Abstract
Irritability, defined as proneness to anger, is among the most common reasons youth are seen for psychiatric care. Youth with irritability demonstrate aberrant processing of anger-related stimuli; however, the neural mechanisms remain unknown. We applied a drift-diffusion model (DDM), a computational tool, to derive a latent behavioral metric of attentional bias to angry faces in youth with varying levels of irritability during functional magnetic resonance imaging (fMRI). We examined associations among irritability, task behavior using a DDM-based index for preferential allocation of attention to angry faces (i.e., extra-decisional time bias; Δt0), and amygdala context-dependent connectivity during the dot-probe task. Our transdiagnostic sample, enriched for irritability, included 351 youth (ages 8–18; M = 12.92 years, 51% male, with primary diagnoses of either attention deficit/hyperactivity disorder [ADHD], disruptive mood dysregulation disorder [DMDD], an anxiety disorder, or healthy controls). Models accounted for age, sex, in-scanner motion, and co-occurring symptoms of anxiety. Youth and parents rated youth’s irritability using the Affective Reactivity Index. An fMRI dot-probe task was used to assess attention orienting to angry faces. In the angry-incongruent vs. angry-congruent contrast, amygdala connectivity with the bilateral inferior frontal gyrus (IFG), insula, caudate, and thalamus/pulvinar was modulated by irritability level and attention bias to angry faces, Δt0, all ts350 > 4.46, ps < 0.001. In youth with high irritability, elevated Δt0 was associated with a weaker amygdala connectivity. In contrast, in youth with low irritability, elevated Δt0 was associated with stronger connectivity in those regions. No main effect emerged for irritability. As irritability is associated with reactive aggression, these results suggest a potential neural regulatory deficit in irritable youth who have elevated attention bias to angry cues.
Similar content being viewed by others
Introduction
Irritability, characterized by increased proneness to anger, is among the most common reasons that families seek psychiatric care [1, 2]. Irritability is highly impairing and common, implicated in multiple clinical conditions in youth, and is associated with negative outcomes in adulthood including high suicidality, depression and anxiety disorders, low income, and poor academic performance [3, 4]. However, the cognitive and neural underpinnings of irritability remain largely unknown [1, 2, 5, 6]. Using clinically-relevant stimuli (e.g., angry faces), research shows that youth with irritability and elevated trait anger demonstrate cognitive biases towards anger-related cues [7,8,9,10]. Likewise, a low threshold for negative cue detection has been associated with reactive aggression and temper outbursts [11], characteristics of irritable youth. Here, we applied a Drift Diffusion Model (DDM), a class of computational modeling to identify associations among irritability, anger-related attention bias, and neural connectivity during angry-face processing.
Attention orienting, the ability to detect and modulate responses to salient negative environmental cues, is mediated by amygdala-prefrontal circuitry [6, 12,13,14,15] and is impaired in youth with psychopathology [16, 17]. Irritable youth show an attention bias toward angry faces [7, 9, 10], which has been associated with emotional dysregulation and aggressive behaviors [7]. The dot-probe task is a canonical paradigm that assesses selective attention and attention biases [16, 18]; the participant responds to a probe that appears in a location previously occupied by an emotionally-relevant (congruent) or neutral (incongruent) cue. Attentional biases have been typically operationalized as the difference between mean reaction time to probes appearing in emotional (congruent) versus neutral (incongruent) stimulus locations [18, 19]. However, this approach provides a general, coarse metric of bias that shows low retest reliability [20, 21] and does not model key performance-relevant data.
Computational modeling has been harnessed to parse more nuanced cognitive parameters associated with attentional processes [22,23,24]. These approaches account for the full distribution of behavioral reaction times and response choices, and can be used to compute discrete parameters reflecting different cognitive components associated with task performance. Recent work shows that an attention bias index derived from the extra-decisional time component of the DDM yields improved test-retest and split-half reliabilities relative to the aforementioned classic attention bias score [22], suggesting that this component may provide a more reliable metric of attention deployment to emotionally-relevant stimuli.
Using a DDM to compute an attention bias index, this fMRI study examines the neurobiology mediating attentional deployment in the angry-incongruent vs. angry-congruent contrast for youth with varying levels of irritability. Neural circuitry involving the amygdala and the prefrontal cortex has been associated with attention allocation [14, 15, 17, 25,26,27] and with the processing and the regulation of negative emotions [28,29,30,31,32] and anger [33,34,35]. Specifically for youth with psychopathology, decreased amygdala-medial prefrontal cortex connectivity has been found in irritability in the context of negative cues [12]. Additionally, a translational model of irritability [11] posits that amygdala-frontal dysfunction mediates irritability. Based on this theoretical framework and preliminary findings, we anticipated aberrancies in this circuitry in irritable youth during attentional processing of angry-face stimuli. Additionally, the inferior frontal gyrus (IFG) is a region with a critical role in the allocation and shifting of attentional resources [36,37,38]. Thus, we predicted that highly irritable youth, characterized by difficulty in regulating anger, will present decreased amygdala-prefrontal connectivity, and specifically amygdala-IFG connectivity in the angry-incongruent vs. angry-congruent contrast.
