Abstract
This study is the first randomized controlled trial to test the effects of ketamine in Borderline Personality Disorder (BPD). BPD remains undertreated in the community and no medication has FDA approval for this indication. People with BPD experience chronic mood disturbances with depressed mood, suicidal ideation, and severe social difficulties. In this double-blind, randomized controlled pilot study, we tested the effects of one infusion of ketamine (0.5 mg/kg, n = 10) or the psychoactive comparator drug midazolam (0.04 mg/kg, n = 12) in adults with BPD. Infusions were well tolerated in both groups. Dissociative symptoms during infusion were more intense with ketamine than midazolam (t(12.3) = 3.61, p = 0.01), but they resolved by 40 min after infusion in both groups. Post-infusion adverse events were at the expected low levels in both groups. For our primary outcome measure of suicidal ideation and our secondary outcome measure of depression, we found numerical reduction but not significant group or group x timepoint difference (p > 0.05). For our secondary outcome measures of anxiety and BPD symptoms, we did not observe group or group x timepoint differences. There was a group x timepoint effect for socio-occupational functioning (F(1,20.12) = 5.16, p = 0.03, at Day 14, ketamine group showed more improvement than midazolam group). An exploratory analysis revealed that improvement in socio-occupational functioning was correlated with improvement in depression in the ketamine group (r(8) = 0.65, p = 0.04) but not midazolam group (r(9) = 0.41, p = 0.216). This pilot study provides the first randomized controlled evidence of the effects of antidepressant-dosed ketamine in people with BPD. Our results provide reason for optimism that antidepressant-dosed ketamine will be well-tolerated in larger studies and may provide clinical benefit for mood symptoms and related impairments in people with BPD.
Similar content being viewed by others
Introduction
Borderline Personality Disorder (BPD) is a common psychiatric condition with no FDA-approved treatment [1]. People with BPD experience profound mood disturbances, suicidal ideation, and social difficulties, and they comprise up to 40% of high utilizers of psychiatric services [2]. As many as 10% of people with BPD complete suicide [3]. Evidence-based psychotherapies (usually 6–12 months long) are the mainstay of treatment for BPD, and they support improvements in BPD symptoms and psychological well-being [4, 5]. However, currently available treatments for BPD leave an unmet need for rapid substantial symptom reduction.
As evidence has grown for the use of ketamine as a rapid-acting treatment for depression and suicidal ideation [6], and ketamine treatment has become widely available, anecdotal reports have suggested benefit for people with BPD [7]. A post-hoc analysis of ketamine-treated patients with treatment-resistant depression showed similar decreases in depression and suicidal ideation at 24 h post-infusion in people with high BPD traits compared to those with low BPD traits [8]. However, no randomized study has been conducted to test the tolerability or efficacy of ketamine in people with BPD.
Evidence supports the use of ketamine for treatment-resistant symptoms of depression [6]. Antidepressant medications have been far less effective in BPD than in Major Depressive Disorder (MDD), even in the case of BPD with co-morbid MDD [5]. When ketamine has been tested for mental health problems other than MDD, results have been mixed about its ability to improve symptoms other than depression and suicidal ideation [9,10,11,12]. Given that symptoms of BPD include transient psychotic-like experiences and dissociative states, both of which can be induced by depression-dosed ketamine, people with BPD could be at greater risk of poorly tolerating ketamine. Therefore, it is not clear that the net benefits of ketamine for other patient groups will extend to those with BPD. Systematically-collected evidence about the efficacy and safety of ketamine in this population is needed.
This pilot study’s primary aim was to collect initial evidence of whether ketamine is well-tolerated in people with BPD and thus a potential treatment option worth further examination. We conducted preliminary analyses examining effects of ketamine on suicidal ideation in people with BPD as the primary outcome of interest, as well as examining effects of ketamine on mood symptoms, core symptoms of BPD, and socio-occupational functioning.
To explore these effects of ketamine treatment in BPD, we conducted a double-blind randomized controlled pilot study. Adults with BPD and suicidal ideation were randomized to one intravenous dose of either ketamine or psychoactive comparator (midazolam) and followed for four weeks. We selected midazolam as a psychoactive comparator in line with other studies of ketamine in psychiatric populations [13]. We assessed safety and tolerability in ten ketamine- and twelve midazolam-treated participants. We then measured suicidal ideation, mood symptoms, BPD symptoms, and socio-occupational functioning.
Methods
Ethics
This study was conducted according to a protocol reviewed and approved by the Yale University Human Investigation Committee (protocol # 2000021457). Informed consent was conducted according to standard procedures, including assessment of capacity to provide consent.
Participants
Participants were recruited from the community via advertisements and clinical referrals. Inclusion criteria were age 21–60 and having a current mental health treater, current suicidal ideation, and current Borderline Personality Disorder (see assessments below). Exclusion criteria included lifetime diagnosis of psychotic disorder for the participant or for a first-degree relative, lifetime history of NMDA antagonist (ketamine or dextromethorphan) or opiate abuse, history of major medical illness, current acute risk of suicide, current moderate or severe substance use disorder, and day-of-infusion positive urine test for either drugs of abuse or pregnancy. We excluded people exposed to ketamine in the past year. Given evidence suggesting possible synergistic response of lithium and lamotrigine with ketamine, and possible interference of response with topiramate [14,15,16,17], we also excluded people who were currently taking topiramate, lithium, or lamotrigine. Medications were held steady for four weeks prior to treatment and for the duration of the trial.
