Abstract
Recent studies indicate that stimulation of the rostromedial tegmental nucleus (RMTg) can drive a negative affective state and that nociceptin/orphanin FQ (N/OFQ) may play a role in affective disorders and drug addiction. The N/OFQ precursor prepronociceptin encoding genes Pnoc are situated in RMTg neurons. To determine whether N/OFQ signaling contributes to the changes in both behavior phenotypes and RMTg activity of alcohol withdrawn (Post-EtOH) rats, we trained adult male Long-Evans rats, randomly assigned into the ethanol and Naïve groups to consume either 20% ethanol or water-only under an intermittent-access procedure. Using the fluorescence in situ hybridization technique combined with retrograde tracing, we show that the ventral tegmental area projecting RMTg neurons express Pnoc and nociceptin opioid peptide (NOP) receptors encoding gene Oprl1. Also, using the laser capture microdissection technique combined with RT-qPCR, we detected a substantial decrease in Pnoc but an increase in Oprl1 mRNA levels in the RMTg of Post-EtOH rats. Moreover, RMTg cFos expression is increased in Post-EtOH rats, which display anxiety- and depression-like behaviors. Intra-RMTg infusion of the endogenous NOP agonist nociceptin attenuates the aversive behaviors in Post-EtOH rats without causing any notable change in Naïve rats. Conversely, intra-RMTg infusion of the NOP selective antagonist [Nphe1]nociceptin(1-13)NH2 elicits anxiety- and depression-like behaviors in Naïve but not Post-EtOH rats. Furthermore, intra-RMTg infusion of nociceptin significantly reduces alcohol consumption. Thus, our results show that the deficiency of RMTg NOP signaling during alcohol withdrawal mediates anxiety- and depression-like behaviors. The intervention of NOP may help those individuals suffering from alcohol use disorders.
Similar content being viewed by others
Introduction
Repeated cycles of excessive alcohol drinking and withdrawal induce a negative affective state and aberrant behaviors, including anxiety and depression. These psychiatric ailments act as negative reinforcers, promoting relapse in drinking and the development of alcohol use disorders (AUDs) [1]. Clinical and preclinical studies suggest hypofunction of mesolimbic dopaminergic neurons is a critical adaptation in depression [2, 3] and withdrawal from many abused drugs, including cocaine, morphine, and alcohol [4,5,6].
The rostromedial tegmental nucleus (RMTg), also known as the tail of VTA (tVTA), has become a hotspot in the addiction research field due to its physiological function as a GABAergic master brake for dopamine systems [7,8,9]. RMTg sends strong GABAergic projections to the VTA dopaminergic neurons. Increased RMTg neuronal activity will increase the inhibition of dopaminergic neurons [10]. RMTg involves punishment learning and aversive valence encoding [11,12,13] and is strongly influenced by many abused drugs [14,15,16,17]. Notably, RMTg inactivation relieves affective disorders such as anxiety and depression in rats withdrawn from chronic alcohol exposure [10] and reduces alcohol consumption [18,19,20]. Still, we know little about the underlying molecular mechanisms.
N/OFQ is structurally related to the opioid peptide dynorphin A but binds with high affinity at NOP receptors [21]. N/OFQ and NOP are widely distributed throughout the brain at exceptionally elevated levels in limbic and limbic-related regions [22, 23]. The N/OFQ is well accepted as a core component of the brain’s anti-stress system by counteracting the endogenous corticotropin-releasing factor (CRF) action [24, 25]. Emerging evidence shows that N/OFQ-NOP receptor system involves regulating stress and addiction-related behavior [26, 27].
A series of studies also demonstrated that activation of N/OFQ system blunts ethanol’s reinforcing and motivation effects across various behavioral measures, including ethanol intake [28,29,30], conditioned place preference [31], and stress-induced reinstatement [32]. Moreover, systemic N/OFQ administration attenuates anxiety-like behavior, producing more potent anxiolytic effects during early withdrawal in alcohol-dependent rats [33], suggesting N/OFQ-NOP system acts as an adaptive compensatory mechanism alleviating innate negative affective states.
A recent elegant study revealed the Pnoc gene is highly expressed in the RMTg [34]. Thus, N/OFQ may exert an anti-stress effect by inhibiting RMTg GABAergic neurons, suppressing their inhibition in VTA dopaminergic neurons, and consequently alleviating the hypofunction of dopaminergic neurons during alcohol withdrawal and the anxiety- and depression-like behaviors. The present study investigated the change of N/OFQ-NOP-related gene expression and the RMTg N/OFQ-NOP system’s role in regulating affective behaviors associated with alcohol withdrawal in adult male Long-Evans rats.
Methods and materials
Animals
A total of 178 male Long-Evans rats (180–220 g at the start of the experiments) were obtained from the Laboratory Animal Center of Sun Yat-sen University and used in the investigation. For a complete description of the subjects, see Table 1 in the Supplementary information. All procedures were performed according to the Chinese National Health and Medical Research Council animal ethics guidelines and with the approval of the Sun Yat-sen University Animal Experimentation Ethics Committee. Rats were housed individually in home cages in a climate-controlled room (20 °C) under a 12-h light/dark cycle. Animals were allowed to acclimatize to the housing conditions and handling before the start of each experiment. Food and water were available ad libitum.
Intermittent access to 20% ethanol in the two-bottle free choice (IA2BC) drinking procedure
As reported, we trained Long-Evans rats to drink alcohol in the IA2BC paradigm for 8–12 weeks [19, 35]. Briefly, rats had 24-h concurrent access to two bottles, one with 20% ethanol (EtOH, v/v) and another with water, starting on Monday afternoon. After 24 h, we replaced the ethanol bottle with a water bottle for the next 24 h. This pattern was repeated on Wednesdays and Fridays. On other days, the rats had unlimited access to two water bottles. In each ethanol-drinking session, the placement of the ethanol bottle was alternated to control for side preferences. The amount of ethanol or water consumed was determined by weighing the bottles before and after 24 h access. Ethanol consumption was determined by calculating grams of alcohol consumed per kilogram of body weight. Weekly “drip” averages (loss of fluid in a cage with no animal present) were subtracted from individual fluid intakes. The spillage was always <1.0 mL (<2.5% of the total fluid intake) for 24 h. The body weight of all rats was recorded weekly. Rats under this paradigm escalated their ethanol intake and preference [19].
Stereotaxic surgery and microinjection procedure
We performed surgery using a stereotaxic apparatus (RWD Life Science, Shenzhen, China) on rats under anesthesia, induced by ketamine/xylazine (80/20 mg/kg, i.p.) and maintained with isoflurane, as described previously [10]. For microinfusion chemicals to the RMTg, bilateral guide cannulas (62022, RWD Life Science, Shenzhen, China) were placed 2 mm above the RMTg in coordinates (in mm: A/P: −7.3 from Bregma; M/L: ± 0.3; D/V: −6.2 from the skull surface, coordinates was in reference to the Paxinos and Watson rat brain atlas [36]) and were secured on the skull using dental cement. To label VTA projecting-RMTg neurons, we delivered Green Retrobeads IX (300 nL/side) with a micropipette into the VTA (in mm: A/P: −5.7, M/L: ± 0.8, D/V: −8.5) of rats [10] very slowly (50 nL/10 min), to minimize tissue damage. After injection, the micropipette remained in place for an additional 10 min to prevent backflow. Then, burr holes were sealed with sterile bone wax. Before recovery from anesthesia, rats were given meloxicam (1.0 mg/kg, s.c.) and returned to the homecage. We sacrificed the animals 9–11 days after tracer injections to obtain optimal tracer expression in the target region.