This study comprises a large transdiagnostic sample of pediatric patients and healthy controls, capturing a wide spectrum of irritability. First, we leveraged computational modeling to identify the precise component of anger-related attention bias, using the extra-decisional time parameter of the DDM. Second, we examined whether this behavioral metric is associated with amygdala seed-based connectivity and tested whether this association varies with level of irritability. We focused on context-dependent amygdala functional connectivity, performing a generalized psychophysiological interaction (gPPI) [39,40,41]. Identifying the neural circuitry associated with anger-related attentional processing in irritability may advance neurocognitive, mechanism-based interventions.
As irritability and anxiety symptoms commonly co-occur [42] and research demonstrates aberrant attentional processing in anxiety [15, 22, 43], it is essential to account for anxiety when exploring irritability. Therefore, anxiety was accounted for in statistical analyses.
Materials and methods
Participants
A total of 464 youth enrolled. N = 113 were excluded primarily due to poor behavioral or neural data (eMethods 1). The final sample consisted of 351 youth (Age: 8.00–18.00 years; M = 12.92; standard deviation [SD] = 2.66). Of these 351, data from N = 190 (54.13%) were previously published in a study examining overlapping vs. unique neural correlates of irritability and anxiety, using a bifactor analytic approach to parse clinical phenomena [6]. The present study, which almost doubles the participants included in Kircanski and colleagues [6], applies an advanced computational approach to behavioral performance to probe aberrant attentional processing in irritability. Specifically, the sample includes a larger number of patients with ADHD and DMDD, psychopathologies particularly associated with irritability (see Table 1). Data from N = 161 (N = 96 patients) have not been examined. Parents/children gave written informed consent/assent. Participants received monetary compensation. The NIMH IRB approved all study procedures. Data were acquired between June 30, 2012, and November 30, 2019.
Clinical characteristics
Patients were diagnosed with a primary anxiety disorder (generalized, social, or separation anxiety disorder; N = 115), attention-deficit/hyperactivity disorder (ADHD; N = 62), or disruptive mood dysregulation disorder (DMDD; N = 65), diagnoses associated with irritability [44, 45]. Sample included 109 healthy controls to maximize variability in irritability, the clinical dimension of interest. For detailed inclusion/exclusion criteria see eMethods 1. Table 1 summarizes participant characteristics and provides information on pubertal status based on the Tanner scale [46]. Most participants were in early- to mid- pubertal stages, with no significant difference between females and males (t(231) = 1.28, p = 0.20).
Symptom measures
Irritability was assessed using the Affective Reactivity Index (ARI) [47] and anxiety using the Screen for Child Anxiety Related Emotional Disorders (SCARED) [48] (eMethods 2). Averaged scores between youth- and parent-reported ARI and SCARED were generated [12, 49]. There were positive correlations between youth- and parent-reported irritability (r351 = 0.56, p < 0.001) and between average scores of youth- and parent-reports on irritability and anxiety (r351 = 0.46, p < 0.001). The Conners’ Parent Rating Scale [50] was used for post-hoc analyses covarying for ADHD (eResults 3).
Dot-Probe task
Participants performed a dot-probe task adapted from the Tel-Aviv University/NIMH ABMT Initiative (http://people.socsci.tau.ac.il/mu/anxietytrauma/research/) during fMRI (Fig. 1). Task includes three trial types: (1) angry-congruent; (2) angry-incongruent; (3) neutral-neutral (eMethods 1).
Dot-Probe Task Schematic. A pair of faces (either angry-neutral or neutral-neutral) is presented on each trial, followed by a probe (< or >). Participants indicate the probe direction using a button press. The task was provided by the Tel-Aviv University/NIMH Attention Bias Modification Treatment Initiative (http://people.socsci.tau.ac.il/mu/anxietytrauma/research/).
Drift Diffusion Model
A DDM was applied to quantify distinct components of task performance (eMethods 1) [51]. Previous studies applying DDM to the dot-probe task have focused on estimating the drift rate parameter (v), reflecting efficiency of information processing, and the extra-decisional time parameter (t0), reflecting a nondecision component that includes perception duration, stimulus encoding, and motor execution [51, 52]. t0 is particularly relevant for the measurement of attention processing [22] because the affective cues are presented prior to the probe and the initiation of the target-dependent decision process. The difference in t0 between incongruent and congruent trials (Δt0) includes the time during incongruent trials to orient attention away from the angry face display to the target probe in the opposite location, compared to the congruent condition. The angry-incongruent reflects a condition where the probe is in a different place than where the angry face was previously presented. Therefore, Δt0 represents the influence of the anger-related stimulus on attention orienting [22, 52, 53].
Models were fit to the data using fast-dm software version 30.2 [54]. Δt0 scores were calculated by subtracting t0 parameters in the congruent condition from t0 parameters in the incongruent condition (t0 incongruent − t0 congruent) [22]. Larger scores indicate greater anger-related attention bias.
Behavioral analyses included a Spearman correlation and t-tests to assess associations between irritability and age or sex, respectively. To measure associations between anger-related attentional bias and irritability, controlling for covariates, a multivariate linear regression model was applied, including irritability, anxiety, age, and sex as predictors. Δt0 was the outcome variable. To assess associations between irritability and the t0 parameter across specific task conditions (congruent/incongruent/neutral), general linear models (GLMs) were used to model t0 for each task condition as a function of irritability, covarying for anxiety and age.
