Abstract
Recent evidence suggests an association between benzodiazepines (BZDs) use and lower brain amyloid load, a hallmark of AD pathophysiology. Other AD-related markers include hippocampal atrophy, but the effect of BZDs on hippocampal volume remains unclear. We aimed at 1) replicating findings on BZDs use and brain amyloid load and 2) investigating associations between BZDs use and hippocampal volume, in the MEMENTO clinical cohort of nondemented older adults with isolated memory complaint or light cognitive impairment at baseline. Total Standardized Uptake Value Ratio (SUVR) of brain amyloid load and hippocampal volume (HV) were obtained, respectively, from 18F Florbetapir positron emission tomography (PET) and magnetic resonance imaging (MRI), and compared between BZD chronic users and nonusers using multiple linear regressions adjusted for age, sex, educational level, ApoE ε4 genotype, cognitive and neuropsychiatric assessments, history of major depressive episodes and antidepressant intake. BZD users were more likely to manifest symptoms of depression, anxiety and apathy. In the MRI subgroup, BZD users were also more frequently females with low education and greater clinical impairments as assessed with the clinical dementia rating scale. Short- versus long-acting BZDs, Z-drugs versus non-Z-drugs BZDs, as well as dose and duration of BZD use, were also considered in the analyses. Total SUVR and HV were significantly lower and larger, respectively, in BZD users (n = 38 in the PET subgroup and n = 331 in the MRI subgroup) than in nonusers (n = 251 in the PET subgroup and n = 1840 in the MRI subgroup), with a medium (Cohen’s d = −0.43) and low (Cohen’s d = 0.10) effect size, respectively. Short-acting BZDs and Z-drugs were more significantly associated with larger HV. We found no effect of dose and duration of BZD use. Our results support the involvement of the GABAergic system as a potential target for blocking AD-related pathophysiology, possibly via reduction in neuronal activity and neuroinflammation. Future longitudinal studies may confirm the causal effect of BZDs to block amyloid accumulation and hippocampal atrophy.
Similar content being viewed by others
Introduction
Epidemiological studies have questioned the chronic use of GABAergic agents such as benzodiazepines (BZDs) and Z-Drugs, as they have been associated with an increased risk of neurocognitive disorders, including Alzheimer’s Disease (AD) [1]. However, the risk of neurocognitive disorder in BZDs users remains inconclusive, mostly because of inconsistent results. Two recent large epidemiological studies are representative of this inconsistency, with, on the one hand, a large Taiwanese study finding a greater risk of dementia with BZDs [2], whereas, on the other hand, a large Danish study finding no effect of BZDs on dementia incidence and rather a lower incidence of dementia in chronic Z-Drugs users [3]. Potential explanation for these recurrent inconsistencies may rely on how well data are controlled for psychiatric disorders which constitute potential confounding factors, the latter Danish study having more strictly controlled for psychiatric disorders than the Taiwanese report for instance, an effect possibly accounted by cultural differences in self-reporting psychiatric symptoms which constitute a potential challenge to the optimal control of psychiatric disorders in Asian populations [4].
While epidemiological studies appear inconsistent, findings on a potential neuroprotective effect of BZDs appear more robust, especially in preclinical studies in which the use of BZDs was associated with neuroprotective effects as shown by lower amyloid deposition [5,6,7] and lower hippocampal cell death in mice [6], these two processes being considered hallmarks of the pathophysiology of AD. Moreover, two recently published neuroimaging studies have consistently found that chronic use of BZDs was associated with lower amyloid deposition in human. Indeed, a first pilot study in the ADNI cohort found lower amyloid load as assessed with 18F Florbetapir positron emission tomography (PET) in 15 BZDs users compared to matched BZD nonusers [8]. More recently, our group, in which most of the authors of the present report were involved, published a second larger PET study based on the MAPT cohort, in which the 47 BZDs users exhibited lower amyloid load compared to the 221 BZDs nonusers [9]. Because only two studies on the impact of BZDs on brain changes are available in clinical population, the potential neuroprotective effect of BZDs remains to be investigated in humans, and replication studies are needed, while other markers of neurodegeneration, such as hippocampal volume, may also be investigated in addition to amyloid load.
In this study, we have investigated associations between BZDs use and two AD-related markers, namely amyloid load in PET imaging and hippocampal volume in Magnetic Resonance Imaging (MRI), in the large French MEMENTO cohort at baseline, which primary goal was to identify new phenotypes of participants who will develop dementia over time. All participants underwent MRI examination whereas only a subgroup underwent amyloid PET imaging. We hypothesized to find lower amyloid load and larger hippocampal volume in BZD users compared to nonusers. In addition, we investigated whether certain characteristics of BZD use, including short- versus long-acting BZDs, as well as Z-drugs versus non-Z-drugs BZD, dose and duration of use, influenced the association with neuroimaging markers.