Participants were initially screened for inclusion/exclusion criteria by phone, then semi-structured interviews were conducted by trained raters in person or by videoconference. See Fig. 1 for consort diagram. We used the SCID-II personality questionnaire for BPD [18] to screen potential participants on the phone, and Diagnostic Interview for Personality Disorders [19] and Diagnostic Interview for BPD Revised [20] at interview to determine BPD diagnosis for inclusion. The Mini-International Neuropsychiatric Interview [21] was used to determine general psychopathology for inclusion/exclusion decisions (see co-morbidities in Table 1). Study enrollment occurred between May 2018 and March 2022.
Medication
Participants were randomized by the Yale Investigational Drug Service (IDS) to receive either 0.5 mg/kg ketamine or 0.04 mg/kg midazolam. Randomization was done at a 1:1 ketamine to midazolam ratio, with fixed block size. Medication was prepared by IDS; clinical staff and participants remained blinded. Medication was delivered over 40 min through an intravenous line. Blood samples were collected for pharmacokinetic analyses at 30 and 60 min after initiation of the infusion. Ketamine (30-min timepoint) and norketamine (60-min timepoint) serum concentrations were measured by the Columbia Biomarkers Core Lab.
Assessment procedures
Baseline measures were collected −7 to −1 days from infusion. Clinical raters were blinded to infusion medication and to intra-infusion experience. Self-report questionnaire scales were selected from the common and well-validated tools used to measure outcomes in recent ketamine trials and recent BPD trials. Infusion day measures of psychotic-like experience and dissociation were collected before the infusion was started, then at 20 min (mid-infusion), 40 min (as infusion stopped), and 80 min (40 min after infusion stop). Outcome measures for adverse events and clinical response were collected at seven timepoints: Baseline, Hour 1 (60 min after infusion stop), Day 1, Day 3, Day 7, Day 14, and Day 28 for each of the measures except for the ZAN-BPD-SRV, which was not measured at Hour 1 and the SAS-SR which was measured in two-week intervals (Baseline, Day 14, Day 28).
Measures of safety and tolerability
Adverse events
To assess for adverse events, raters asked participants about their well-being and we specifically queried common side effects of ketamine at each timepoint.
Dissociative experiences during infusion
To measure psychotomimetic and dissociative experiences during infusion, we used two scales: The Brief Psychiatric Rating Scale (BPRS) Positive Symptom subscale and the Clinician Administered Dissociative State Scale (CADSS). The BPRS Positive Symptom subscale has four items from the full BPRS (Unusual Thought Content, Conceptual Disorganization, Hallucinations, and Grandiosity) with subscale range 4 to 28 [22]. The CADSS has 23 items scored on a Likert scale from 0 to 4, increasing with symptom severity, and the full scale range is 0 to 92. [23].
Clinical assessments
Suicidal ideation
For suicidal ideation, we collected one measure with enough range to detect even subtle baseline ideation and also subsequent change: the Beck Scale for Suicide Ideation (BSS, 19 items, each scored on a 4-point Likert scale with full scale range 0–57 [24]).
Depression
For depression, we collected the Beck Depression Inventory (BDI) [25]. This 21-item self-report questionnaire scores each item on a 4-point Likert scale where higher scores indicate more depressive symptoms. It can detect a wide range of symptom levels (scores 0–63) and is sensitive to change.
Anxiety symptoms
To measure current anxiety, we used the Beck Anxiety Inventory, which has 21 items scored on a four point Likert for symptom intensity (scale range 0–63) [26].
BPD symptoms
To measure current BPD symptom severity, we used the Zanarini Scale for Borderline Personality Disorder self-report version, which has 9 items scored on a five point Likert for symptom intensity (ZAN-BPD-SRV, total scale range 0–36) [27]. The items correspond to DSM criteria.
Socio-occupational functioning
To measure problems with socio-occupational functioning, we collected Baseline, Day 14, and Day 28 reports on the Social Adjustment Scale - Self Report (SAS-SR) about the previous two weeks’ experience [28]. This scale includes 24 items, with branching logic (yes/no questions lead to Likert scales with 5- and 6-point Likert scales). Total scale range is 0–125 and higher scores indicate more socio-occupational problems.
Statistical approach and preregistration
This study was pre-registered on ClinicalTrials.gov (Identifier: NCT03395314). The analyses here are exploratory, fitting our pilot study and small final sample size. Statistics were implemented in R; code is available upon request.
For between-group comparison testing randomization effects, we used Students’ t-tests; for those comparing treatment effects with likely differences in population variances, we used Welch’s t-test. At baseline, there were no significant group differences in age, gender, race, education, or baseline clinical variables (Table 1).
Linear mixed models with random effect of individual and control for baseline value were used to test for ketamine vs. midazolam impacts on clinical scores from Baseline through the two weeks after infusion when symptoms reductions were expected. We tested for potential group x timepoint effects on suicidal ideation (BSS), depression (BDI), anxiety (BAI), BPD symptoms (ZAN-BPD-SRV), and socio-occupational functioning (SAS-SR). For the clinical outcomes (BSS, BDI, BAI, and ZAN-BPD-SRV), the analyses included symptoms at Baseline, Day 1, Day 3, Day 7, and Day 14. For socio-occupational functioning, the model tested Baseline compared to Day 14. These models were implemented using the lme4 package functions lmer and anova (to summarize main and interaction effects of lmer models) in R. Effect sizes were computed from these data. We ran one model per outcome measure as follows, where “clinical_score” was replaced with BSS, BDI, BAI, ZAN-BPD-SRV, or SAS for each model respectively.