Neuroimaging acquisition, preprocessing, and analysis
fMRI data were acquired on General Electric 3-T MR750 imaging systems (Waukesha, Wisconsin, USA) with either an 8- or 32-channel head coil. Functional images were analyzed using Analysis of Functional NeuroImages (AFNI) [55]. See Supplement for details on acquisition and first-level analyses (eMethods 3). AFNI’s 3dMVM [56] was used for group-level analyses. GLMs estimating BOLD response and gPPI were used to assess voxel-wise functional connectivity of each left and right amygdala seed as a function of task condition [40, 41]. Specifically, gPPI was used to estimate the magnitude of the seed-time series of amygdala connectivity in the context of the task conditions. Since the angry-incongruent vs. angry-congruent was the a-priori contrast of interest, gPPI was restricted to this contrast, calculated as incongruent minus congruent. Models included Δt0, irritability, and the interaction between Δt0 and irritability (grand-mean centered) as between-subject independent variables. Age, average in-scanner motion, and anxiety score (all grand-mean centered), as well as sex and head-coil during acquisition (dummy coded) were covariates.
Results were thresholded voxel-wise at p < 0.005. Cluster correction was used to control for multiple tests via 3dClustSim (nearest neighbor = 1) and was set to α = 0.05 for activation and to α = 0.025 for functional connectivity (Bonferroni corrected for 2 seeds). We used AFNI’s 3dFWHMx with -acf flag to estimate the smoothness of the residuals via Monte Carlo cluster-size simulation with a Gaussian plus mono-exponential spatial autocorrelation function. Parameters were estimated and averaged for all participants, yielding an effective smoothness of FWHM = 9.14 mm (ACF parameters, a = 0.58, b = 3.42, and c = 10.81), resulting in a cluster-size threshold of k > 60 (1078 mm3) for activation and k > 71 (1313 mm3) for connectivity.
For post-hoc analyses, mean activity and connectivity values for significant clusters of the incongruent vs. congruent contrast were extracted using AFNI’s 3dROIstat program. Using SPSS (version 23.0; SPSS Inc) [57], multivariate linear regression models were conducted using the same variables as the fMRI group analyses. fMRI analyses were then replicated adding medication usage (dummy-coded) as a covariate to the model to test for potential influence of medication status on the results.
Three sets of additional analyses were conducted (eResults 1–3, eTable 2–3). First, fMRI analyses were replicated adding an anxiety-by-irritability interaction as an additional term; these yielded similar results to the initial models, which included anxiety as a covariate. Second, analyses examined associations of brain function with irritability and Δt0, covarying for ADHD symptoms. All initial findings survived this addition. Third, behavioral and fMRI analyses with the traditional anger-related attention-bias scores were performed.
Results
Demographics and behavior
Age was negatively associated with irritability (rs351 = −0.15, p = 0.005). Sex was significantly associated with irritability (t349 = −2.50, p = 0.013); males exhibited higher irritability levels compared to females. No associations were found between irritability and race, ethnicity, IQ, socioeconomic status (SES) (all ps > 0.554), or medication status (η2 = 0.240). Adjusting for covariates, no significant findings emerged for the association between irritability and Δt0 (p = 0.890). Using a categorical approach to compare Δt0 among diagnostic groups, no significant differences were found (p = 0.240). Additionally, t0 across task conditions was not significantly related to irritability (ps > 0.244). See eTable 1 for descriptive statistics of DDM parameters.
Spearman-Brown split-half reliability of Δt0 in the current sample was 0.20; for the conventional bias score it was 0.02. Fisher’s r-to-z test comparing these reliability scores was significant (z = 2.41, p = 0.016), suggesting that though the reliability of Δt0 is low, it may nonetheless be a somewhat improved index compared to the conventional bias.
Amygdala seed-based gPPI functional connectivity
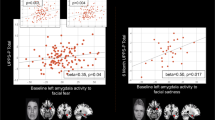
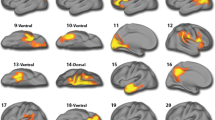
Greater Δt0 was associated with increased left amygdala functional connectivity with bilateral IFG and insula in the angry-incongruent vs. angry-congruent contrast (βs350 > 4.71, ts350 > 2.78, ps = 0.001). However, this main effect was qualified by significant higher-order interactions. Significant interactions were observed between Δt0 and irritability for left amygdala functional connectivity with bilateral IFG and insula (Fig. 2A, B; right IFG: β350 = −1.84, t350 = −4.74, p < 0.001; left IFG: β350 = −1.89, t350 = −4.69, p < 0.001; right insula: β350 = −1.92, t350 = −5.53, p < 0.001; left insula: β350 = −1.71, t350 = −4.47, p < 0.001). As irritability increased, the association between Δt0 and amygdala functional connectivity with these regions became more negative. To further explore these interactions, the sample was grouped into tertiles of low (ARI score cutoff: 1.5), medium (ARI score cutoff: 4.5) and high irritability (ARI score cutoff: above 4.5). For youth with relatively elevated irritability, greater Δt0 was associated with a weaker left amygdala connectivity to these regions, whereas for youth with relatively low irritability, greater Δt0 was associated with stronger connectivity (Table 2, Fig. 3A, B).