Materials and methods
Participants
The participants were part of the MEMENTO Cohort (registration: NCT01926249), a 5-year longitudinal clinic-based study whose principal objective was to identify new phenotypes of participants who will develop dementia over time. The study design of the MEMENTO cohort has been extensively described elsewhere [10]. There were no overlapping participants among the MEMENTO and the MAPT cohorts and no risk of overlapping results with our previously published study in the MAPT cohort. Briefly, 2323 nondemented adults with either an isolated memory complaint (if aged ≥60) or a light cognitive impairment (defined as test performance 1 SD below age, sex and education-level norms) while not demented (Clinical Dementia Rating [CDR] < 1), were recruited in 28 French memory clinics from 2011 to 2014. Main exclusion criteria were contraindication or refusal to perform magnetic resonance imaging (MRI), neurological disease such as treated epilepsy, treated Parkinson’s disease, Huntington disease, or brain tumor, history of head trauma with neurological sequelae, stroke occurring in the past three months, history of schizophrenia, or illiteracy. Baseline data collection included clinical examinations, neuropsychological testing, blood sampling and brain MRI. In addition, we included participants with 18F Florbetapir PET scans (n = 289) [11, 12], so as to replicate the previous findings on amyloid load and benzodiazepine use, from 2 ancillary studies (Insight-PreAD, AMYGING, NCT02164643). All participants signed an informed consent to participate in the study that was approved by the ethics committee “Comité de Protection des Personnes Sud-Ouest et Outre Mer III.”
Benzodiazepine use and clinical assessments
Participants were identified as chronic BZD users if they had used any type of BZD or Z-drug for at least 3 months in the immediate period prior to study enrollment. The duration of BZD use was calculated as follows: end date of use (or date of PET/MRI scan, if still on medication at PET/MRI scan date) minus starting date of use. The BZD dose was standardized by converting the various BZD doses to diazepam dose equivalents. We also distinguished between short- (half-life ≤20 h) and long-acting (half-life >20 h) BZDs because previous epidemiological and imaging studies used this classification and found a differential effect of long-acting versus short-acting BZDs on AD-related pathophysiology [9]. In case of multiple BZDs in a participant, we considered sum of the doses and durations of use of each BZD. In addition, in the eventuality of different BZD classes in a participant, we classified the participant as a long-acting BZD user if at least one long-acting BZD was taken by the participant and as a short-acting BZD user if only short-acting BZD were reported. Finally, in case of pro re nata BZD prescription, dose and duration of use were calculated based on the approximation of a daily use.
Clinical assessment included age, sex, educational level, cognitive and dementia status assessed with the Mini-Mental State Examination (MMSE) and the Clinical Dementia Rating (CDR), NPI depression, anxiety, apathy subscales, history of major depressive episodes, history of antidepressant intake and apolipoprotein E ε4 (ApoE ε4) genotype. NPI allows assessments for 12 different behavioral and psychological disorders, scored whether they are absent (score = 0) or based on their frequency (score ranged 1–4) and severity (score ranged 1–3). Total score for a given disorder is calculated as frequencyXseverity for a total score ranging between 0 and 12. Implementation of the NPI in the MEMENTO cohort was limited to a systematic assessment of depression, anxiety and apathy, whereas assessments of other disorders, such as sleep disorder, were optional. Sleep disorder scoring had many missing data and no reliable statistics could be performed with this NPI dimension notably.
MRI evaluation
Brain magnetic resonance images were acquired after a standardization of the imaging processes by a dedicated neuroimaging specialized team (CATI for “Centre pour l’Acquisition et le Traitement des Images”, http://cati-neuroimaging.com/) [13, 14]. MRI machines of 1.5 and 3 Tesla were used for this study (the complete list of machines is provided in Supplementary 2 Appendix A). All MRI scans were centralized, quality checked, and postprocessed by the CATI to obtain standardized measurements for each participant. The datasets underwent two steps of quality check in the process to yield HV. MRI raw data were first quality checked to evaluate if no artefact (system or patient related) could endanger the reliability of subsequent image processing pipelines. Then the result of the segmentation tool was quality checked to ensure that no major segmentation failure could make the estimated volume unreliable. Both steps of the quality check rely on systematic visual evaluation of trained raters. Segmentation results are graded on a 0–4 range, with 0 meaning completely wrong segmentation, 2 borderline segmentation (segmentation mistakes may have an influence on the resulting volume) and 4 perfect segmentation; volumes for grades lesser than 2 were not included in the analyses. The MRI protocol included 3D-T1 1 mm isometric sequences that were used to assess the total intracranial volume (TIV) with Statistical Parametric Mapping [15] and hippocampal volumes with the SACHA software [16]. The principal MRI outcome was the hippocampal volume (HV - calculated as the sum of the right and left hippocampi in each participant) divided by TIV as a control for head size inter-subject variability. 2,171 participants with hippocampus volumes were included in the MRI analyses, as HV was not available for 152 participants, because no MRI was available or exclusion after CATI quality check.