The linear mixed models included all the available data (12 midazolam and 10 ketamine participants). Some data were missing for each of the clinical scales (BSS, BDI, BAI, ZAN-BPD-SRV, SAS-SR): Day 1, one midazolam participant; Day 3, two midazolam participants. For BAI, data were missing from one additional midazolam participant at Day 3. For SAS-SR, data from one midazolam participant was missing at Day 14.
In an exploratory analysis, we computed the Pearson correlation between BDI and SAS-SR Baseline to Day 14 change scores.
Results
Twenty-two participants were randomized. The ketamine (n = 10) and midazolam (n = 12) groups did not differ significantly on age, race, years of education, depression severity, or BPD severity (details in Methods, Table 1). Participants were predominantly white and female. Baseline depression severity scores were in the severe depression range [29]. Baseline suicidal ideation severity scores were in line with current ideators in previous studies using this scale [30]. Baseline BPD severity scores were in the range of those with severe impairment [31].
People with BPD tolerated the infusions well with no serious adverse events and similar low frequencies of expected adverse events in both groups (Table 2). Two participants in the study, both in the ketamine group, experienced acute distress and suicidal ideation in the fourth week after infusion leading to acute psychiatric evaluation. One was discharged after overnight evaluation in the emergency department; the other received further ketamine infusions as part of inpatient treatment.
Intra-infusion dissociative symptoms were transient in both groups (Fig. 2A) and did not correlate with peak change in depressive symptoms (Fig. 2B). We found significantly greater CADSS score at 20 min in the ketamine versus midazolam group (t(12.3) = 3.61, p = 0.01). The six ketamine-group participants with history of dissociative experiences had higher mid-infusion dissociative (CADSS, 2C) and positive psychotic symptom (BPRS positive, 2D) scale scores than the four ketamine-group participants without history of dissociation. However, these effects were transient: ketamine group participants with history of dissociative symptoms did not experience more prolonged dissociative experiences after ketamine infusion (Fig. 2C, D).
Intra-infusion dissociative symptoms were transient in both groups (A) and did not correlate with peak change in depressive symptoms (B). Ketamine-group participants with history of dissociative symptoms experienced more intense but not more prolonged dissociative experiences during the infusion (C CADSS total score) or positive symptoms of psychosis (D BPRS positive symptom scale). For A, C, and D, error bars represent standard error of the mean. For B, shaded regions represent 95% confidence intervals. Abbreviations: Clinician-Administered Dissociative States Scale (CADSS), minutes (min), Beck Depression Inventory (BDI), Day 3 timepoint (D3), Brief Psychiatric Rating Scale Positive Symptom subscale (BPRS Positive Sx).
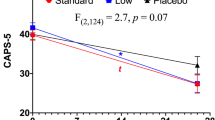
For the primary clinical outcome of suicidal ideation, we observed a numerical but not significant effect of group x timepoint, where mean post-infusion scores decreased more in the ketamine than midazolam group from Baseline to post-infusion timepoints (Fig. 3A). BSS scores decreased in both groups after infusion (main effect of timepoint, F(4, 78.28) = 9.30, p < 0.001), but there was not a statistically significant effect of group (F(1, 19.18) = 0.14, p = 0.71) or group x timepoint (F(6, 116.46) = 0.65, p = 0.69, ƞp = 0.03). While not significant, the timepoint at Day 1 had the greatest magnitude of group effect on change in suicidal ideation from baseline, with greater change for ketamine group (t(135.79) = 1.24, p = 0.22, Cohen’s d = 0.020794). Seven participants (two ketamine and five midazolam) had very low Baseline suicidal ideation severity scores (BSS less than 4). Sensitivity analysis after removing these participants did not change the results.
Change from baseline in suicidal ideation (BSS score), A depression (BDI score), B anxiety (BAI score), C BPD symptoms (ZAN-BPD-SRV), D, and socio-occupational functioning (SAS-SR score, E) from before (BL) to each timepoint after infusion. Error bars represent standard error of the mean. In F, we show correlation between socio-occupational functioning at Day 14 (when group difference was observed) and depression score. Shaded areas represent 95% confidence intervals. For all panels, solid black lines with solid dots indicate ketamine group results, and dashed line with open triangles indicate midazolam group results. Abbreviations: Beck Scale for Suicide Ideation (BSS), Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI), Zanarini Scale for Borderline Personality Disorder Self Report Version (ZAN_BPD_SRV), Borderline Personality Disorder (BPD), Social Adjustment Scale–Self Report (SAS-SR), Baseline (BL), 1 h (1HR), Day 1 (D1), Day 3 (D3), Day 7 (D7), Day 14 (D14), Day 28 (D28).
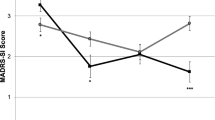
For the secondary clinical outcome measures of depression, anxiety, and BPD symptoms, we also observed significant post-infusion decreases in symptoms (main effect of timepoint) for each measure (Fig. 3, BDI: F(4, 77.91) = 36.93, p < 0.001; BAI: F(4, 74.03) = 26.56, p < 0.001; ZAN-BPD-SRV F(4, 78.13) = 27.23, p < 0.001). For depression (Fig. 3B), we found a numerical but not statistically significant effect of group by timepoint where BDI scores decreased more in the ketamine than midazolam group from before to after infusion but there was no significant effect of group or group x timepoint (F(6,116.10) = 0.47, p = 0.83, ƞp = 0.02). While not significant, the timepoint at Day 3 had the greatest magnitude of group effect on change in depression severity from baseline, with greater change for ketamine group (t(18.15) = 0.41, p = 0.69, Cohen’s d = 0.18). For anxiety (Fig. 3C) and BPD symptoms (Fig. 3D), we did not observe group or group x timepoint differences.