Functional left amygdala connectivity bilaterally to the IFG during orienting to angry stimuli is shown in A. Functional left amygdala connectivity bilaterally to the insula during orienting to angry stimuli is shown in B. Functional right amygdala connectivity to the right caudate and the right thalamus/pulvinar during orienting to angry face stimuli is shown in C; Note: Z, Y, X indicate the peak location. Note: these are not individual data; informed consent for publishing the data was obtained from the participants.
A It shows the correlation coefficients for the interaction between extra-decisional time bias (Δt0) and residualized functional connectivity for the left amygdala bilaterally with the IFG and insula; and the right amygdala with the right caudate and the right thalamus/pulvinar, for varying levels of irritability. B It illustrates the data for the correlation coefficients listed in table A for left amygdala functional connectivity with the left IFG during orienting to angry faces. C This illustrates the data for the correlation coefficients listed in Table A for right amygdala functional connectivity with the right thalamus/pulvinar during orienting to angry faces. The effects of motion, age, sex, head-coil channel during acquisition, and SCARED scores were partialled out from mean change in connectivity. Resultant residual change in connectivity during orienting to angry face stimuli by Δt0 for individuals grouped into tertiles of ARI scores (averaged across youth and parent report). Correlation coefficients are given for the plotted data. Primary diagnosis abbreviations are as follows: Anxiety Diagnosis (AD), Attention Deficit Hyperactivity Disorder (ADHD), Healthy Volunteers (Control), Disruptive Mood Dysregulation disorder (DMDD). a P = 0.001. b P = 0.06. c P = 0.002. d P = 0.50. e P < 0.001. f P = 0.60. g P = 0.02. h P = 0.40. i P = 0.39. j P = 0.43. k P = 0.005.
Greater Δt0 was also associated with increased right amygdala functional connectivity with the right caudate and right thalamus/pulvinar in the angry-incongruent vs. angry-congruent contrast (βs350 > 5.27, ts350 > 3.67, ps < 0.001). Again, this was qualified by significant interactions between Δt0 and irritability for right amygdala connectivity with right caudate and right thalamus/pulvinar (Table 2, Fig. 2C; right caudate: β350 = −1.81, t350 = −5.30, p < 0.001; right thalamus/pulvinar: β350 = −1.70, t350 = −5.06, p < 0.001). In youth with relatively elevated irritability, greater Δt0 was associated with a weaker right amygdala connectivity to these regions, whereas for youth with relatively low irritability, greater Δt0 was associated with stronger connectivity (Fig. 3A, 4c). No other significant findings for amygdala connectivity emerged.
Post-hoc analyses adding medication usage as a covariate revealed similar results to the initial model of significant interactions between Δt0 and irritability for amygdala connectivity (βs350 < −1.70, ts350 < −4.43, ps < 0.001).
Activation
Whole-brain analysis revealed that greater Δt0 scores were negatively associated with activation in the right precuneus (β350 = −0.71, t350 = −4.61, p < 0.001) in the angry-incongruent vs. angry-congruent contrast (eFigure 3). No findings emerged for individual differences in irritability. Table 2 presents brain regions showing a significant main effect of Δt0. Post-hoc analyses adding medication usage as a covariate revealed a similar result (β350 = −0.70, t350 = −4.09, p < 0.001).
Discussion
The current study applied computational modeling to examine neural function during the dot-probe task in a large sample of youth with varying levels of irritability. Leveraging DDMs to parse behavior, we discovered a complex three-way interaction between context-dependent amygdala functional connectivity, irritability, and attention bias to angry faces. As irritability increased, the relation between attention bias (i.e., Δt0) and amygdala functional connectivity in the angry-incongruent vs. angry-congruent contrast became more negative. Indeed, as one progresses along the spectrum from participants with the least irritability to those with the most irritability, the association between Δt0 and amygdala-connectivity shifts from a positive to a negative correlation. Thus, while on average participants showed greater Δt0 (i.e., indicates being slower in the incongruent vs. the congruent condition), youth with relatively high irritability demonstrated decreased engagement of regulatory circuitry for the incongruent vs. congruent contrast, while youth with relatively low irritability demonstrated increased engagement of neural regulatory circuitry.
Our findings are consistent with prior connectivity-based studies showing that amygdala connectivity to frontal regions, specifically the IFG, is associated with regulatory processing of emotional cues [25, 26, 58,59,60]. Here, youth who were relatively high on both the irritability spectrum and Δt0 showed a relatively weak coupling between neural regions implicated in effective emotion regulation [61]. Non-irritable youth did not show this differential neural engagement. One potential interpretation is that decreased neural regulation in the context of aberrant attentional processing is maladaptive and may be a risk factor for irritability. It is also possible that reduced top-down engagement develops as a consequence of prolonged irritability, suggesting that experiencing clinical symptoms may have downstream neural effects. Both interpretations speak to aberrant neural regulation as characteristic of irritability uncovered by the current study. Future studies aiming to replicate these findings may include a longitudinal component to help elucidate a potential causal association.