18F Florbetapir PET analysis
Control procedures for PET assessment were carried out by the CATI, similarly to MRI. Participants were examined using 5 different hybrid PET-CT scanners, including one PET CT 690 (GE Healthcare), one Discovery RX VCT (General Electric), two True Point HiRez (Siemens Medical Solutions) and one Biograph 4 Emission Duo LSO (Siemens Medical Solutions), 50 (±5) min after the injection of roughly 370 MBq (333–407 MBq) of 18F-Florbetapir. PET acquisition were performed 50 (±5) min after the injection of roughly 370 MBq (333–407 MBq) of 18F-Florbetapir, and consisted of 3 × 5-min frames, during a 128 × 128 acquisition matrix, with a voxel size of 2 × 2 × 2 mm3.
The method for images processing with partial volume effect correction on untransformed PET images has been extensively described elsewhere [17]. Briefly, a quality check was performed by the CATI team: frames were realigned, averaged and visually inspected for possible artefacts, including subject motion, mismatch between CT and emission scans and attenuation correction artefacts. The Rachel software, developed by the CATI, was used for 18F Florbetapir PET image processing. Structural MRI images were coregistered to 18F Florbetapir-PET images using SPM12 with visual inspection to detect any coregistration errors. Parametric PET images were then created for every individual, by dividing each voxel with the mean activity extracted from a combination of the whole cerebellum and pons as the reference region. Images were corrected from the Partial Volume Effect using the RBV-sGTM method. Finally, standardized uptake value ratios (SUVRs) were calculated by averaging the mean activity of all cortical regions of interest within the individual PET native space. Cerebellum and pons were selected as the reference region based on prior work on the MEMENTO cohort that found that the combination of these 2 regions was the best to distinguish between amyloid positive and amyloid negative subject [17]. Total SUVR was the principal PET outcome of this study because previous studies indicated a global effect on amyloid with BZDs rather than region-specific effects.
Statistical analysis
Clinical variables were described and compared according to the use of BZDs. Quantitative variables were described with medians and interquartile ranges and compared using Mann–Whitney’s tests. Qualitative variables were described with counts and percentages and compared using the chi-squared test.
We used linear mixed models to estimate the effect of BZD use (the independent variable) on total SUVR and HV (the dependent variables). The models included adjustments for age, sex, educational level, CDR, MMSE, NPI depression, NPI anxiety, NPI apathy, history of major depressive episodes, history of antidepressant intake, and ApoE ε4 genotype to account for the potential confounding effects of these factors. These covariates were chosen because of their potential associations with amyloid and hippocampal volume, as well as because they may influence BZD use, which may modify the results of linear regressions. Indeed, older women with low education may be more likely to use BZD [18], while at the same time being potentially more likely to exhibit higher amyloid load and smaller hippocampus. Similarly, BZD use may be more frequently associated with low cognitive performance (in particular because BZD may impair cognitive functions such as memory and attention [19] and more psychiatric symptoms (in particular because BZD can be prescribed in patients with anxiety and/or depression), while these factors have been associated with higher amyloid [20] and smaller hippocampus [21]. Prescription of antidepressants could also be considered a potential bias because individuals with BZD may be more frequently prescribed antidepressants as a co-prescription for anxiety or depressive disorders for example, as well as potentially influencing amyloid accumulation [20] and hippocampal volume [21]. ApoE ε4 genotype was also included because of a strong association toward greater amyloid accumulation [22] and smaller hippocampus volume [23]. Differences in hippocampus measurements could also be observed depending on MRI machines and this factor may also constitute a potential bias in our study. Scanner types for PET imaging were less likely to influence the amyloid measurement because the different PET scanners involved in the MEMENTO cohort have been rigorously configured to limit variability across centers [24].