For socio-occupational functioning (Fig. 3E), we observed a significant group x timepoint effect (F(1,20.12) = 5.16, p = 0.03, ƞp = 0.20) in addition to main effects of group (F(1, 19.07) = 5.03, p = 0.04) and timepoint (F(1, 20.12) = 22.07, p < 0.001). The groups differed significantly at Day 14 (t(18.98) = 1.72, p = 0.10, Cohen’s d = 0.18). Furthermore, the observed post-infusion improvement in socio-occupational function was significantly correlated with reductions in depression at two weeks after infusion in the ketamine group (r(8) = 0.65, p = 0.04) but not midazolam group (r(9) = 0.41, p = 0.216) (Fig. 3F).
In an exploratory analysis, we found that ketamine and norketamine levels were quite variable across ketamine-infused participants, and we did not observe a linear relationship between serum drug levels and change in depressive symptoms (Supplementary Fig. 1, for the correlation between ketamine level and Day 3 BDI score, r(8) = 0.01, p = 0.99; for the relationship between norketamine at the 60 min timepoint and Day 3 BDI score, r(8) = 0.31, p = 0.38).
Discussion
This pilot study provides the first prospective randomized and controlled evidence supporting the use of ketamine in people with BPD.
We found that ketamine was well-tolerated. Adverse events were similar in the two groups with no serious events on infusion day or in the two weeks after infusion. The two people in the ketamine group who experienced acute psychiatric distress during the study did not have this occur until week four after infusion, suggesting that these events may relate more closely to psychological effects of study termination than to pharmacologic effects of the medication. Consistent with others’ results in patients with treatment-resistant MDD (TRD, [32]), history of dissociation in this study was associated with stronger intra-infusion dissociation in this cohort of people with BPD but all participants experienced rapid resolution of dissociative symptoms. Patients with history of dissociation may expect stronger intra-infusion dissociative experience but can also expect the ketamine-induced dissociation to fully and rapidly resolve after infusion. This is of particular importance given that for people with BPD, efforts to resolve enduring dissociative symptoms can lead to self-harm [33], and acute dissociation may interfere with emotional learning [34] and psychotherapy treatment response [35,36,37]. Taken together, our results and the literature provide preliminary evidence that ketamine is safe and tolerable in people with BPD. As intense fluctuating distress and suicidal ideation are common in people with BPD [38], any research and clinical work should include safety planning for times when these symptoms intensify that provides support without over-reaction to these expected events.
Ketamine led to a numerical but not statistically significant decrease in both suicidal ideation and depression that was greater in the ketamine than midazolam group. These results are exciting for a pilot study: novel treatments are needed that can work alone and in concert with BPD psychotherapy [39]. Further study is needed in larger cohorts powered to detect ketamine-specific effects of small-medium size (as observed here). While the effect sizes we report here should not be overly interpreted given the low powered sample and lack of significance, these estimates can provide initial guidance for clearly-needed larger follow-up studies examining ketamine as a treatment for BPD.
BPD symptoms and anxiety improved in both groups with no significant difference between ketamine and psychoactive comparator. We measured BPD symptoms here with the ZAN-BPD-SRV self-report questionnaire, which measures the recent intensity of each of the DSM5 BPD criteria. The lack of observed effect could reflect a slower pace of change for some of the BPD-specific criteria, like unstable identity, and change in BPD symptoms might be more likely with repeated ketamine infusions and/or longer follow-up period. Also, these results are similar to those from some studies in populations beyond TRD such as PTSD and OCD where ketamine was found to have transdiagnostic efficacy to reduce depressive and suicidal symptoms, but was less effective for disorder-specific symptoms [9, 10] (though data are mixed, and some studies have found benefit of ketamine for PTSD symptoms [11, 40]).
There are several factors that likely contributed to the observed results. The high level of inter-individual variance (see individual trajectories in Supplementary Fig. 2) precludes identification of between-group differences in this small sample. Higher frequency of intra-individual variance in symptoms in BPD may also contribute [41, 42]; future work could benefit from high density sampling methods such as daily diaries or ecologic momentary assessment [41, 43], as well as assessment over a longer baseline period to more reliably capture pre-treatment symptom levels. Floor effects may also have influenced results: participants in the study were outpatients with relatively low levels of baseline suicidality, such that both groups had marked reductions in suicidal ideation during study participation to the point of many reporting no ideation at all for several of the timepoints. Also, non-pharmacologic effects of study participation may have promoted significant enduring symptom reduction in both groups that obscured between-group effects of drug. A recent review of 10 intravenous ketamine studies found a stronger impact of placebo response on suicidal ideation compared to other study outcomes [44]. This effect was exaggerated for midazolam-placebo compared to saline-placebo trials. Several aspects of placebo response may be especially relevant for our study. Clinical contact may be particularly impactful in BPD [45, 46]: this study involved seven meetings in five weeks with a pair of supportive interested clinical raters. Data from body ownership illusion tasks (e.g., rubber hand illusion) suggest that people with BPD may be more suggestible in some settings [47]. One observation in this study that fits with the effect of suggestion is that ketamine-induced dissociative experiences peaked at the 20 min timepoint, when only half the medication had been delivered, and decreased markedly by the 40 min timepoint, just after the infusion stopped. Others have observed something similar over the course of multiple-infusion series’, where participants had decreased intensity of dissociative experiences with each infusion, suggesting that ketamine tolerance or changes in expectancy may influence the ketamine-related changes in mental states [48, 49]. Future work on ketamine response in BPD should consider and try to limit these non-pharmacologic contributions; designs that evoke less change in the control group may be better able to reveal any ketamine-specific effects.