We observed similar patterns of connectivity between the amygdala and subcortical regions implicated in the integration of emotional and attentional processing [62,63,64]. The pulvinar has been previously linked to fast, automatic visual recognition of aversive stimuli, both in non-human primates and in humans [65,66,67]. The insula has been previously identified as a region associated with threat processing and bottom-up detection of salient events [68]. Amygdala connectivity with the insula is assumed to allow the engagement of the salience network to deploy attention when threats appear [69, 70].
The consistency of the current findings across various brain regions implicated with emotional and attentional processing highlights robustness in identifying a potential global neural regulatory deficit in a sub-group of irritable youth. Our findings are aligned with previous phenotypic, behavioral, and neural observations. Clinically, irritable youth have a low threshold for reactive aggression, and demonstrate approach responses to emotionally-relevant stimuli [71]. Behaviorally, youth with irritability or elevated trait anger perceive neutral and ambiguous faces as more threatening and angry [7, 9, 72]. Neurobiologically, when processing angry faces, youth with irritability demonstrate aberrant amygdala, prefrontal, and executive attention network activation [6, 11].
Though the current study did not identify a direct link between irritability and attention bias, we found this association to be qualified by brain connectivity, reflected by the presence of a three-way interaction. Additionally, previous studies showed cognitive biases towards anger-related cues in youth with irritability and elevated trait anger [7,8,9,10]. Accumulating data on the neural bases of attentional processing associated with angry cues and its relation to irritability could potentially contribute targets for intervention in the future; however, more research and replications are needed. There is a significant literature demonstrating the efficacy of attention bias modification training (ABMT) using the dot-probe task in decreasing attention bias in anxiety [73]. This training aims to teach individuals to shift attention away from task-irrelevant negative cues. For example, White et al. [27] showed that ABMT was most effective in anxious patients with elevated attentional bias who also presented abnormal amygdala-insula connectivity. Interestingly, we found an analog association among Δt0, decreased amygdala-insula connectivity, and irritability. Therefore, future work might examine whether irritable youth with disrupted amygdala-insula/IFG connectivity may benefit from a similar intervention. However, additional work is first needed to further explore the behavior and neural underpinnings of attention bias in irritability.
This study has several strengths. First, our task engaged relevant attentional and regulatory brain regions. The brain activation finding, showing association between greater Δt0 and decreased activation in the right precuneus, is consistent with previous studies demonstrating the role of the precuneus during attentional shifting to emotional content [74], and with visual attention and inhibitory control [75,76,77]. Second, our data includes a large sample of well-characterized youth presenting a wide variation in irritability. Third, the current results in combination with those of Price and colleagues [22] validate the t0 parameter as a measure of attentional orienting with a standard DDM and support its added value in characterizing aberrant attention processing in pediatric psychopathology. This study provides converging evidence that cue-related processes occurring before probe onset are implicated in pediatric affective psychopathology. It has important theoretical implications for interpreting the dot-probe task and demonstrates the utility of computational modeling for interpreting cognitive tasks. Fifth, integrating computational strategies may offer a more precise methodological approach to capture anger-related attentional processing in a well-studied task, and its associations with clinical symptoms. The few findings we observed with the traditional attention bias scores based on raw response time (eResults 3) were distinct from those found with Δt0. DDM can be used in future studies to quantify anger-related attention bias and assess changes in this parameter following interventions.
This study also has limitations. First, we found no direct association between irritability and DDM-derived attention bias at the behavioral level. While this is the first study examining Δt0 in irritability, the non-significant association between these variables is inconsistent with previous data [7,8,9,10]. However, our three-way interaction reveals a more nuanced relationship between attention bias, irritability, and amygdala functional connectivity, indicating that neural abnormalities vary as a function of irritability and Δt0. Discrepancies might also be associated with differences in task sensitivity across different levels of analyses, consistent with recent reports indicating that the subtraction score typically used to measure attention bias in the dot-probe task has poor psychometrics [78, 79]. By using a DDM approach to generate a latent parameter of attention bias, we overcome the limitations associated with relying on a difference value-based measure. More studies are needed to establish the reliability of Δt0 and its potential superiority. Relative to the behavioral level, research demonstrates that neural connectivity during the task yields a more sensitive output [27]. In line with this, in the present study, task-based measures showed consistent associations with amygdala connectivity. Future studies aiming to replicate the current findings may enhance current knowledge regarding the sensitivity of the task across different levels of analyses.
Second, in a standard DDM, the t0 component represents both the attentional orienting process of interest and other processes including response preparation and motor execution. By contrasting congruent vs. incongruent conditions, we were able to isolate these processes related to the condition differences; however, we cannot precisely disentangle the different extra-decisional processes that may have influenced the findings, if indeed these are affected by congruency. Additionally, findings should prompt further modeling of the attentional effects on decision processing itself [23]. A question remains whether attentional shifts are reflected solely in t0, or also in the drift rate during evidence accumulation. To best identify the effects of attentional dynamics across the whole dot-probe trial, a revision to the standard DDM is likely required. This avenue could be further explored in future studies using other diffusion models [80].