The use of BZDs was considered as a binary variable (BZD users versus BZD nonusers) and as a 3-category variable for different half-life groups (short versus long-acting BZD users versus BZD nonusers) and as a 3-category variable for investigating the effects of Z-drugs (Z-drugs versus non-Z-drugs BZD versus BZD nonusers). In each case, non exposure to BZD was used as the reference level. Finally, to investigate potential effects of dose and duration of BZD use, the doseXduration variable (dose of BZD multiplied by duration of use) was coded in 4 categories (BZD nonusers as reference and the three tertiles of the variable to distinguish for small, moderate and large doseXduration BZD use) and integrated in adjusted multiple linear regressions.
Results
Sample characteristics
The clinical and demographic characteristics of the study participants are shown in Table 1.
In the MRI subgroup, n = 1840 participants were identified as BZD nonusers and n = 331 were identified as BZD users. The BZD list included in the analyses is provided in Supplementary Material 1. There were no significant differences between BZD users and BZD nonusers in age, ApoE ε4 and MMSE score. In contrast, BZD users were more frequently females, with lower educational level and higher CDR and NPI anxiety, depression and apathy scores, and were more likely to have experienced depression and to use antidepressants.
In the 18F Florbetapir PET subgroup, n = 251 participants were identified as BZDs nonusers and n = 38 were identified as BZD users. The BZD list included in the analyses is provided in Supplementary Material 1. There were no significant differences between BZD users and BZD nonusers in clinical characteristics, except for history of depression and NPI anxiety, depression and apathy scores, which were greater in BZD users.
Associations between benzodiazepine use and brain amyloid load
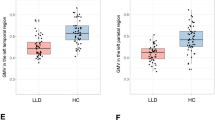
Distribution of SUVR according to BZD use showed a lower amyloid load in BZD users (mean = 0.72, SD = 0.11) compared to nonusers (mean = 0.81, SD = 0.22) (Fig. 1). The difference was moderate, with a raw Cohen’s d at 0.43. The first model included amyloid SUVR in the total cortex as the dependent variable and BZD use, age, sex, educational level, MMSE, CDR sum of boxes, history of depression, antidepressant intake, ApoE ε4 status and NPI scores (apathy, depression, anxiety), as explicative variables. We found a significant effect of BZD use (beta = −0.073, IC95% = [−0.142; −0.003], p = 0.042) on total cortex SUVR. We found no significant effect of doseXduration on total SUVR (p = 0.39), neither for dose solely (p = 0.24), nor duration (p = 0.45).
BZD: Benzodiazepine; SUVR: standardized uptake value ratio. Thresholds for amyloid positivity with the PET imaging processing used in the MEMENTO cohort were 0.79 (liberal threshold) and 0.88 (conservative threshold) [17]. The total amyloid load was significantly lower in BZD users compared to BZD nonusers (beta = −0.073, p = 0.042) after controlling for age, sex, educational level, CDR, MMSE, NPI scores (depression, anxiety and apathy) history of major depressive episodes, history of antidepressant intake and ApoE ε4.
In the second model, where we distinguished short and long-acting BZDs, we found no significant effects of either short- or long-acting BZDs on the total cortex SUVR, although there was a statistical trend for short-acting BZDs (beta = −0.078, IC95% = [−0.170; 0.014], p = 0.097).
In the third model, where we distinguished Z-drugs and non-Z-drugs BZD, we found no significant effects (p = 0.119).
Associations between benzodiazepine use and hippocampal volume
Figure 2 depicts the ratio of the hippocampal volume over TIV, with a larger HV in BZD users (mean = 3.98, SD = 0.57) compared to BZD nonusers (mean = 3.92, SD = 0.57). Raw Cohen’s d was 0.10. After adjustment for age, sex, educational level, MMSE, CDR, history of depression, antidepressant intake, ApoE ε4 status and NPI score (apathy, depression, anxiety), we found a significant effect of BZD use (beta = 0.072, IC95% = [0.008; 0.136], p = 0.027).
BZD Benzodiazepine, HV hippocampal volume, TIV total intracranial volume. The hippocampal volume (ratio of the sum of the left and right hippocampal volumes over TIV, multiplied by 1000 to facilitate interpretation) was significantly larger in BZD users compared to BZD nonusers (beta = 0.072, p = 0.027) after controlling for age, sex, educational level, CDR, MMSE, NPI scores (depression, anxiety and apathy) history of major depressive episodes, history of antidepressant intake and ApoE ε4, type of MRI.