Four to five days before the behavior test, to minimize stress and habituate the subjects to the microinjection procedures, animals were brought to the experimental room and handled for 10 min/daily for 3 to 5 sessions until the test day. During this phase, animals became accustomed to the experimenter, the experimental room, and the manipulation procedure. Microinjections of NOP agonist or antagonist were given 5–10 min before behavioral tests. Obstructers were carefully removed from a gently restrained rat, and the injectors were inserted bilaterally to a depth of 2 mm beyond the end of the guide cannulae. Drug or vehicle control was infused into the RMTg through a 26-gauge internal cannulae connected to the Hamilton 1.0 μL syringe driven by a syringe pump (R462, RWD Life Science). Chemicals were infused over 1 min, and the injectors were left in place for an additional 60 s to allow for diffusion. After the removal of the injector, a new sterile obstructer was inserted.
Chemicals and application
N/OFQ, and selective NOP receptor antagonist [Nphe1]nociceptin(1-13)NH2 ([Nphe1]NC(1-13)NH2) were purchased from Tocris Bioscience (UK). Upon arrival, the chemicals were dissolved in the artificial cerebral spinal fluid (aCSF), and the aliquots were stored at −80 °C. On the test day, drugs were diluted with aCSF. N/OFQ (0.01 nmol/100 nL/side) and [Nphe1]NC(1-13)NH2 (5 nM/100 nL/side) were injected into the RMTg. Doses of these chemicals were chosen based on previous studies [37,38,39]. All other chemicals were purchased from Aladdin (China) and listed in the Supplementary information.
Behavioral procedure
The test battery included the elevated plus maze test (EPM), open field test (OFT), marble burying test (MBT) for measuring anxiety-like behaviors; sucrose preference test (SPT), and forced swimming test (FST) for measuring depression-like behaviors in naïve rats and rats at 48 h withdrawal from last drinking session (Post-EtOH) as previously described [10, 40]. The materials and methods of Supplementary information describe the exact condition of the above assays.
The effects of NOP agonist and antagonist on the anxiety- and depression-like behaviors were tested in separate subgroups of Naïve or Post-EtOH rats in a counterbalanced manner. For animals subjected to multiple behavioral tests, there was a 1-week interval between tests.
RNA fluorescence in situ hybridization (FISH) and image analysis
The RNA FISH procedure for Pnoc and Oprl1 was performed with the RNA FISH kit (GenePharma Inc. Shanghai, China), according to the manufacturer’s instructions. Probes were transcribed from rat Pnoc cDNA (Accession No: NM_013007.1) or Oprl1 cDNA (Gene ID: 29256). Briefly, rats under deep anesthetized (with isoflurane) were euthanized by decapitation. The brains were immediately harvested and flash-frozen at −80 °C. The 30 μm thick coronal sections were collected with a cryostat under RNase-free conditions, fixed in 4% PFA for 15 min at 4 °C. The frozen slices were rehydrated in the citrate buffer, followed by a 3 × 5-min rinse in PBS. Next, the slices were digested by proteinase K digestion at 37 °C for 20 min, followed by citrate buffer. After that, the slices were dehydrated through gradient ethanol for 3 min, subsequently by immense denaturation solution at 78 °C for 8 min, followed by gradient ethanol dehydration and complete air-dry. The hybridization was carried out overnight (12–16 h) at 37 °C using a hybridization buffer added with denatured 5′ CY3-labeled Pnoc or Oprl1 probes. After washing, sections were mounted on glass slides using an antifade mounting medium and observed under the ZEISS LSM 780 confocal microscopy.
The percentages of retrobeads-labeled cells expressing Pnoc/Oprl1 and Pnoc/Oprl1 fluorescence intensity of retrobeads-labeled cells were measured using methods described previously [10, 41]. Briefly, the images of retrobeads-labeled cells (Green) in RMTg expressing Pnoc or Oprl1 (Red) were obtained at 63 × magnifications. The retrobeads-labeled cells in RMTg expressing Pnoc or Oprl1 within two groups (Naïve vs. Post-EtOH) were counted by two investigators who were blinded to the treatment history. The Pnoc or Oprl1 expressing cells with retrobeads were selected, and Pnoc or Oprl1 fluorescence intensity of these retrobeads-labeled cells was measured in the red channel using NIH ImageJ software.
Laser capture microdissection (LCM), RNA extraction, and real-Time quantitative PCR
We used an LCM system (Leica Microsystems, Germany) to harvest RMTg brain tissue for the molecular experiment, based on the procedure published previously with minor modifications [42,43,44]. Briefly, deeply anesthetized rats (with isoflurane) were decapitated. Brains were rapidly harvested and placed in optimal cutting temperature (OCT) medium (Sakura Fineteck U.S.A., Torrance, CA), then cooled to −20 °C. The brain tissue blocks containing RMTg were cut using a cryostat to 30 μm coronal sections and mounted on room-temperature RNase-free polyethylene naphthalene (PEN) membrane slides (Leica Microsystems, Germany). The slide was lightly stained by cresylic violet followed by gradient ethanol dehydration, then proceeded immediately to LCM. Laser parameters were set up as follows: magnification 5×; power, 56; aperture, 9; speed, 3; specimen balance, 17; pulse frequency 501. The anatomical identification of the RMTg region was according to the earlier study [45], and tissue was collected in the 0.5 mL Eppendorf tube and then stored at −80 °C until RNA extraction.
Total RNA from LCM samples was extracted using the RNeasy micro kit (cat. no. 74004, Qiagen Inc.). RMTg tissue was resuspended in 30 μL lysis buffer and vortexed for 30 s, then treated following the instructions of the RNeasy Micro Kit for Microdissected Cryosections, and the total RNA was eluted with 14 μL of RNase-free water. RNA was quantified using a spectrophotometer Nanodrop One (Thermo scientific) in absorbance ratios at 260–280 nm of 1.8–2.0. The cDNA was synthesized using the EasyScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (One-Step gDNA Removal) (TransGen Biotech.). The primers were obtained from the previous study [33]. The quantitative PCR was performed on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, 1855196) using the PerfectStartTM Green qPCR SuperMix (TransGen Biotech.) under the following conditions: 3 s at 94 °C followed by 40 cycles of 5 s at 94 °C, 15 s at 60 °C and 10 s at 72 °C. Real-time PCR was performed in triplicate for each sample. The mRNA expression of genes of interest to the endogenous control gene Gapdh was compared between groups.
Immunofluorescence
We performed immunofluorescence to evaluate the effect of manipulation of NOP receptors on RMTg cFos expression. The detailed protocol is provided in Supplementary information, as we reported [10].
Statistical methods
All data were expressed as a mean ± SEM (standard error of the mean). Animal sample sizes were chosen to ensure adequate statistical power based on our preliminary studies [10]. Animals were randomly assigned to different studies. Investigators were blinded to group allocations in the pharmacological behavioral experiments. Before the analysis, all data were checked for normality and homogeneity of variances. The relative mRNA expression of Pnoc and Oprl1 were analyzed using an unpaired Student’s t-test between naive and Post-EtOH rats. The cFos immunoreactive cell number and behavioral tests, including drinking, anxiety- and depression-like behaviors, between naive and Post-EtOH rats were analyzed using one or two-way repeated-measures (RM) ANOVA, followed by Bonferroni post-hoc tests. Statistical significance was declared at p < 0.05.
Results
Alcohol withdrawal impairs Pnoc and Oprl1 in VTA-projecting RMTg neurons
The schematic in Fig.1A depicts the experimental design. The male Long-Evans rats consuming 20% alcohol under an 8-week IA2BC paradigm [10, 35], gradually and significantly escalated the ethanol intake and preference, reaching 5.40 ± 0.68 g/kg/24 h and 43.57 ± 3.99% at the 24th session, and maintained at the stable levels (Fig. 1B, C). Compared to the withdrawal physical signs score of naïve counterparts (0.94 ± 0.14), the rats subjected to 48 h of abstinence from the last drinking session (Post-EtOH) exhibited a higher score (1.35 ± 0.17) but lacked statistical significance between groups (p = 0.0915, Fig. S1A).