Despite the above considerations, socio-occupational functioning improved more in the ketamine than midazolam group, with the degree of improvement strongly correlated with improvement in depression symptoms. An important question for any proposed BPD treatment is how much it can help to support meaningful recovery. The natural history of BPD is to experience decreasing numbers of symptoms over time, but social and occupational recovery are less frequent [50, 51]. For BPD, the potential for a medication to not only provide short-term symptom relief, but to also improve social functioning, would be particularly exciting.
Our findings and the available literature suggest that a larger multi-dose study would be useful to better characterize the effects of ketamine in people with BPD. In depression [52] and PTSD [10], a single sub-anaesthetic dose of intravenous ketamine provides transient relief from depressive symptoms and suicidality. Symptomatic improvements last two to fourteen days in ketamine responders with Major Depressive Disorder and repeat dosing schedules increase both the fraction of responders and the duration of the effect in those patients [53]. Repeat dosing could be a path to improvement in core BPD symptoms in addition to effects on suicidality and depression.
Interpretation of the results of our study with respect to current clinical ketamine practice is limited by our small sample size and our single-dose protocol. A better-powered study would help to understand if we may have under-estimated effects (regression to the mean could have reduced apparent group differences as our randomly assigned ketamine group had higher baseline symptoms). The gender and ethnic makeup of this pilot sample size is a limitation for generalizability, and recruitment of a diverse sample should be an aim for future studies. Careful consideration of how to ensure adequate support for study participants while evoking less placebo response would also be important to understand drug-specific effects, though our results may suggest medication/psychotherapy combination approaches to be an especially important direction.
The rapidly increasing uptake of ketamine as an intervention for suicide and depression means that patients with BPD are likely currently receiving ketamine and do not have clear data to guide decision-making. At the same time, all too often researchers and providers are hesitant to explicitly study or treat BPD given the perception of these individuals as difficult and risky to work with. Thus, initial findings of no serious adverse events and no enduring dissociation will hopefully facilitate needed larger investigation of ketamine in BPD. In conclusion, this first randomized double-blind placebo-controlled clinical trial of ketamine for adults with BPD provides initial safety data and suggests that ketamine may help to improve depressive symptoms and promote functional recovery in this underserved clinical group.
Funding
The project described was primarily supported by Grant Number YIG-0-034-15 from the American Foundation for Suicide Prevention (AFSP). Research reported in this publication was also supported by the National Institute of Mental Health (NIMH) of the National Institutes of Health under award number 1K23MH123760 which provided support for Dr. Fineberg’s research-focused effort toward training in clinical research, and by CTSA Grant Number UL1 TR001863 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). This work was funded in part by the State of Connecticut, Department of Mental Health and Addiction Services and by the Yale University Department of Psychiatry. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of AFSP, NIH, Yale University, the Connecticut Department of Mental Health and Addiction Services, or the State of Connecticut.
References
Masland SR, Price D, MacDonald J, Finch E, Gunderson J, Choi-Kain L. Enduring effects of one-day training in good psychiatric management on clinician attitudes about borderline personality disorder. J Nerv Ment Dis. 2018;206:865–9. https://doi.org/10.1097/NMD.0000000000000893
Comtois KA, Carmel A. Borderline personality disorder and high utilization of inpatient psychiatric hospitalization: concordance between research and clinical diagnosis. J Behav Health Serv Res. 2016;43:272–80. https://doi.org/10.1007/s11414-014-9416-9
Paris J. Suicidality in borderline personality disorder. Medicina. 2019;55:223 https://doi.org/10.3390/medicina55060223
Storebo OJ, Stoffers-Winterling JM, Vollm BA, Kongerslev MT, Mattivi JT, Jorgensen MS, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2020;5:CD012955 https://doi.org/10.1002/14651858.CD012955.pub2
Binks CA, Fenton M, McCarthy L, Lee T, Adams CE, Duggan C. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006:CD005653. https://doi.org/10.1002/14651858.CD005653
McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, et al. Synthesizing the evidence for Ketamine and Esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;178:383–99. https://doi.org/10.1176/appi.ajp.2020.20081251