Third, participants differed in psychotropic medication exposure. Although this speaks to ecological validity, it might confound our results, as medications may have differential effects on young brains and we could not directly disentangle the effects of medication usage from psychopathology. However, most participants (74.18%) were medication free. Importantly, our post-hoc analyses including medication status as a covariate indicated that all reported findings remained significant. We also replicated our behavioral and fMRI analyses limited to the large unmedicated sub-set of participants (see eResults 4). All original reported patterns remained within this sample.
A fourth limitation in the current study relates to the generalizability of the findings. Nearly a quarter of the acquired sample were excluded from analyses for a variety of reasons specified in the Supplement, including poor quality of the data or a failure to converge for the DDM. Although considered appropriate exclusion criteria, and consistent with previous literature [6, 27, 81], these may affect the generalizability of the findings. An additional consideration is the age range and the puberty status of the current sample. Little is known about the effects of pubertal status on neural systems of threat/anger processing. Some prior research suggests that pubertal maturation is associated with aberrant neural activity in brain regions implicated in emotional processing [82, 83]. In the current data, we adjusted for age, as well as for pubertal status, with the original findings surviving these inclusions (see eResults 5). The current sample consisted of mainly young adolescents in the early- to mid- stages of puberty (see Table 1). Longitudinal studies are needed to elucidate the effect of pubertal maturation on neural emotional processing, and our findings cannot be generalized to the entire span of puberty.
Fifth, findings should be interpreted with caution considering recent work indicating that preprocessing and modeling choices can meaningfully influence results in neuroimaging analyses in general, and gPPI specifically [84, 85]. In the current study, all specifications were determined a-priori, and established and off-the-shelf procedures were selected, reflecting the standard in both neuroimaging and DDM [6, 27, 49, 54]. These factors potentially reduce sensitivity to discrepancies [86]. The relatively large sample size in the present study may also mitigate some sensitivity concerns. Future studies applying multiverse approaches [87] and independent replications are needed to further evaluate the robustness of the results. Similarly, while our focus on amygdala connectivity was hypothesis-driven, seed-based connectivity approaches have limitations. Other approaches examining connectivity of large-scale networks across the brain may serve as a next step in providing an additional explanation of individual differences in irritability [88]. For example, Scheinost and colleagues [88] used a connectome-based predictive modeling to identify predictive networks of irritability. Whereas the present study explored the role of amygdala circuitry in attentional processing, future studies applying data-driven whole-brain networks analyses are needed to identify which additional brain regions play a role for aberrant processing as it relates to irritability.
In sum, this is the first study that applies DDM on an established attention task during concurrent fMRI recording to examine the neurobiology of anger-related attentional processing in a transdiagnostic sample of youth with varying levels of irritability. The current findings reflect an interplay between irritability and the way in which attentional processing of angry faces is linked to brain circuits, suggesting that youth with high irritability present with a dysfunction in the way that those circuits function when attention deployment is biased towards angry face stimuli. Future studies could further determine the extent to which this aberrant neuro-behavioral coupling underlies reactive aggression in irritability, test the efficacy of attentional training on irritability, and examine changes in amygdala connectivity to regions implicated in emotional and attentional regulation over the course of effective treatment and symptom remission.
References
Brotman MA, Schmajuk M, Rich BA, Dickstein DP, Guyer AE, Costello EJ, et al. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 2006;60:991–7.
Dougherty LR, Smith VC, Bufferd SJ, Kessel EM, Carlson GA, Klein DN. Disruptive mood dysregulation disorder at the age of 6 years and clinical and functional outcomes 3 years later. Psychol Med. 2016;46:1103–14.
Pickles A, Aglan A, Collishaw S, Messer J, Rutter M, Maughan B. Predictors of suicidality across the life span: the Isle of Wight study. Psychol Med. 2010;40:1453–66.
Stringaris A, Vidal-Ribas P. Probing the irritability-suicidality nexus. J Am Acad Child Adolesc Psychiatry. 2019;58:18–19.
Kessler RC, Avenevoli S, Costello EJ, Georgiades K, Green JG, Gruber MJ, et al. Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69:372–80.
Kircanski K, White LK, Tseng WL, Wiggins JL, Frank HR, Sequeira S, et al. A latent variable approach to differentiating neural mechanisms of irritability and anxiety in youth. JAMA Psychiatry. 2018;75:631–39.
Hommer RE, Meyer A, Stoddard J, Connolly ME, Mogg K, Bradley BP, et al. Attention bias to threat faces in severe mood dysregulation. Depress Anxiety. 2014;31:559–65.
Smith PW, Waterman M. Processing bias for aggression words in forensic and nonforensic samples. Cognition Emot. 2003;17:681–701.
Maoz K, Adler AB, Bliese PD, Sipos ML, Quartana PJ, Bar-Haim Y. Attention and interpretation processes and trait anger experience, expression, and control. Cogn Emot. 2017;31:1453–64.
Salum GA, Mogg K, Bradley BP, Stringaris A, Gadelha A, Pan PM, et al. Association between irritability and bias in attention orienting to threat in children and adolescents. J Child Psychol Psychiatry. 2017;58:595–602.
Brotman MA, Kircanski K, Stringaris A, Pine DS, Leibenluft E. Irritability in youths: a translational model. Am J Psychiatry. 2017;174:520–32.