A schematic of the experimental design depicting the morphology and molecular study time points. Male adult Long-Evans rats were trained to consume alcohol for >8 weeks in the two-bottle free choice and intermittent access to ethanol (20%, v/v) (IA2BC) paradigm. A separate cohort of drinking water served as Naïve control. Morphology and molecular experiments were conducted at 48 h withdrawal (WD) of the last drinking session (Post-EtOH). These rats’ ethanol intake and preference were robustly escalated during the 8–10 weeks of drinking in the IA2BC paradigm. B, C. The representative fluorescence images show the RMTg (red box in the schematic picture). Pnoc (red, D) or Oprl1 (red, E) mRNA expressed in the retrograde labeled VTA-projecting RMTg neurons in Naïve and Post-EtOH rats. Scale bar = 20 μm (left) and 1 mm (right). F no difference is found between the number of retobeads+ neurons in Post-EtOH rats and Naive control (8.50 ± 0.27 in Naive, 8.60 ± 0.30 in Post-EtOH, t = 0.2489, p = 0.8041, n = 6 rats/group). G, H, the histograms show the % of retrogradely labeled RMTg neurons expressing Pnoc is decreased, but Oprl1 fluorescence intensity of the retrogradely labeled RMTg neurons is increased in Post-EtOH rats. I the representative images of coronal unfixed cryostat slices of RMTg area after laser capture microdissection (LCM) tissue removal. The areas sampled by LCM are seen as circular light patches. Scale bar = 1 mm; J, K, illustrates the increased Oprl1 but decreased Pnoc relative mRNA levels in Post-EtOH rats. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 vs. 1st session, one-way RM ANOVA followed by Bonferroni post hoc test, n = 12 rats; ^p < 0.05, ^^p < 0.01, and ^^^^p < 0.0001 vs. Naïve, two-tailed unpaired t-test. n = 6 rats/group. All data were presented in mean ± SEM.
It has been shown that the Pnoc gene is highly expressed in the RMTg [34], and N/OFQ-NOP signaling is involved in AUD [26]. To determine whether chronic intermittent alcohol administration alters Pnoc and Oprl1 expression in VTA-projecting RMTg neurons, we used the retrograde-tracing technique combined with RNA FISH (Fig. 1D, E). The retrobeads in VTA injection sites are depicted in Fig. S1B. An unpaired t-test revealed no difference between the number of VTA-projecting RMTg neurons in rats of Post-EtOH and naive groups (Fig. 1F). Interestingly, as shown in Fig. 1G, compared to Naïve rats, the FISH data showed the proportion of Pnoc expressing retrobeads labeled neurons was significantly lower in Post-EtOH rats (90.90 ± 1.72% in Naive, 71.24 ± 2.22% in Post-EtOH, t = 6.816, p < 0.0001), and the fluorescence intensity of Pnoc expressing retrobeads labeled neurons was significantly decreased (t = 3.802, p = 0.0035, Fig. S1C). By contrast, the fluorescence intensity of Oprl1 expressing retrobeads labeled neurons was elevated in Post-EtOH rats (t = 2.243, p = 0.0488, Fig. 1H), although there was no significant difference in the numbers of Oprl1 expressing retrogradely labeled neurons (86.74 ± 1.80% in Naive, 85.79 ± 2.41% in Post-EtOH, t = 0.3157, p = 0.7542, Fig. S1D).
Meanwhile, we examined the correlation between ethanol intake and the number of retrobeads and Pnoc (or Oprl1) co-expressing RMTg cells (Fig. S1E, F) and the correlation between ethanol intake and Pnoc (or Oprl1) fluorescence intensity of VTA-projecting RMTg cells (Fig. S1G, H). There was a negative correlation trend between the Pnoc fluorescence intensity and ethanol intake (Fig. S1F, p = 0.0682), while a positive correlation trend was between Oprl1 fluorescence intensity and ethanol intake (Fig. S1H, p = 0.0932).
Moreover, we collected RMTg tissues in Post-EtOH and Naïve rats using laser capture microdissection (See Method LCM) for the RT-qPCR. Figure 1I depicts the representative images of the coronal brain section captured in the RMTg area. RT-qPCR tests showed compared to the Naïve rats, Oprl1 mRNA levels were increased (t = 2.894, p = 0.0159, Fig. 1J) while Pnoc levels were decreased (t = 3.295, p = 0.0080, Fig. 1K) in Post-EtOH rats.
In addition, to understand whether the signaling change was caused by long-term drinking or acute withdrawal, RMTg N/OFQ-NOP levels were also evaluated in a separate non-abstinent group of animals (Fig. S1I). The results showed no significant change in Oprl1 mRNA level but (t = 0.1525, p = 0.8819) decrease in the Pnoc level (t = 4.428, p = 0.0013) in non-abstinent rats compared to Naïve counterparts (Fig. S1J, K). Thus, we infer the change in NOP levels occurs as a response to the removal of alcohol, in keeping with a previous report [33]. Together, these results indicate that decreased RMTg N/OFQ levels might result from escalated alcohol consumption. In contrast, the up-regulated RMTg NOP level might be derived from ethanol withdrawal in the RMTg-VTA circuit.
Stimulating RMTg NOP receptors mitigates anxiety-like behaviors in alcohol withdrawn rats
Anxiety symptoms are often observed after prolonged alcohol abuse, particularly during acute withdrawal [33, 46], which promotes relapse [46]. A recent study has shown that RMTg inhibition reverses withdrawal-induced anxiety-like behaviors [18]. To determine if NOP regulates withdrawal-associated anxiety-like behaviors, we infused the NOP receptor endogenous agonist N/OFQ or the selective antagonist [Nphe1]NC(1-13)NH2 into the RMTg. We assessed the anxiety-like behaviors using the elevated plus maze test (EPM), open field test (OFT), and marble burying test (MBT) in naïve and Post-EtOH rats. The schematic in Fig. 2A shows the experimental design for pharmacological behavioral assays. Figure 2B illustrates the representative traces in the EPM and OFT (Fig. S2A). Figure 2C–F summarized the anxiety-like behaviors after N/OFQ administration in EPM and MBT, respectively. The anxiety level of post-EtOH rats was higher than that of their Naïve counterparts. Two-way RM ANOVA analysis revealed significant main effects of groups (Naïve vs. Post-EtOH) on the percentage time spent in open arms (OA) divide to closed arms (CA) of the EPM (Fig. 2C, F1,16 = 10.13, p = 0.0058), the duration in (Fig. S2B, F1,17 = 26.53, p < 0.0001) and entries to the center (Fig. S2C, F1,17 = 34.13, p < 0.0001) of OFT, as well as the number of marbles buried (Fig. 2F, F1,11 = 61.24, p < 0.0001), which are consistent with our previous reports [10, 40].