Nandan NK, Soni PK, Parsaik A, Hashmi A. “Esketamine” in borderline personality disorder: a look beyond suicidality. Cureus. 2022;14:e24632 https://doi.org/10.7759/cureus.24632
Chen KS, Dwivedi Y, Shelton RC. The effect of IV ketamine in patients with major depressive disorder and elevated features of borderline personality disorder. J Affect Disord. 2022;315:13–16. https://doi.org/10.1016/j.jad.2022.07.054
Bloch MH, Wasylink S, Landeros-Weisenberger A, Panza KE, Billingslea E, Leckman JF, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012;72:964–70. https://doi.org/10.1016/j.biopsych.2012.05.028
Abdallah CG, Roache JD, Gueorguieva R, Averill LA, Young-McCaughan S, Shiroma PR, et al. Dose-related effects of ketamine for antidepressant-resistant symptoms of posttraumatic stress disorder in veterans and active duty military: a double-blind, randomized, placebo-controlled multi-center clinical trial. Neuropsychopharmacology. 2022;47:1574–81. https://doi.org/10.1038/s41386-022-01266-9
Feder A, Costi S, Rutter SB, Collins AB, Govindarajulu U, Jha MK, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178:193–202. https://doi.org/10.1176/appi.ajp.2020.20050596
Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38:2475–83. https://doi.org/10.1038/npp.2013.150
Wilkinson ST, Farmer C, Ballard ED, Mathew SJ, Grunebaum MF, Murrough JW, et al. Impact of midazolam vs. saline on effect size estimates in controlled trials of ketamine as a rapid-acting antidepressant. Neuropsychopharmacology. 2019;44:1233–8. https://doi.org/10.1038/s41386-019-0317-8
Wilkowska A, Wiglusz MS, Jakuszkowiak-Wojten K, Cubala WJ. Ketamine and Lamotrigine combination in psychopharmacology: systematic review. Cells. 2022;11:645 https://doi.org/10.3390/cells11040645
Chiu CT, Scheuing L, Liu G, Liao HM, Linares GR, Lin D, et al. The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress. Int J Neuropsychopharmacol. 2014;18:pyu102 https://doi.org/10.1093/ijnp/pyu102
Price JB, Yates CG, Morath BA, Van De Wakker SK, Yates NJ, Butters K, et al. Lithium augmentation of ketamine increases insulin signaling and antidepressant-like active stress coping in a rodent model of treatment-resistant depression. Transl Psychiatry. 2021;11:598 https://doi.org/10.1038/s41398-021-01716-w
Motaghinejad M, Motevalian M, Fatima S, Beiranvand T, Mozaffari S. Topiramate via NMDA, AMPA/kainate, GABA(A) and Alpha2 receptors and by modulation of CREB/BDNF and Akt/GSK3 signaling pathway exerts neuroprotective effects against methylphenidate-induced neurotoxicity in rats. J Neural Transm. 2017;124:1369–87. https://doi.org/10.1007/s00702-017-1771-2
First MB, Spitzer, RL, Gibbon, M, Williams, JBW Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition. In: Institute BRDNYSP, editor. New York, New York 2007.
Zanarini M, Frankenburg FR, Chauncey DL, Gunderson JG. The diagnostic interview for personality disorders: Interrater and test-retest reliability. Compr Psychiatry. 1987;28:467–580.
Zanarini MC, Gunderson JG, Frankenburg FR, Chauncey DL. The revised diagnostic interview for borderlines: discriminating BPD from other axis II disorders. J Pers Disord. 1989;3:10–8.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4-57
Overall JE The brief psychiatric rating scale in psychopharmacology research. 1974.
Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress. 1998;11:125–36. https://doi.org/10.1023/A:1024465317902
Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consul Clin Psychol. 1979;47:343.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71.
Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7.
Zanarini MC, Weingeroff JL, Frankenburg FR, Fitzmaurice GM. Development of the self-report version of the Zanarini Rating Scale for Borderline Personality Disorder. Personal Ment Health. 2015;9:243–9. https://doi.org/10.1002/pmh.1302
Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Arch Gen Psychiatry. 1976;33:1111–5.
Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation;; 1996.
Beck AT, Brown GK, Steer RA. Psychometric characteristics of the Scale for Suicide Ideation with psychiatric outpatients. Behav Res Ther. 1997;35:1039–46. https://doi.org/10.1016/s0005-7967(97)00073-9
Zanarini MC, Vujanovic AA, Parachini EA, Boulanger JL, Frankenburg FR, Hennen J. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. J Pers Disord. 2003;17:233–42. https://doi.org/10.1521/pedi.17.3.233.22147
Mello RP, Echegaray MVF, Jesus-Nunes AP, Leal GC, Magnavita GM, Vieira F, et al. Trait dissociation as a predictor of induced dissociation by ketamine or esketamine in treatment-resistant depression: Secondary analysis from a randomized controlled trial. J Psychiatr Res. 2021;138:576–83. https://doi.org/10.1016/j.jpsychires.2021.05.014
Perez S, Lorca F, Marco JH. “Dissociation, posttraumatic stress symptoms, emotional dysregulation, and invalidating environments as correlates of NSSI in borderline personality disorder patients”. J Trauma Dissociation. 2020;21:520–35. https://doi.org/10.1080/15299732.2020.1719262
Ebner-Priemer UW, Mauchnik J, Kleindienst N, Schmahl C, Peper M, Rosenthal MZ, et al. Emotional learning during dissociative states in borderline personality disorder. J Psychiatry Neurosci. 2009;34:214–22.