We found no significant effect of doseXduration on HV (p = 0.33), dose solely (p = 0.87) or duration (p = 0.39).
In the second model, where we distinguished short and long-acting BZDs, we found significant effects of short-acting BZDs on hippocampal volume (beta = 0.099, IC95% = [0.023; 0.174], p = 0.037), whereas the effect of long-acting BZDs on hippocampal volume was not significant (beta = 0.02, IC95% = [−0.083; 0.122]).
In the third model, where we distinguished Z-drugs and non-Z-drugs BZD, we found significant effects of Z-drugs on hippocampal volume (beta = 0.114, IC95% = [0.016; 0.211], p = 0.047), whereas the effect of non-Z-drugs BZD on hippocampal volume was not significant (beta = 0.048, IC95% = [−0.03; 0.126]).
Univariate analyses
Supplementary material 3 provides the detailed statistics of the uni and multivariate regressions for the 3 models. For SUVR and BZD use multivariate analysis, only age and APOE covariate were associated, and in a weaker extend MMSE score. Thus, non-adjusted and adjusted associations were similar. HV was associated with sex, age, APOE, CDR, MMSE, apathy (only in univariate regression), history of depression (only in univariate regression) and antidepressant intake. BZD use was not significantly associated with HV in univariate analysis (p = 0.18) but became significantly associated in multivariate analysis (p = 0.027) with the influence of the antidepressant intake variable which was strongly associated with the BZD use variable (Chi [2], p < 10−4). Interestingly, the association HV/antidepressant intake was negative. Therefore, the BZD use variable probably captured at the same time the BZD effect and the antidepressant effect, which were opposite, resulting in a nonsignificant univariate association for BZD use. In adjusted regression, the proper effect of BZD was revealed as a significant positive association with HV, independent of antidepressant.
Discussion
We replicated the findings that nondemented older adults with either isolated memory complaint or light cognitive impairment who chronically use BZDs had a reduced brain amyloid load compared to BZD nonusers, independent of potential confounding factors such as cognitive impairment, history of depression and antidepressant intake. In addition, we extended these findings to other neuroimaging markers of AD, by showing that nondemented older adults with BZDs had a significantly larger hippocampal volume compared to BZD nonusers. These results are consistent with the recent literature on the protective effect of GABAergic agents on AD-related pathophysiology, both in clinical [8, 9] and preclinical studies [6].
As discussed elsewhere, the principal hypotheses to account for amyloid blockade with BZDs refer to modulation of neuronal hyperactivity and neuroinflammation [25, 26]. BZD act as positive allosteric modulators of GABA-A receptors and depress neuronal activity via the increase in intracellular chlorine ions through chloride channels, hyperpolarizing the cell and decreasing its probability of firing. Interestingly, growing evidence suggest that blocking excessive neuronal activity could limit amyloid progression and ultimately prevent the development of AD [27]. Indeed, excessive neuronal firing increase amyloid formation [28, 29] and neuronal hyperactivity has been suggested to constitute an early dysfunction in the pathophysiological cascade of AD [30], while its modulation could reduce amyloid accumulation, since a reduction in neuronal activity results in decreased amyloid production [31], as well as reduced axonal dystrophy and synaptic loss in areas near amyloid plaques [32]. Based on accumulating findings on the link between excessive neuronal activity and brain amyloid accumulation, some authors have proposed that targeting neuronal hyperactivity with pharmacological treatment such as antiepileptic drugs may attenuate amyloid progression and ultimately prevent the development of AD [27].
Other possible mechanisms related to neuroprotective properties of BZDs include modulation of neuroinflammation, a process involved in the pathophysiology of AD, via their action as Translocator protein (18 kDa) (TSPO) ligands. TSPO, formerly known as the peripheral benzodiazepine receptor, is an outer mitochondrial membrane protein involved in steroid metabolism and other mitochondrial functions, including cell proliferation and differentiation, mitochondrial respiration and regulation of cytochrome C release, caspase activation, and apoptosis [33]. Pleiotropic neuroprotective effects have been associated with TSPO ligands, including reduction of amyloid accumulation [34].