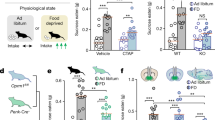
Rats at 48 h alcohol withdrawal showed anxiety-like behaviors measured using elevated plus maze (EPM) and marble burying test (MBT) compared to Naïve counterparts. The N/OFQ (0.01 nM/100 nL/site), the endogenous agonist of nociceptin receptor (NOP) or aCSF in the same volume, was microinjected into the RMTg of Naïve and Post-EtOH rats 5–10 min before the behavioral test. A the schematic diagram shows the experimental timeline. B illustrates a representative behavioral tracking trace of RMTg aCSF (blue) and NOP activating (purple) in Naïve (left) and Post-EtOH (right) rats. Closed arms of the EPM area are presented in solid lines grayed area. RMTg NOP agonist produced an anxiolytic effect, reflected by elevating the percentage of time spent in OA/CA (C) but without significantly altering the percentage entries to OA/CA (D) and total entries to both arms (E) in EPM, and the decreasing number of marbles buried (F) in MBT. The representative behavioral track was traced on EPM (G) and OFT (H) from RMTg aCSF (blue) and NOP antagonizing (green) in Naïve (left) and Post-EtOH (right) rats. RMTg infusion of [Nphe1]NC(1-13)NH2 (5 nM/100 nL/site) elicits an anxiogenic effect, reflected by reducing the duration and entries to the center in OFT (I, J), reducing the percentage of time spent in and entries to OA/CA (K, L) without altering total entries to both arms in EPM (M), and the increasing number of marbles buried (N) in MBT. O Verification of cannulae placements. A representative image of cannulae tracks 2 mm above the RMTg at the level of −6.9 from Bregma. Scale bar = 1 mm (left) and 500 μm (right). IPN interpeduncular nucleus, ml medial lemniscus, Pn pontine nucleus, cp cerebellar peduncle. P schematic drawings of coronal sections of the rat brain showing the tips of injector placements from individual Naïve (black circle) and Post-EtOH rats (blue square). #p < 0.05, ##p < 0.01, ###p < 0.001 and ####p < 0.0001vs naïve group; *p < 0.05, **p < 0.01 and ***p < 0.001 vs aCSF within group, revealed by two-way RM ANOVA followed by Bonferroni’s post hoc test. n = 6–10 rats/group. All data are shown as mean ± SEM.
Importantly, activating RMTg NOP attenuated anxiety-like behaviors significantly in Post-EtOH but not naïve rats. The two-way RM ANOVA analysis revealed the main treatment effects (N/OFQ vs. aCSF) on percentage time spent in OA/CA of the EPM (Fig. 2C, F1,16 = 10.37, p = 0.0053) and number of marbles buried (Fig. 2F, F1,11 = 10.21, p = 0.0085). Within Post-EtOH rats, compared to aCSF, intra-RMTg N/OFQ treatment significantly increased the percentage of time spent in OA/CA in EPM (p = 0.0018) and decreased marbles buried in MBT (p = 0.0033). The two-way RM ANOVA did not find the main treatment effect on the locomotion test in OFT (Fig. S2D, F1,17 = 0.9222, p = 0.3504) and total entries to both arms in EPM (Fig. 2E, F1,16 = 1.908, p = 0.1861), suggesting that the anxiolytic effect might not be attributed to the drug-induced change in locomotor activity.
We next investigated the role of NOP for anxiety by bilateral intra-RMTg infusion of [Nphe1]NC(1-13)NH2. Figure 2G, H illustrates the representative behavioral traces of Naïve and Post-EtOH rats. Figure 2I–N summarized the anxiety-like behavior after [Nphe1]NC (1-13) NH2 administration in the EPM, OFT, and MBT. Two-way RM ANOVA revealed the group × treatment effects on percentage time spent in OA/CA in EPM (Fig. 2I, F1,16 = 8.730, p = 0.0093), duration in (Fig. 2L, F1,15 = 7.477, p = 0.0154) and entries to (Fig. 2M. F1,15 = 9.462, p = 0.0077) center area in OFT, as well as on number of marbles buried (Fig. 2N, F1,11 = 11.74, p = 0.0057) in MBT, and also revealed the main treatment effect on percentage entries to OA/CA of EPM test (Fig. 2J, F1,16 = 6.482, p = 0.0216). The post-hoc test found that naïve rats that received [Nphe1]NC(1-13)NH2 showed a significantly lower percentage of time spent in (Fig. 2I, p = 0.0013) and entries to (Fig. 2J, p = 0.0409) OA/CA in EPM, shorter duration in (Fig. 2L, p = 0.0042) and fewer entries to the center area (Fig. 2M, p = 0.0007) in OFT, and buried more marbles (Fig. 2N, p = 0.0016) than those received aCSF infusion. However, post-hoc tests did not find a significant difference between aCSF and [Nphe1]NC(1-13)NH2 treatments on the above assays among Post-EtOH rats. Additionally, neither aCSF nor [Nphe1]NC(1-13)NH2 alters locomotor activity in OFT (Fig. S3E) and EPM (Fig. 2K) regardless of alcohol exposure history. The histological study confirmed all cannulae tips are within the RMTg (Fig. 2O, P).
In addition, considering the anatomical position of the VTA is close to the RMTg and has high NOP expression, we also tested the effect of intra-VTA N/OFQ on anxiety-like behaviors in a separate group of Post-EtOH rats (n = 10 rats) to examine whether RMTg N/OFQ infusion is possibly mediated by modulation of NOP receptors in neighboring nuclei (Fig. S3A). Unlike what we observed in the RMTg, intra-VTA N/OFQ failed to rescue the anxiety symptoms in EPM and OFT, even displaying an anxiogenic effect in MBT in Post-EtOH rats (Fig. S3B–I). Thus, these results suggest that RMTg NOP activation alleviates anxiety-like behaviors in Post-EtOH rats, while RMTg NOP inhibition produces anxiety-like behavior in Naïve rats.
Activating RMTg NOP reduces depression-like behaviors in alcohol-withdrawn rats
Depression is common among people who abuse alcohol. Anhedonia and depression contribute significantly to relapse [1]. We previously reported that RMTg inactivation alleviates depressive-like behaviors in Post-EtOH rats [10]. To determine whether the RMTg N/OFQ-NOP system is involved in depressive-like behaviors, we used the forced swimming test (FST) and sucrose preference test (SPT) in the Post-EtOH, and naïve rats (Fig. 3A).
A the schematic diagram shows the experimental timeline. Rats at 48 h alcohol withdrawal showed despair and anhedonia, measured by forced swimming test (FST, B–D) and sucrose preference test (SPT, E) compared to Naïve rats. Intra-RMTg infusion of N/OFQ (0.01 nM/100 nL/site) significantly shortened immobility (B), increased the swimming time (C), and increased the preference for sucrose (E) in Post-EtOH rats. Conversely, [Nphe1]NC(1-13)NH2 (5 nM/100 nL/site) infusion significantly increased immobility (F) but did not alter other FST behaviors (G, H) and sucrose preference (I) in Naïve rats. J schematic drawings of coronal sections of the rat brain showing the tips of injector placements from individual Naïve (black circle) and Post-EtOH rats (blue square). #p < 0.05, ##p < 0.01, ###p < 0.001 and ####p < 0.0001 vs Naïve group; *p < 0.05, **p < 0.01 and ***p < 0.001 vs aCSF within group, revealed by two-way RM ANOVA followed by Bonferroni’s post hoc test. n = 6-8 rats/group. All data are shown as mean ± SEM.
As shown in Fig. 3B–E, the two-way RM ANOVA analysis revealed significant group main effects on duration of immobility (Fig. 3B, F1,12 = 5.936, p = 0.0314), climbing (Fig. 3D, F1,12 = 7.260, p = 0.0195) in FST, as well as the sucrose preference (Fig. 3E, F1,11 = 11.13, p = 0.0066) in SPT. The post-hoc tests found that Post-EtOH rats displayed a longer immobility duration (p = 0.0009) and shorter swimming period (p = 0.0303) than naive counterparts, indicating depression-like symptoms. Besides, Post-EtOH rats showed anhedonia, as reflected by the marked decline in sucrose preference (p = 0.0099). These observations are consistent with our earlier report [10].
Notably, the two-way RM ANOVA analysis revealed intra-RMTg infusion of N/OFQ had a group × treatment interaction effect on the duration of immobility (Fig. 3B, F1,12 = 13.83, p = 0.0029) and swimming (Fig. 3C, F1,12 = 8.419, p = 0.0132) and in FST, as well as a significant main treatment effect on sucrose preference (Fig. 3E, F1,11 = 7.511, p = 0.0192). Also, post-hoc tests revealed that within the Post-EtOH group but not the Naïve group, intra-RMTg N/OFQ significantly shortened the immobility time (p = 0.0013), elevated swimming duration (p = 0.0137), and sucrose preference (p = 0.0343), compared to intra-RMTg aCSF infusion.