Kleindienst N, Limberger MF, Ebner-Priemer UW, Keibel-Mauchnik J, Dyer A, Berger M, et al. Dissociation predicts poor response to Dialectial Behavioral Therapy in female patients with Borderline Personality Disorder. J Pers Disord. 2011;25:432–47. https://doi.org/10.1521/pedi.2011.25.4.432
Kleindienst N, Priebe K, Gorg N, Dyer A, Steil R, Lyssenko L, et al. State dissociation moderates response to dialectical behavior therapy for posttraumatic stress disorder in women with and without borderline personality disorder. Eur J Psychotraumatol. 2016;7:30375 https://doi.org/10.3402/ejpt.v7.30375
Arntz A, Stupar-Rutenfrans S, Bloo J, van Dyck R, Spinhoven P. Prediction of treatment discontinuation and recovery from Borderline Personality Disorder: Results from an RCT comparing Schema Therapy and Transference Focused Psychotherapy. Behav Res Ther. 2015;74:60–71. https://doi.org/10.1016/j.brat.2015.09.002
Gunderson JG, Herpertz SC, Skodol AE, Torgersen S, Zanarini MC. Borderline personality disorder. Nat Rev Dis Prim. 2018;4:18029 https://doi.org/10.1038/nrdp.2018.29
Stoffers-Winterling J, Storebo OJ, Lieb K. Pharmacotherapy for borderline personality disorder: an update of published, unpublished and ongoing studies. Curr Psychiatry Rep. 2020;22:37 https://doi.org/10.1007/s11920-020-01164-1
Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71:681–8. https://doi.org/10.1001/jamapsychiatry.2014.62
Rizk MM, Choo TH, Galfalvy H, Biggs E, Brodsky BS, Oquendo MA, et al. Variability in suicidal ideation is associated with affective instability in suicide attempters with borderline personality disorder. Psychiatry. 2019;82:173–8. https://doi.org/10.1080/00332747.2019.1600219
Links PS, Eynan R, Heisel MJ, Barr A, Korzekwa M, McMain S, et al. Affective instability and suicidal ideation and behavior in patients with borderline personality disorder. J Pers Disord. 2007;21:72–86. https://doi.org/10.1521/pedi.2007.21.1.72
Kleiman EM, Turner BJ, Fedor S, Beale EE, Picard RW, Huffman JC, et al. Digital phenotyping of suicidal thoughts. Depress Anxiety. 2018;35:601–8. https://doi.org/10.1002/da.22730
Bloomfield-Clagett B, Ballard ED, Greenstein DK, Wilkinson ST, Grunebaum MF, Murrough JW, et al. A participant-level integrative data analysis of differential placebo response for suicidal ideation and non-suicidal depressive symptoms in clinical trials of intravenous Racemic Ketamine. Int J Neuropsychopharmacol. 2022;25:827–38. https://doi.org/10.1093/ijnp/pyac055
Gunderson JG, Choi-Kain LW. Medication management for patients with borderline personality disorder. Am J Psychiatry. 2018;175:709–11. https://doi.org/10.1176/appi.ajp.2018.18050576
Fineberg SK, Gupta S, Leavitt J. Collaborative deprescribing in borderline personality disorder: a narrative review. Harv Rev Psychiatry. 2019;27:75–86. https://doi.org/10.1097/HRP.0000000000000200
Neustadter ES, Fineberg SK, Leavitt J, Carr MM, Corlett PR. Induced illusory body ownership in borderline personality disorder. Neurosci Conscious. 2019;2019:niz017 https://doi.org/10.1093/nc/niz017
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. https://doi.org/10.1016/s0006-3223(99)00230-9
Wlodarczyk A, Cubala WJ, Galuszko-Wegielnik M, Szarmach J. Dissociative symptoms with intravenous ketamine in treatment-resistant depression exploratory observational study. Medicine. 2021;100:e26769 https://doi.org/10.1097/MD.0000000000026769
Zanarini MC, Frankenburg FR, Reich DB, Fitzmaurice G. Attainment and stability of sustained symptomatic remission and recovery among patients with borderline personality disorder and axis II comparison subjects: a 16-year prospective follow-up study. Am J Psychiatry. 2012;169:476–83. https://doi.org/10.1176/appi.ajp.2011.11101550
Zanarini MC, Temes CM, Frankenburg FR, Reich DB, Fitzmaurice GM. Description and prediction of time-to-attainment of excellent recovery for borderline patients followed prospectively for 20 years. Psychiatry Res. 2018;262:40–5. https://doi.org/10.1016/j.psychres.2018.01.034
Dean RL, Hurducas C, Hawton K, Spyridi S, Cowen PJ, Hollingsworth S, et al. Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder. Cochrane Database Syst Rev. 2021;9:CD011612 https://doi.org/10.1002/14651858.CD011612.pub3
Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry. 2019;176:401–9. https://doi.org/10.1176/appi.ajp.2018.18070834
Acknowledgements
We are very grateful to the people who volunteered as study participants for this project. A number of people in our lab helped with recruitment for this project. We particularly wish to acknowledge Nyla Conaway, Max Crosland-Wood, Rena Linden, Dan McGlade, Erica Robinson, and Esther Teo. We are also very grateful to the supportive and excellent team of scheduling staff and nurses at the Yale Center for Clinical Investigation Hospital Research Unit. We thank Kaitlin Maciejewski from the Yale Center for Analytic Science for advice on statistical approaches. We also thank Michael Bloch and Naomi Driesen for advice in project planning. Pharmacokinetic sample processing for this study was performed by the Biomarkers Core Laboratory at the Irving Institute for Clinical and Translational Research, home to Columbia University’s Clinical and Translational Science Award Program hub.