In comparison to amyloid reduction, the effect of BZDs on hippocampal volume has been less studied. One clinical study found an independent positive association of BZD use and hippocampal volume in a population of middle-aged psychiatric patients [35]. Preclinical studies show inconsistent findings on the effect of BZDs on BDNF in the hippocampus of mice, such that acute but not repeated BZD treatment decrease BDNF concentration [36]. Hippocampal volume loss is generally considered a later process in the pathophysiology of AD compared to amyloid accumulation that can occur several years before cognitive decline. More specifically, hippocampal atrophy in AD could occur as the consequence of the neurotoxicity of amyloid that accumulate in the brain [37]. Therefore, the relatively preserved hippocampus volume observed in the BZD users of our study may be accounted by their lower amyloid burden compared to BZD nonusers and the blockade of hippocampal atrophy with BZD may involve an indirect mechanism via the blockade of amyloid accumulation. This hypothesis is consistent with our results of a smaller effect size in the association between BZDs and hippocampal volume compared to the effect on amyloid load, because our participants had low or no cognitive impairments and could exhibit either no or early AD pathophysiology, with only little effects on hippocampus at this stage.
We found a more specific association for short-acting BZD and Z-drugs with larger hippocampus. We previously found that the effect of short-acting BZDs on amyloid was more significant than long-acting BZDs [9], a result that was not replicated in the present study, although we found a statistical trend for a similar result. Interestingly, short-acting BZDs, especially Z-drugs, have a greater affinity for the alpha 1 subunit of GABA receptors, compared to long-acting BZDs, and the alpha 1 GABA receptor is particularly abundant in the hippocampus [38]. A more focused action of short-acting BZDs that have a greater affinity for alpha 1 GABA receptors in the hippocampal region may therefore account for their more specific effect on the hippocampus volume. In addition, because Z-drugs are generally prescribed for insomnia, a superior sleep quality may account for the association between Z-drugs use and larger hippocampus, since chronic insomnia has been associated with hippocampal atrophy [39]. Unfortunately, only a few participants in the MEMENTO cohort were assessed for sleep quality, and we were unable to reliably test this hypothesis.
We found no association between the amyloid load/hippocampus volume and dosage or duration of BZD use, which is consistent with previously published experimental studies [8, 9]. One possible explanation of this result relates to the potential ceiling effect of BZDs on AD-related pathophysiology, suggesting that after a certain dose or time of use, no further benefit in lowering amyloid or protecting hippocampus is achieved with BZDs. Another complementary explanation could be that the maximum protective effect of BZDs occurs rapidly, within a relatively brief duration of use. This hypothesis is consistent with preclinical studies showing that the in vitro effects of BZDs on amyloid formation are rapid and occur after a few hours of exposure [7].
Limitations of our study include that the Memento study was not primarily designed to assess the effect of BZD use on neuroimaging markers of AD, and our results were derived from a secondary analysis, which may limit the strength of our conclusions. In addition, the use of a cross-sectional design to examine the association between BZD use and neuroimaging markers of AD prevents inference of causality; the possible causal relationship between reduced amyloid load, greater hippocampal volume and BZDs remains to be confirmed in longitudinal studies. Moreover, while the association between brain amyloid and BZD use is now confirmed in three different studies and shows a medium effect size, the association between hippocampal volume and BZD use was first identified in our study with only a small effect size and this association requires further replication and longitudinal examination. Furthermore, characteristics of the PET and MRI subpopulations were not strictly identical, regarding clinical impairment in particular, with a greater proportion of participants with CDR 0.5 in the MRI subpopulation. We may conclude from our results that BZD use could block amyloid accumulation in older individuals with low or no clinical impairment (a larger sample size would be more informative as to whether it also apply to more severely cognitively impaired individuals) whereas the small effect to limit hippocampal atrophy with BZD may be observed on individuals with greater clinical impairment. We could not infer whether BZD use has an effect on amyloid in more severely impaired individuals or on hippocampal volume in individuals with no or low clinical impairment. In addition, a recall bias could have limited the accuracy in reporting dose and duration of use, especially because duration of use largely exceed 5 years in more than half of the BZD users and retrospective assessment of such data may be imprecise in most of the patients. Additional limitations regarding dose and/or duration of BZD use include that we used the approximation of a daily use for pro re nata BZD prescription, and that these variables were calculated based on the immediate period prior to study enrollment, whereas lifetime exposure of BZD was not available in our study. These approximations in our data may explain why we could not identify the critical time period of BZD exposure that impacts amyloid deposition or hippocampal cell loss. Longitudinal well-controlled studies for dose and duration of BZD use may be more informative as to whether dose and/or duration of use influence the effect of BZD on amyloid accumulation and hippocampal volume and may ultimately reveal potential mechanisms involved in the BZD effect. Finally, we cannot exclude that other factors not included in our study may have confounded our results, such as history of anxiety disorders (which were not systematically assessed in the MEMENTO cohort, in contrast to depression) or sleep disturbance for which there was too much missing data since this dimension was not systematically asked and only rarely informed in the NPI.