We next determined the role of RMTg NOP in depression-like phenotypes by local infusion of [Nphe1]NC(1-13)NH2 in Naïve and Post-EtOH rats. The two-way RM ANOVA revealed that intra-RMTg infusion of [Nphe1]NC(1-13)NH2 had significant main treatment effects on the duration of immobility (Fig. 3F, F1,12 = 12.80, p = 0.0038) and swimming (Fig. 3G, F1,12 = 4.763, p = 0.0497) in FST, and sucrose preference in SPT (Fig. 3I, F1,13 = 15.15, p = 0.0019). The post-hoc test found [Nphe1]NC(1-13)NH2 significantly enhanced immobility time (p = 0.0254) in FST in Naive rats.
In addition, like our observation mentioned in the anxiety assay, intra-VTA N/OFQ shows an aggregation trend of anhedonia and despair phenotypes in a separate group of Post-EtOH rats in the FST and SPT (Fig. S3J–N). Overall, these results suggest that RMTg NOP might control despair behaviors.
Activation of RMTg NOP reduces voluntary alcohol consumption of rats
After verifying the roles of RMTg NOP in anxiety- and depression-like behaviors, we next tested the hypothesis that activating RMTg NOP might limit alcohol consumption. In a separate cohort of rats, the alcohol drinking levels were escalated and maintained at 5.38 ± 0.46 g/kg/24 h at the end of 24 sessions under the IA2BC paradigm, keeping with earlier reports [19, 35]. N/OFQ, [Nphe1]NC(1-13)NH2, or aCSF were infused into the RMTg 5–10 min before the onset of the drinking session (Fig. 4A). Figure 4B–I summarize the effects of these chemicals on ethanol consumption during 24 h following the infusion. Two-way RM ANOVA detected that intra-RMTg N/OFQ produced a significant treatment × time interaction effect on ethanol intake (F3,72 = 4.095, p = 0.0097, Fig. 4B), the main treatment effect on ethanol preference (F1,72 = 4.424, p = 0.0389, Fig. 4C). The post-hoc test revealed that N/OFQ reduces 24 h ethanol intake compared to aCSF (p < 0.0001) but did not alter water and total liquid intake. By contrast, [Nphe1]NC(1-13)NH2 did not affect ethanol and water intake (all p > 0.05). Nevertheless, intra-VTA N/OFQ infusion did not significantly affect alcohol drinking or water consumption tested in a separated cohort of Post-EtOH rats (Fig. S4). The result indicates that activating the RMTg NOP receptors could reduce ethanol consumption.
A the schematic diagram shows the experimental timeline. The male adult Long-Evans rats were trained to consume alcohol under the IA2BC drinking paradigm for >10 weeks until the ethanol intake reached and was maintained at plateau levels. These rats were randomly divided into 2 subgroups to test the effect of pharmacological manipulation of RMTg NOP on drinking behaviors. Bar graphs summarizing the change of ethanol drinking behaviors from 20 min to 24 h in response to RMTg N/OFQ (B–E) and [Nphe1]NC (1-13)NH2 (F–I) administration. J schematic drawings of coronal sections of rat brains showing the tips of injector placements from individual Naïve (black circle) and Post-EtOH rats (blue square). ****p < 0.0001 vs. aCSF, revealed by two-way RM ANOVA followed by Bonferroni’s post hoc test. n = 10 rats/group. All data are shown as mean ± SEM.
Manipulating RMTg NOP on RMTg cell activity
To further understand the cellular mechanisms underlying the behavioral changes, we measured neuronal activity with c-Fos immunoreactivity (c-Fos-IR) after intra-RMTg infusion of N/OFQ and [Nphe1]NC(1-13)NH2, respectively (Fig. 5A). As shown in Fig. 5B, the two-way ANOVA revealed that intra-RMTg chemical injection had a significant group × treatment interaction effect on RMTg c-Fos-IR cell numbers (F2,67 = 5.012, p = 0.0088). The post-hoc test found that compared to Naïve rats, c-Fos-IR cell number was significantly increased in the RMTg of Post-EtOH rats (p < 0.0001), which was reduced after intra-RMTg N/OFQ infusion (p < 0.0001). By contrast, intra-RMTg N/OFQ infusion did not affect RMTg c-Fos-IR cell number among Naive rats (p = 0.8123). Conversely, compared to aCSF, [Nphe1]NC(1-13)NH2 elevated c-Fos-IR cell numbers in Naive rats (p = 0.0014) but not in Post-EtOH rats (p = 0.1991, Fig. 5C).
A The schematic diagram shows the experimental timeline. B Representative images of the c-Fos-immunoreactivity (IR) in the RMTg in the Naïve and 48 h withdrawn rats (Post-EtOH), in response to aCSF, N/OFQ, and [Nphe1]NC(1-13)NH2. C The bar graph illustrates the RMTg c-Fos-IR cell numbers of Naive and Post-EtOH. **p < 0.01 vs. aCSF within the group; ^p < 0.05, ^^^^p < 0.0001 vs. N/OFQ within the group, ####p < 0.0001 vs. Naïve aCSF, revealed by two-way ANOVA followed by Bonferroni’s post hoc test. n = 5 rats/group. Scale bar = 100 μm. All data are shown as mean ± SEM.
Discussion
Here, we showed increased Oprl1 and decreased Pnoc in VTA-projecting RMTg neurons of Post-EtOH rats. Activating RMTg NOP alleviated anxiety- and depression-like behaviors and reduced relapse-like drinking in Post-EtOH rats, while inhibiting RMTg NOP elicited anxiety- and depression-like behaviors in Naïve rats. The changes in behaviors mirror changes in RMTg c-Fos expression. Significantly, activating RMTg NOP reduced ethanol consumption. These data suggest that the RMTg N/OFQ-NOP system involves negative affective behaviors during alcohol withdrawal and relapse-like drinking.
The RMTg, a GABAergic structure, sends dense projections to the midbrain dopamine neurons and plays significant roles in aversive learning, addiction, and other motivated behaviors [11, 13, 47]. We previously reported that increased RMTg neuronal activity drives affective disorders in Post-EtOH rats [10]. However, we know little about the underlying molecular mechanisms. The recently published RNA sequencing data showed that Pnoc was enriched in the RMTg [34]. Since N/OFQ is derived from the prepronociceptin encoded by Pnoc, we conducted the current study to determine whether RMTg Pnoc encoded protein N/OFQ regulates the negative aversive behaviors and RMTg activity.
To delineate the VTA-projecting RMTg neurons, we infused green retrobeads into the VTA [10]. The FISH and immunofluorescence results showed that Pnoc or N/OFQ were situated in the RMTg neurons, in keeping with a recent report [34]. We found about 80% Pnoc expressing cells were colocalized with VTA-projecting RMTg cells in the coordinate (in the range of −6.8 ~ −7.1 mm from Bregma) (according to a previous report [45]). This is higher than that (55%) reported in a recent study, measured in the range of −6.2 ~ −7.4 mm from Bregma [34]. In addition, given the three-dimensional architecture of the RMTg, a significant challenge is to isolate RMTg tissue precisely using the conventional punch method. To circumvent this challenge, we applied LCM combined with qPCR [48] to strictly collect RMTg in frozen tissue to test how the N/OFQ-NOP signaling changed by chronic alcohol exposure and alcohol abstinence.
N/OFQ and its cognate receptors NOP are widely expressed in the brain. N/OFQ is a 17-amino acid neuropeptide structurally related to opioid peptide dynorphin A. Despite its structural homology, N/OFQ does not bind to mu, delta, and kappa opioid receptors, nor do opioid peptides activate NOP [21]. The roles of the N/OFQ-NOP system was extensively investigated in substance abuse, including alcohol addiction, which was well addressed in an elegant review article [49].