Stoddard J, Tseng WL, Kim P, Chen G, Yi J, Donahue L, et al. Association of irritability and anxiety with the neural mechanisms of implicit face emotion processing in youths with psychopathology. JAMA Psychiatry. 2017;74:95–103.
Kryza-Lacombe M, Brotman MA, Reynolds RC, Towbin K, Pine DS, Leibenluft E, et al. Neural mechanisms of face emotion processing in youths and adults with bipolar disorder. Bipolar Disord. 2019;21:309–20.
Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216.
Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–76.
Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IMH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1–24.
Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–7.
MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. J Abnorm Psychol. 1986;95:15–20.
Bar-Haim Y, Lamy D, Glickman S. Attentional bias in anxiety: a behavioral and ERP study. Brain Cogn. 2005;59:11–22.
Waechter S, Nelson AL, Wright C, Hyatt A, Oakman J. Measuring attentional bias to threat: reliability of dot probe and eye movement indices. Cogn Ther Res. 2014;38:313–33.
Staugaard SR. Reliability of two versions of the dot-probe task using photographic faces. Psychol Sci Q. 2009;51:339–50.
Price RB, Brown V, Siegle GJ. Computational modeling applied to the dot-probe task yields improved reliability and mechanistic insights. Biol Psychiatry. 2019;85:606–12.
Nishiguchi Y, Sakamoto J, Kunisato Y, Takano K. Linear ballistic accumulator modeling of attentional bias modification revealed disturbed evidence accumulation of negative information by explicit instruction. Front Psychol. 2019;10:2447.
Pe ML, Vandekerckhove J, Kuppens P. A diffusion model account of the relationship between the emotional flanker task and rumination and depression. Emotion. 2013;13:739–47.
Ironside M, Browning M, Ansari TL, Harvey CJ, Sekyi-Djan MN, Bishop SJ, et al. Effect of prefrontal cortex stimulation on regulation of amygdala response to threat in individuals with trait anxiety: a randomized clinical trial. JAMA Psychiatry. 2019;76:71–78.
Gold AL, Morey RA, McCarthy G. Amygdala-prefrontal cortex functional connectivity during threat-induced anxiety and goal distraction. Biol Psychiatry. 2015;77:394–403.
White LK, Sequeira S, Britton JC, Brotman MA, Gold AL, Berman E, et al. Complementary features of attention bias modification therapy and cognitive-behavioral therapy in pediatric anxiety disorders. Am J Psychiatry. 2017;174:775–84.
Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83.
Nomura M, Ohira H, Haneda K, Iidaka T, Sadato N, Okada T, et al. Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: an event-related fMRI study. Neuroimage. 2004;21:352–63.
Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501.
Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87.
Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–14.
Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167:61–9.
Blair RJR. Considering anger from a cognitive neuroscience perspective. Wiley Interdiscip Rev Cogn Sci. 2012;3:65–74.
Thomas LA, Brotman MA, Muhrer EJ, Rosen BH, Bones BL, Reynolds RC, et al. Parametric modulation of neural activity by emotion in youth with bipolar disorder, youth with severe mood dysregulation, and healthy volunteers. Arch Gen Psychiatry. 2012;69:1257–66.
Tops M, Boksem MA. A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Front Psychol. 2011;2:330.
Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–26.
Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nat Neurosci. 2010;13:507–12.
Horien C, Greene AS, Constable RT, Scheinost D. Regions and connections: complementary approaches to characterize brain organization and function. Neuroscientist. 2020;26:117–33.
Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29.
McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–86.
Cornacchio D, Crum KI, Coxe S, Pincus DB, Comer JS. Irritability and severity of anxious symptomatology among youth with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2016;55:54–61.
Hardee JE, Benson BE, Bar-Haim Y, Mogg K, Bradley BP, Chen G, et al. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol Psychiatry. 2013;74:273–9.
Stoddard J, Stringaris A, Brotman MA, Montville D, Pine DS, Leibenluft E. Irritability in child and adolescent anxiety disorders. Depress Anxiety. 2014;31:566–73.
Shaw P, Stringaris A, Nigg J, Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry. 2014;171:276–93.
Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283-&.
Stringaris A, Goodman R, Ferdinando S, Razdan V, Muhrer E, Leibenluft E, et al. The Affective Reactivity Index: a concise irritability scale for clinical and research settings. J Child Psychol Psychiatry. 2012;53:1109–17.
Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–53.
Tseng WL, Deveney CM, Stoddard J, Kircanski K, Frackman AE, Yi JY, et al. Brain mechanisms of attention orienting following frustration: associations with irritability and age in youths. Am J Psychiatry. 2019;176:67–76.
Conners CK. Conners 3rd edition manual. Multi-Health Systems, Inc., North Tonawanda, Canada, 2008.
Voss A, Voss J, Lerche V. Assessing cognitive processes with diffusion model analyses: a tutorial based on fast-dm-30. Front Psychol. 2015;6:336.
Voss A, Nagler M, Lerche V. Diffusion models in experimental psychology: a practical introduction. Exp Psychol. 2013;60:385–402.
Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput. 2008;20:873–922.
