structure and Function (2022)