Regarding the alcohol-induced change of N/OFQ-NOP-related genes, significant increases and decreases in gene transcription in several brain regions in rats were reported, depending on various ethanol administration routes, ethanol intake, and rat lines used in the experiments [30, 33, 50]. Here, we show that alcohol withdrawal increased Oprl1 mRNA levels in the RMTg. This finding is generally consistent with previous reports that enhanced the NOP gene expression in BNST of alcohol-preferring rats at 24 h abstinence [51] and CeA and lateral hypothalamus at 1 and 3 weeks of abstinence [33]. Besides, similarly increased NOP expression patterns and binding activity was reported in alcohol-preferring rats [30]. Moreover, we speculate that the enhanced NOP gene expression may reflect a cascade of neuroadaptive change triggered by ethanol withdrawal since no significant RMTg NOP gene expression change between naïve and continuous drinking rats. Although recent human research based on PET study on alcoholic subjects revealed no change in NOP receptor had been found but does necessarily rule out alterations in nociceptin transmission in alcohol dependence [52].
In addition, the decreased trend of Pnoc mRNA levels is in keeping with previous studies showing decreased Pnoc and N/OFQ in human alcoholics [53] and withdrawal animals [1, 27, 54]. Considering the stressful situation of affective states during withdrawal mobilizes the N/OFQ system might occur in weeks [33], we interpreted the decrease as likely an initial transit change. A future study must evaluate the Pnoc change at extended periods during abstinence.
Additionally, compared to naïve animals, alcohol exposure decreased RMTg Pnoc expression whether the subjects underwent non-abstinence or withdrawal, while Oprl1 only increased during withdrawal period rats with no significant change during non-abstinent. These observations imply a fluctuation change of RMTg N/OFQ-NOP signaling at the different stages of alcohol drinking.
Similar fluctuation patterns were also found in molecules involved in alcohol drinking, including growth factors that modulate alcohol consumption [55, 56]. For example, Gdnf acts as an alcohol-responsive gene, showing up-regulation during short-term alcohol intake but down-regulated during withdrawal from excessive alcohol intake in VTA [57]. Functionally, Gdnf exerts acute inhibitory effects on reducing alcohol consumption and seeking behavior [58]. Besides, Bdnf displays a more complicated expression pattern at the different stages of alcohol exposure in various brain regions. Generally, Bdnf signaling in dorsolateral striatum activation is required for maintaining moderate drinking while suppressing high intake in corticostriatal circuitry [55].
Additionally, N/OFQ appears to buffer the brain stress state, influencing the vulnerability to the development and perpetuation of addiction [1]. We observed high anxiety and depression levels in Post-EtOH rats, keeping with our previous reports [10, 46]. Given that these rats were unhabituated to the anxiety assessment apparatus, this observation suggests that rats chronically exposed to alcohol have a higher innate sensitivity to stress induced by novel circumstances than naive animals [59]. Moreover, we noticed that these aberrant behaviors were more evident in Post-EtOH rats than in the Naïve counterparts or the rats in the IA2BC paradigm without abstinence [46], suggesting that alcohol withdrawal plays a significant role in affective disorders.
We used local pharmacology to determine the function of the RMTg N/OFQ-NOP system in the affective disorders associated with alcohol withdrawal. Activating RMTg NOP shows potent anxiolytic and antidepressant effects in Post-EtOH rats. Inactivating RMTg NOP elicits anxiety while partly extending immobility in forced swim tests in Naïve animals. These results suggest NOP and endogenous N/OFQ in the RMTg might regulate negative moods. This idea is supported by the view that the N/OFQ system plays a central role in the anxiolytic effect against stress response [60], as evidenced in several experimental models [33, 60,61,62,63], utilized by intracranial infusion of N/OFQ [61, 64,65,66] or NOP exogenous agonist [67,68,69,70]. Moreover, the current data is consistent with previous reports showing that N/OFQ reduces symptoms and anxiety during alcohol withdrawal [30, 71], particularly in dependent animals [72].
Interestingly, intra-RMTg administration of the NOP antagonist did not alter the percent time spent and the entries of OA/CA in EPM, and the times of entries and time in the central area in the OFT of Post-EtOH rats. Notably, these parameters were low even when these animals received aCSF injections. Thus, it is possible that alcohol withdrawal induces a floor effect that did not leave enough room for the chemical to act. Conversely, Post-EtOH rats that received aCSF injection buried a high number of marbles, which may cause a ceiling effect that did not leave enough room for the chemical to act.
Our result differs from a previous study that reported that NOP receptor antagonists administered into the lateral ventricle were anxiolytic [70]. The difference suggests that the anxiolytic function of RMTg NOP receptors is overridden by the anxiogenic effects somewhere else in the brain. Like, dorsal raphe and amygdala NOP activation suppress local 5-HT and NE levels, displaying depressogenic results [73].
Relapse drinking is a significant hurdle in AUD treatment and is driven by the negative affective state accompanying alcohol withdrawal, characterized by depression and anxiety phenotypes [74]. Decreased alcohol consumption following intra-RMTg infusion of N/OFQ may be resulted from alleviating anxiety and depression. We did not evaluate the effect of RMTg NOP activation on consummatory behavior. However, since nociceptin injection did not change water intake or locomotion, nociceptin-induced decreased alcohol consumption is unlikely to be secondary to consummatory suppression or locomotion interference.
A series of preclinical studies have reported that N/OFQ is efficacious against the rewarding property of alcohol, shedding light on its potential therapeutic effect in AUD. Systemic N/OFQ administration reduced alcohol intake in alcohol-preferring rats and blunted the reinforcing and motivating behaviors in the conditional place preference paradigm and operant self-administration of ethanol [29, 31, 32]. Remarkably, intracerebroventricular N/OFQ infusion significantly inhibited relapse drinking induced by foot shock in male Wistar rats [75], highlighting that N/OFQ has treatment potential in controlling anxiety-related drinking behavior.
It has been well accepted that alcohol withdrawal produced a deficit in dopamine in which basal dopamine concentration and tonic dopamine signals were disproportionately lower than the phasic dopamine signals, triggering the pursuit of abused substances [1]. Glover et al. and we previously showed that increased RMTg activity could account, at least partially, for the reduced activity of dopamine neurons during acute alcohol withdrawal [10, 18].
NOP inhibits VTA neurons by hyperpolarization [76]. Thus, NOP may also inhibit RMTg cells by the exact mechanism. The observation supports the possibility that N/OFQ normalized RMTg c-Fos-IR numbers increased during acute alcohol withdrawal [10]. A future study using the patch-clamp technique will determine whether NOP causes hyperpolarization of RMTg cells.
Importantly, to exclude the effect of N/OFQ infusion into the RMTg is not mediated by modulating NOP receptors in neighboring nuclei, we assessed the effect of intra-VTA N/OFQ infusion in Post-EtOH rats. Unlike what was displayed by intra-RMTg infusion, intra-VTA N/OFQ causes an anxiogenic action, and depressogenic action, without affecting ethanol drinking. There is recent evidence that NOP antagonism in the VTA attenuates alcohol drinking and relapse [77, 78] and that N/OFQ acts as a rewarding stop signal in the VTA [76]. These studies suggest that the VTA NOP receptors might have a complicated role in reward-related behaviors due to the complex heterogeneity of neuron types and anatomical localization in the VTA. Overall, these opposite behavioral phenotype patterns might implicate the distinct regulation trend of NOP between RMTg and VTA in affective disorders, supporting the notion of the role of RMTg as a mirror VTA function [7, 8].
N/OFQ has a general inhibitory effect on excitatory neurotransmissions through pre-and post-synaptic mechanisms. NOP activation by nociceptin and its analogs might negatively impact adenylyl cyclase, activating inward rectifying K+ channels and inhibiting Ca2+[79]. We speculate that intra-RMTg N/OFQ alleviates anxiety- and depression-like behaviors might involve a reduction of glutamatergic transmission from the LHb, which were elevated in Post-EtOH animals [40, 80], although this assumption needed to be tested in future studies.