Voss A, Voss J. Fast-dm: a free program for efficient diffusion model analysis. Behav Res Methods. 2007;39:767–75.
Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73.
Chen G, Adleman NE, Saad ZS, Leibenluft E, Cox RW. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. Neuroimage. 2014;99:571–88.
IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp.
Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22.
Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav Rev. 2009;33:613–30.
Ray RD, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci Biobehav Rev. 2012;36:479–501.
Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–12.
Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol. 2017;34:300–6.
Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–55.
Zhou H, Schafer RJ, Desimone R. Pulvinar-cortex interactions in vision and attention. Neuron. 2016;89:209–20.
Ward R, Danziger S, Bamford S. Response to visual threat following damage to the pulvinar. Curr Biol. 2005;15:571–3.
Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, et al. A direct brainstem-amygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage. 2005;24:235–43.
Le QV, Isbell LA, Matsumoto J, Le VQ, Hori E, Tran AH, et al. Monkey pulvinar neurons fire differentially to snake postures. PLoS One. 2014;9:e114258.
Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67.
Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61.
Nieuwenhuys R. The insular cortex: a review. Prog Brain Res. 2012;195:123–63.
Carver CS, Harmon-Jones E. Anger is an approach-related affect: evidence and implications. Psychol Bull. 2009;135:183–204.
Stoddard J, Sharif-Askary B, Harkins EA, Frank HR, Brotman MA, Penton-Voak IS, et al. An open pilot study of training hostile interpretation bias to treat disruptive mood dysregulation disorder. J Child Adolesc Psychopharmacol. 2016;26:49–57.
Jones EB, Sharpe L. Cognitive bias modification: a review of meta-analyses. J Affect Disord. 2017;223:175–83.
Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83.
Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–91.
Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23:3990–8.
Ferri J, Schmidt J, Hajcak G, Canli T. Emotion regulation and amygdala-precuneus connectivity: focusing on attentional deployment. Cogn Affect Behav Neurosci. 2016;16:991–1002.
Kappenman ES, Farrens JL, Luck SJ, Proudfit GH. Behavioral and ERP measures of attentional bias to threat in the dot-probe task: poor reliability and lack of correlation with anxiety. Front Psychol. 2014;5:1368.
Thigpen NN, Gruss LF, Garcia S, Herring DR, Keil A. What does the dot-probe task measure? A reverse correlation analysis of electrocortical activity. Psychophysiology. 2018;55:e13058.
White CN, Ratcliff R, Starns JJ. Diffusion models of the flanker task: discrete versus gradual attentional selection. Cogn Psychol. 2011;63:210–38.
Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, et al. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry. 2010;68:982–90.
Spielberg JM, Olino TM, Forbes EE, Dahl RE. Exciting fear in adolescence: does pubertal development alter threat processing? Dev Cogn Neurosci. 2014;8:86–95.
Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev Neuropsychol. 2011;36:429–52.
Botvinik-Nezer R, Holzmeister F, Camerer CF, Dreber A, Huber J, Johannesson M, et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature. 2020;582:84–8.
Bloom PA, VanTieghem M, Gabard-Durnam L, Gee DG, Flannery J, Caldera C, et al. Age-related change in task-evoked amygdala-prefrontal circuitry: a multiverse approach with an accelerated longitudinal cohort aged 4–22 years. BioRxiv. 2021.
Gelman A, Loken E. The statistical crisis in science. Am Sci. 2014;102:460–5.
Harder JA. The multiverse of methods: extending the multiverse analysis to address data-collection decisions. Perspect Psychol Sci. 2020;15:1158–77.
Scheinost D, Dadashkarimi J, Finn ES, Wambach CG, MacGillivray C, Roule AL, et al. Functional connectivity during frustration: a preliminary study of predictive modeling of irritability in youth. Neuropsychopharmacology. 2021;46:1300–06.
Acknowledgements
The authors appreciate the role of Drs. Ellen Leibenluft and Daniel S. Pine in early consultation and discussion about the findings and potential implications. The authors would like to thank the patients and families for their time and participation. This work was supported by the Intramural Research Program (IRP) of the National Institute of Mental Health, National Institutes of Health (NIMH/NIH), ZIAMH002781 (Pine), ZIAMH002786 (Leibenluft), ZIAMH002778 (Leibenluft), and conducted under NIH Clinical Study Protocols 01-M-0192 and M-00-M-0021 [ClinicalTrials.gov identifiers: NCT00018057 (Pine) and NCT00025935 (Brotman)]. This body had no role in the study design, writing the manuscript, or the decision to submit the paper for publication. This research received no specific external grant from any funding agency, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MAB is the principal investigator for this project. RN and MAB conceptualized the initial study’s questions and analytic approach. All authors contributed to the conception of the work and to the interpretation of the data. RN wrote the first and successive drafts of the manuscript. MAB, SPH, JOL, AJ, JS, MJ, AH, KK, YBH contributed to writing, editing, and revising the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naim, R., Haller, S.P., Linke, J.O. et al. Context-dependent amygdala–prefrontal connectivity during the dot-probe task varies by irritability and attention bias to angry faces. Neuropsychopharmacol. 47, 2283–2291 (2022). https://doi.org/10.1038/s41386-022-01307-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01307-3