Author information
Authors and Affiliations
Contributions
We describe author contributions here using the CRediT system (https://www.elsevier.com/authors/policies-and-guidelines/credit-author-statement). SF: conceptualization, methodology, formal analysis, investigation, visualization, writing of original and revised drafts. Supervision, project administration, and funding acquisition. EC: project administration, investigation (interviews and blind raters), formal analysis, and visualization. RST: project administration, investigation (interviews and blind ratings), formal analysis, and visualization. KD: project administration, investigation (interviews and blind ratings). EN: methodology, investigation (interviews and blind raters), data curation, writing—edited manuscript. Madison Sakheim: methodology, data curation. Kaylee Null: data curation, investigation (interviews). DT-D: investigation (extensive interviewing). JR: formal analysis, writing—edited manuscript. GP: formal analysis, writing—edited manuscript. Jessica Peters: formulation, formal analysis, and writing – edited manuscript. PRC: provided close mentorship on conceptualization and funding acquisition. JHK: mentorship on conceptualization and funding acquisition, close mentorship on formal analysis and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
SKF discloses work with the pharmaceutical company Boehringer Ingelheim as site PI for a multinational clinical trial and for consulting on advisory boards (< $10,000 in 2022). PRC is co-founder of Tetricus Labs. JHK consults for Aptinyx Inc., Biogen Idec MA, Bionomics Ltd., Boehringer Ingelheim International, Clearmind Medicine, Inc., Cybin IRL, Enveric Biosciences, Epiodyne, Inc., EpiVario, Inc., Janssen Research & Development, Jazz Pharmaceuticals, Inc., Otsuka America Pharmaceutical, Inc., Perception Neuroscience, Inc., Praxis Precision Medicines, Inc., Spring Care, Inc., and Sunovion Pharmaceuticals, Inc.; is a scientific advisor of Biohaven Pharmaceuticals, BioXcel Therapeutics, Inc. (Clinical Advisory Board), Cerevel Therapeutics, LLC, Delix Therapeutics, Inc., Eisai, Inc., EpiVario, Inc., Freedom Biosciences, Inc., Jazz Pharmaceuticals, Inc., Numara Therapeutics, Inc., Neurocrine Biosciences, Inc., Novartis Pharmaceuticals Corporation, Perception Neuroscience, Inc., Praxis Precision Medicines, Inc., PsychoGenics, Inc., Takeda Industries, Tempero Bio, Inc., and Terran Biosciences, Inc.; holds stock or stock options with Biohaven Pharmaceuticals, Clearmind Medicine, Inc., Spring Care, Inc., EpiVario, Inc., Neumora Therapeutics, Inc., Tempero Bio, Inc., and Terran Biosciences, Inc.; and is editor of Biological Psychiatry. JHK was also awarded the following patents: (i) Seibyl JP, Krystal JH, Charney DS. Dopamine and Noradrenergic Reuptake Inhibitors in Treatment of Schizophrenia. U.S. Patent No. 5447948. September 5, 1995; (ii) Vladimir C, Krystal JH, Sanacora G. Glutamate Modulating Agents in the Treatment of Mental Disorders. U.S. Patent No. 8778979 B2. Patent Issue Date July 15, 2014. U.S. Patent Application No. 15/695,164. Filing Date September 5, 2017; (iii) Charney D, Krystal JH, Manji H, Matthew S, Zarate C. Intranasal Administration of Ketamine to Treat Depression. U.S. Patent Application No. 14/197767 filed on March 5, 2014. U.S. Application or Patent Cooperation Treaty International Application No. 14/306382 filed on June 17, 2014; (iv) Zarate C, Charney DS, Manji HK, Mathew SJ, Krystal JH, Department of Veterans Affairs. Methods for Treating Suicidal Ideation. Patent Application No. 14/197767 filed on March 5, 2014, by Yale University Office of Cooperative Research; (v) Arias A, Petrakis I, Krystal JH. Composition and Methods to Treat Addiction. Provisional Use Patent Application No. 61/973/961. April 2, 2014. Filed by Yale University Office of Cooperative Research; (vi) Chekroud A, Gueorguieva R, Krystal JH. Treatment Selection for Major Depressive Disorder. U.S. Patent and Trademark Office Docket No. Y0087.70116US00. Filed June 3, 2016. Provisional patent submission by Yale University; (vii) Gihyun Y, Petrakis I, Krystal JH. Compounds, Compositions, and Methods for Treating or Preventing Depression and Other Diseases. U.S. Provisional Patent Application No. 62/444552. Filed on January 10, 2017 by Yale University Office of Cooperative Research OCR 7088 US01; (viii) Abdallah C, Krystal JH, Duman R, Sanacora G. Combination Therapy for Treating or Preventing Depression or Other Mood Diseases. U.S. Provisional Patent Application No. 62/719935. Filed on August 20, 2018, by Yale University Office of Cooperative Research OCR 7451 US01. (ix) Krystal, JH, Pearlson, G, O’Malley, S, Potenza, M, Gasparini, F, Gomez-Mancilla, B, Malaterre, V. Mavoglurant in treating gambling and gaming disorders. U.S. Provisional Patent Application No. 63/125,181filed on December 14, 2020 by Yale University Office of Cooperative Research OCR 8065 US00. AstraZeneca Pharmaceuticals provides the drug, Saracatinib, for research related to NIAAA grant “Center for Translational Neuroscience of Alcoholism [CTNA-5]”. Novartis provides the drug, Mavoglurant, for research related to NIAAA grant “Center for Translational Neuroscience of Alcoholism [CTNA-5]”. Cerevel provides the drug PF-06412562 for A Translational and Neurocomputational Evaluation of a D1R Partial Agonist for Schizophrenia (1 U01 MH121766-01). EYC, RST, KD, EN, MS, KN, DTD, JR, GFP, and JRP declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fineberg, S.K., Choi, E.Y., Shapiro-Thompson, R. et al. A pilot randomized controlled trial of ketamine in Borderline Personality Disorder. Neuropsychopharmacol. 48, 991–999 (2023). https://doi.org/10.1038/s41386-023-01540-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01540-4
This article is cited by
-
Ketamine and rapid antidepressant action: new treatments and novel synaptic signaling mechanisms
Neuropsychopharmacology (2024)