Lastly, a limitation of the current study is that we tested only the males, although few previous studies reported sex differences in NOP receptors regulating ethanol drinking [49]. Future study in females is needed to enhance the translational value of preclinical findings [81].
In summary, we report that alcohol withdrawal decreases Pnoc but enhances Oprl1 mRNA levels in the VTA-projecting RMTg neurons in male rats. Pharmacological manipulation RMTg NOP modulates cFos expression and affective mood disorders in animals withdrawn from chronic excessive alcohol drinking and reducing relapse-like drinking. These results highlight the role of the RMTg-VTA circuit in the NOP receptors modulating alcohol drinking and its associated anxiety and depression symptoms.
References
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 2016;3:760–73.
Martinot M, Bragulat V, Artiges E, Dolle F, Hinnen F, Jouvent R, et al. Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. Am J Psychiatry 2001;158:314–6.
Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013;493:537–41.
Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharm. 1992;221:227–34.
Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci USA. 1993;90:7966–9.
Diana M, Muntoni AL, Pistis M, Melis M, Gessa GL. Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur J Neurosci. 1999;11:1037–41.
Jhou TC. The rostromedial tegmental (RMTg) “brake” on dopamine and behavior: A decade of progress but also much unfinished work. Neuropharmacology 2021;198:108763.
Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC. Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. J Neurosci. 2012;32:14094–101.
Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800.
Fu R, Zuo W, Shiwalkar N, Mei Q, Fan Q, Chen X, et al. Alcohol withdrawal drives depressive behaviors by activating neurons in the rostromedial tegmental nucleus. Neuropsychopharmacology 2019;44:1464–75.
Vento PJ, Burnham NW, Rowley CS, Jhou TC. Learning from one’s mistakes: a dual role for the rostromedial tegmental nucleus in the encoding and expression of punished reward seeking. Biol Psychiatry 2017;81:1041–49.
Li H, Pullmann D, Cho JY, Eid M, Jhou TC. Generality and opponency of rostromedial tegmental (RMTg) roles in valence processing. Elife. 2019;8. https://doi.org/10.7554/eLife.41542.
Li H, Pullmann D, Jhou TC. Valence-encoding in the lateral habenula arises from the entopeduncular region. Elife. 2019;8. https://doi.org/10.7554/eLife.41223.
Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, et al. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–12.
Huff ML, LaLumiere RT. The rostromedial tegmental nucleus modulates behavioral inhibition following cocaine self-administration in rats. Neuropsychopharmacology 2015;40:861–73.
Steidl S, Myal S, Wise RA. Supplemental morphine infusion into the posterior ventral tegmentum extends the satiating effects of self-administered intravenous heroin. Pharm Biochem Behav. 2015;134:1–5.
Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. gamma-Aminobutyric acid cells with cocaine-induced DeltaFosB in the ventral tegmental area innervate mesolimbic neurons. Biol Psychiatry 2010;67:88–92.
Glover EJ, Starr EM, Chao Y, Jhou TC, Chandler LJ. Inhibition of the rostromedial tegmental nucleus reverses alcohol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology 2019;44:1896–905.
Fu R, Chen X, Zuo W, Li J, Kang S, Zhou LH, et al. Ablation of mu opioid receptor-expressing GABA neurons in rostromedial tegmental nucleus increases ethanol consumption and regulates ethanol-related behaviors. Neuropharmacology 2016;107:58–67.
Sheth C, Furlong TM, Keefe KA, Taha SA. Lesion of the rostromedial tegmental nucleus increases voluntary ethanol consumption and accelerates extinction of ethanol-induced conditioned taste aversion. Psychopharmacol (Berl). 2016;233:3737–49.
Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 1995;270:792–4.
Neal CR Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ Jr. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999;406:503–47.
Neal CR Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, et al. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999;412:563–605.
Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M. Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron. 2012;76:192–208.
Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003;23:9445–51.
Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, et al. The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharm Ther. 2014;141:283–99.
Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34.
Aziz AM, Brothers S, Sartor G, Holm L, Heilig M, Wahlestedt C, et al. The nociceptin/orphanin FQ receptor agonist SR-8993 as a candidate therapeutic for alcohol use disorders: validation in rat models. Psychopharmacol (Berl). 2016;233:3553–63.
Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacol (Berl). 1999;141:220–4.
Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, et al. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry 2008;64:211–8.
Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharm Exp Ther. 2003;304:310–8.
Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, et al. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology. 2004;172:170–8.
Aujla H, Cannarsa R, Romualdi P, Ciccocioppo R, Martin-Fardon R, Weiss F. Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict Biol. 2013;18:467–79.
Smith RJ, Vento PJ, Chao YS, Good CH, Jhou TC. Gene expression and neurochemical characterization of the rostromedial tegmental nucleus (RMTg) in rats and mice. Brain Struct Funct. 2019;224:219–38.
Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–23.
Paxinos G & Watson C. The Rat Brain in Stereotaxic Coordinates, 6th ed. San Diego: Academic Press; 2007.
Bytner B, Huang YH, Yu LC, Lundeberg T, Nylander I, Rosen A. Nociceptin/orphanin FQ into the rat periaqueductal gray decreases the withdrawal latency to heat and loading, an effect reversed by (Nphe(1))nociceptin(1-13)NH(2). Brain Res. 2001;922:118–24.
Fernandez F, Misilmeri MA, Felger JC, Devine DP. Nociceptin/orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacology 2004;29:59–71.
Mallimo EM, Kusnecov AW. The role of orphanin FQ/nociceptin in neuroplasticity: relationship to stress, anxiety and neuroinflammation. Front Cell Neurosci. 2013;7:173.
Li J, Kang S, Fu R, Wu L, Wu W, Liu H, et al. Inhibition of AMPA receptor and CaMKII activity in the lateral habenula reduces depressive-like behavior and alcohol intake in rats. Neuropharmacology 2017;126:108–20.
Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 2013;341:1517–21.
Espina V, Wulfkuhle JD, Calvert VS, VanMeter A, Zhou W, Coukos G, et al. Laser-capture microdissection. Nat Protoc. 2006;1:586–603.
Ellis P, Moore L, Sanders MA, Butler TM, Brunner SF, Lee-Six H, et al. Reliable detection of somatic mutations in solid tissues by laser-capture microdissection and low-input DNA sequencing Nat Protoc. 2021;16:841–71.
Weisz HA, Boone DR, Sell SL, Hellmich HL. Stereotactic atlas-guided laser capture microdissection of brain regions affected by traumatic injury. J Vis Exp. 2017;127. https://doi.org/10.3791/56134.
Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–96.
Fu R, Mei Q, Shiwalkar N, Zuo W, Zhang H, Gregor D, et al. Anxiety during alcohol withdrawal involves 5-HT2C receptors and M-channels in the lateral habenula. Neuropharmacology 2020;163:107863.
Sanchez-Catalan MJ, Faivre F, Yalcin I, Muller MA, Massotte D, Majchrzak M, et al. Response of the tail of the ventral tegmental area to aversive stimuli. Neuropsychopharmacology 2017;42:638–48.
Baskin DG, Bastian LS. Immuno-laser capture microdissection of rat brain neurons for real time quantitative PCR. Methods Mol Biol. 2010;588:219–30.
Ciccocioppo R, Borruto AM, Domi A, Teshima K, Cannella N, Weiss F. NOP-related mechanisms in substance use disorders. Handb Exp Pharm. 2019;254:187–212.
D’Addario C, Caputi FF, Rimondini R, Gandolfi O, Del Borrello E, Candeletti S, et al. Different alcohol exposures induce selective alterations on the expression of dynorphin and nociceptin systems related genes in rat brain. Addict Biol. 2013;18:425–33.
Caputi FF, Stopponi S, Rullo L, Palmisano M, Ubaldi M, Candeletti S, et al. Dysregulation of nociceptin/orphanin FQ and dynorphin systems in the extended amygdala of alcohol preferring marchigian sardinian (msP) rats. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms22052448.
Narendran R, Ciccocioppo R, Lopresti B, Paris J, Himes ML, Mason NS. Nociceptin receptors in alcohol use disorders: a positron emission tomography study using [(11)C]NOP-1A. Biol Psychiatry 2018;84:708–14.
Kuzmin A, Bazov I, Sheedy D, Garrick T, Harper C, Bakalkin G. Expression of pronociceptin and its receptor is downregulated in the brain of human alcoholics. Brain Res. 2009;1305:S80–5.
Ploj K, Roman E, Gustavsson L, Nylander I. Basal levels and alcohol-induced changes in nociceptin/orphanin FQ, dynorphin, and enkephalin levels in C57BL/6J mice. Brain Res Bull. 2000;53:219–26.
Liran M, Rahamim N, Ron D, Barak S. Growth factors and alcohol use disorder. Cold Spring Harb Perspect Med. 2020;10. https://doi.org/10.1101/cshperspect.a039271.
Ron D, Barak S. Molecular mechanisms underlying alcohol-drinking behaviours. Nat Rev Neurosci. 2016;17:576–91.
Barak S, Ahmadiantehrani S, Logrip ML, Ron D. GDNF and alcohol use disorder. Addict Biol. 2019;24:335–43.
Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci USA. 2008;105:8114–9.
Cannella N, Ubaldi M, Masi A, Bramucci M, Roberto M, Bifone A, et al. Building better strategies to develop new medications in Alcohol Use Disorder: Learning from past success and failure to shape a brighter future. Neurosci Biobehav Rev. 2019;103:384–98.
Ciccocioppo R, de Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, et al. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J Neurosci. 2014;34:363–72.
Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ Jr., et al. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci USA. 1997;94:14854–8.
Filaferro M, Ruggieri V, Novi C, Calo G, Cifani C, Micioni Di Bonaventura MV, et al. Functional antagonism between nociceptin/orphanin FQ and corticotropin-releasing factor in rat anxiety-related behaviors: involvement of the serotonergic system. Neuropeptides. 2014;48:189–97.
Vitale G, Arletti R, Ruggieri V, Cifani C, Massi M. Anxiolytic-like effects of nociceptin/orphanin FQ in the elevated plus maze and in the conditioned defensive burying test in rats. Peptides. 2006;27:2193–200.
Gavioli EC, Rae GA, Calo G, Guerrini R, De Lima TC. Central injections of nocistatin or its C-terminal hexapeptide exert anxiogenic-like effect on behaviour of mice in the plus-maze test. Br J Pharm. 2002;136:764–72.
Kamei J, Matsunawa Y, Miyata S, Tanaka S, Saitoh A. Effects of nociceptin on the exploratory behavior of mice in the hole-board test. Eur J Pharm. 2004;489:77–87.
Uchiyama H, Toda A, Hiranita T, Watanabe S, Eyanagi R. Role of amygdaloid nuclei in the anxiolytic-like effect of nociceptin/orphanin FQ in rats. Neurosci Lett. 2008;431:66–70.
Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, et al. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci USA. 2000;97:4938–43.
Varty GB, Hyde LA, Hodgson RA, Lu SX, McCool MF, Kazdoba TM, et al. Characterization of the nociceptin receptor (ORL-1) agonist, Ro64-6198, in tests of anxiety across multiple species. Psychopharmacology 2005;182:132–43.
Lu SX, Higgins GA, Hodgson RA, Hyde LA, Del Vecchio RA, Guthrie DH, et al. The anxiolytic-like profile of the nociceptin receptor agonist, endo-8-[bis(2-chlorophenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octane-3-carboxami de (SCH 655842): comparison of efficacy and side effects across rodent species. Eur J Pharm. 2011;661:63–71.
Duzzioni M, Duarte FS, Leme LR, Gavioli EC, De Lima TC. Anxiolytic-like effect of central administration of NOP receptor antagonist UFP-101 in rats submitted to the elevated T-maze. Behav Brain Res. 2011;222:206–11.
Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, et al. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin Exp Res. 2011;35:747–55.
Aujla H, Nedjadrasul D. Low-dose Nociceptin/Orphanin FQ reduces anxiety-like performance in alcohol-withdrawn, but not alcohol-naive, Male Wistar rats. Neuropharmacology 2015;93:1–6.
Gavioli EC, Calo G. Nociceptin/orphanin FQ receptor antagonists as innovative antidepressant drugs. Pharm Ther. 2013;140:10–25.
Gowing LR, Ali RL, Allsop S, Marsden J, Turf EE, West R, et al. Global statistics on addictive behaviours: 2014 status report. Addiction. 2015;110:904–19.
Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport 2000;11:1939–43.
Zheng F, Grandy DK, Johnson SW. Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br J Pharm. 2002;136:1065–71.
Borruto AM, Fotio Y, Stopponi S, Brunori G, Petrella M, Caputi FF, et al. NOP receptor antagonism reduces alcohol drinking in male and female rats through mechanisms involving the central amygdala and ventral tegmental area. Br J Pharm. 2020;177:1525–37.
Borruto AM, Fotio Y, Stopponi S, Petrella M, De Carlo S, Domi A, et al. NOP receptor antagonism attenuates reinstatement of alcohol-seeking through modulation of the mesolimbic circuitry in male and female alcohol-preferring rats. Neuropsychopharmacology 2021;46:2121–31.
Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT. Nociceptin/Orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharm Rev. 2016;68:419–57.
Kang S, Li J, Bekker A, Ye JH. Rescue of glutamate transport in the lateral habenula alleviates depression- and anxiety-like behaviors in ethanol-withdrawn rats. Neuropharmacology 2018;129:47–56.
Borruto AM, Stopponi S, Li H, Weiss F, Roberto M, Ciccocioppo R. Genetically selected alcohol-preferring msP rats to study alcohol use disorder: Anything lost in translation? Neuropharmacology 2021;186:108446.
Acknowledgements
We thank Professors Yun-Qing Li and Li-Hua Zhou for reading the whole manuscript.
Funding
This work is made possible by the National Natural Science Foundation of China (82071496 to RF), the Young Teacher Foundation of Sun Yat-sen University (59000-18841219 RF), Natural Science Foundation of Guangdong Province (2021 A1515010463 to RF), Shenzhen Science and Technology Program (202205303001697 to RF) the Fellowship of China Postdoctoral Science Foundation (2022M711491 to YT).
Author information
Authors and Affiliations
Contributions
WFL contributed to data collection, analysis, and interpretation and wrote and edited the manuscript. WFL, ZHR, YT, YXF, and SZS performed behavioral tests. WFL, RXD, JWH, YLM, BZ, and YXZ conducted molecular biology and histology experiments. WHZ and JHY contributed to the interpretation of data and editing of the manuscript. RF contributed to experimental design and conception, data interpretation, editing, and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, W., Ren, Z., Tang, Y. et al. Rostromedial tegmental nucleus nociceptin/orphanin FQ (N/OFQ) signaling regulates anxiety- and depression-like behaviors in alcohol withdrawn rats. Neuropsychopharmacol. 48, 908–919 (2023). https://doi.org/10.1038/s41386-022-01482-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01482-3
This article is cited by
-
Attenuated incubation of ethanol-induced conditioned taste aversion in a model of dependence
Psychopharmacology (2024)
-
LPA1 receptors in the lateral habenula regulate negative affective states associated with alcohol withdrawal
Neuropsychopharmacology (2023)
-
The rostromedial tegmental nucleus RMTg is not a critical site for ethanol-induced motor activation in rats
Psychopharmacology (2023)