In conclusion, our results suggest neuroprotective effects of BZDs and support the involvement of the GABAergic system as a potential target for blocking brain amyloid accumulation and hippocampal atrophy, possibly via reduction in neuronal activity and neuroinflammation. However, we do not intend to suggest that BZDs should be used to prevent AD because the chronic use of BZDs has several side effects, including increased risk of fall, dependence and cognitive impairment (attention, memory and executive impairments mostly, at least during the time they are being taken) that certainly overcome the potential benefits on neurodegeneration. It is worth reminding that guidelines for BZD prescription include a short duration of use, which may not exceed 1 month as a hypnotic and 3 months as an anxiolytic. Moreover, blocking amyloid pathology and hippocampal atrophy does not necessarily lead to a decreased incidence of AD, which involves multiple other pathophysiological mechanisms, such as tau pathology and vascular disorders. Nevertheless, our paper suggests that further investigations of GABA and/or TSPO related mechanisms involved in neuronal excitability and neuroinflammation may allow the identification of novel pathophysiological pathways in AD and provide pharmacological targets to reduce amyloid formation and hippocampal atrophy.
References
Islam MM, Iqbal U, Walther B, Atique S, Dubey NK, Nguyen PA, et al. Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology. 2016;47:181–91.
Tseng L-Y, Huang S-T, Peng L-N, Chen L-K, Hsiao F-Y. Benzodiazepines, z-hypnotics, and risk of dementia: special considerations of half-lives and concomitant use. Neurotherapeutics. 2020;17:156–64.
Osler M, Jørgensen MB. Associations of benzodiazepines, Z-drugs, and other anxiolytics with subsequent dementia in patients with affective disorders: a nationwide cohort and nested case-control study. Am J Psychiatry. 2020;177:497–505.
Reyes AT, Constantino RE, Arenas RA, Bombard JN, Acupan AR. Exploring challenges in conducting E-mental health research among Asian American women. AsianPacific Isl Nurs J. 2018;3:139–53.
Quiroga C, Chaparro RE, Karlnoski R, Erasso D, Gordon M, Morgan D, et al. Effects of repetitive exposure to anesthetics and analgesics in the Tg2576 mouse Alzheimer’s model. Neurotox Res. 2014;26:414–21.
Tampellini D, Capetillo-Zarate E, Dumont M, Huang Z, Yu F, Lin MT, et al. Effects of synaptic modulation on -amyloid, synaptophysin, and memory performance in Alzheimer’s disease transgenic mice. J Neurosci. 2010;30:14299–304.
Yamamoto N, Arima H, Sugiura T, Hirate H, Kusama N, Suzuki K, et al. Midazolam inhibits the formation of amyloid fibrils and GM1 ganglioside-rich microdomains in presynaptic membranes through the gamma-aminobutyric acid A receptor. Biochem Biophys Res Commun. 2015;457:547–53.
Chung JK, Nakajima S, Shinagawa S, Plitman E, Chakravarty MM, Iwata Y, et al. Benzodiazepine use attenuates cortical β-Amyloid and is not associated with progressive cognitive decline in nondemented elderly adults: a pilot study using F18-Florbetapir positron emission tomography. Am J Geriatr Psychiatry. 2016;24:1028–39.
Desmidt T, Delrieu J, Lebouvier T, Robert G, David R, Balageas AC, et al. Benzodiazepine use and brain amyloid load in nondemented older individuals: a florbetapir PET study in the Multidomain Alzheimer Preventive Trial cohort. Neurobiol. Aging. 2019;84:61–69.
Dufouil C, Dubois B, Vellas B, Pasquier F, Blanc F, Hugon J, et al. Cognitive and imaging markers in non-demented subjects attending a memory clinic: study design and baseline findings of the MEMENTO cohort. Alzheimers Res Ther. 2017;9:67.
Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, et al. In vivo imaging of amyloid deposition in alzheimer disease using the radioligand 18 F-AV-45 (Flobetapir F 18). J Nucl Med. 2010;51:913–20.
Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–78.
Operto G, Chupin M, Batrancourt B, Habert MO, Colliot O, Benali H, et al. CATI: a large distributed infrastructure for the neuroimaging of cohorts. Neuroinformatics. 2016;14:253–64.
Habert M-O, Marie S, Bertin H, Reynal M, Martini JB, Diallo M, et al. Optimization of brain PET imaging for a multicentre trial: the French CATI experience. EJNMMI Phys. 2016;3:6.
Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51.
Chupin M, Hammers A, Liu RSN, Colliot O, Burdett J, Bardinet E, et al. Automatic segmentation of the hippocampus and the amygdala driven by hybrid constraints: method and validation. Neuroimage. 2009;46:749–61.
Habert M-O, Bertin H, Labit M, Diallo M, Marie S, Martineau K, et al. Evaluation of amyloid status in a cohort of elderly individuals with memory complaints: validation of the method of quantification and determination of positivity thresholds. Ann Nucl Med. 2018;32:75–86.
Haider SI, Johnell K, Weitoft GR, Thorslund M, Fastbom J. The influence of educational level on polypharmacy and inappropriate drug use: a register-based study of more than 600,000 older people: polypharmacy and inappropriate drug use in the elderly. J Am Geriatr Soc. 2009;57:62–69.
Crowe SF, Stranks E. Sci-Hub | The residual medium and long-term cognitive effects of benzodiazepine use: an updated meta-analysis. Arch Clin Neuropsychol. 2017. https://doi.org/10.1093/arclin/acx120. https://sci-hubtw.hkvisa.net/.
Conejero I, Dubois J, Gutierrez LA, Delrieu J, Arbus C, Garcia M, et al. Amyloid burden and depressive symptom trajectories in older adults at risk of developing cognitive decline. J Clin Psychiatry. 2021;82:20m13410.
Chen F, Bertelsen AB, Holm IE, Nyengaard JR, Rosenberg R, Dorph-Petersen KA. Hippocampal volume and cell number in depression, schizophrenia, and suicide subjects. Brain Res. 2020;1727:146546.
Fouquet M, Besson FL, Gonneaud J, La Joie R, Chételat G. Imaging brain effects of APOE4 in cognitively normal individuals across the lifespan. Neuropsychol Rev. 2014;24:290–9.
Jack CR, Petersen RC, Xu YC, O'Brien PC, Waring SC, Tangalos EG, et al. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer’s disease. Ann Neurol. 1998;43:303–10.
Operto G, Chupin M, Batrancourt B, Habert MO, Colliot O, Benali H, et al. CATI: a large distributed infrastructure for the neuroimaging of cohorts. Neuroinformatics. 2016;14:253–64.
Pilipenko V, Narbute K, Pupure J, Rumaks J, Jansone B, Klusa V. Neuroprotective action of diazepam at very low and moderate doses in Alzheimer’s disease model rats. Neuropharmacology. 2019;144:319–26.
Arbo BD, Marques CV, Ruiz-Palmero I, Ortiz-Rodriguez A, Ghorbanpoor S, Arevalo MA, et al. 4′-Chlorodiazepam is neuroprotective against amyloid-beta through the modulation of survivin and bax protein expression in vitro. Brain Res. 2016;1632:91–97.
Haberman RP, Branch A, Gallagher M. Targeting neural hyperactivity as a treatment to stem progression of late-onset Alzheimer’s disease. Neurotherapeutics. 2017;14:662–76.
Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14:750–6.
Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron. 2008;58:42–51.
Busche MA, Konnerth A. Impairments of neural circuit function in Alzheimer’s disease. Philos Trans R Soc B Biol Sci. 2016;371:20150429.
Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–22.
Yuan P, Grutzendler J. Attenuation of -amyloid deposition and neurotoxicity by chemogenetic modulation of neural activity. J Neurosci. 2016;36:632–41.
Veenman L, Papadopoulos V, Gavish M. Channel-Like Functions of the 18-kDa Translocator Protein (TSPO): regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr Pharm Des. 2007;13:2385–405.
Arbo BD, Benetti F, Garcia-Segura LM, Ribeiro MF. Therapeutic actions of translocator protein (18 kDa) ligands in experimental models of psychiatric disorders and neurodegenerative diseases. J. Steroid Biochem Mol Biol. 2015;154:68–74.
Huhtaniska S, Korkala I, Heikka T, Björnholm L, Lehtiniemi H, Hulkko AP, et al. Antipsychotic and benzodiazepine use and brain morphology in schizophrenia and affective psychoses – Systematic reviews and birth cohort study. Psychiatry Res Neuroimaging. 2018;281:43–52.
Licata SC, Shinday NM, Huizenga MN, Darnell SB, Sangrey GR, Rudolph U, et al. Alterations in brain-derived neurotrophic factor in the mouse hippocampus following acute but not repeated benzodiazepine treatment. PLoS ONE. 2013;8:e84806.
Jack CR Jr, Wiste HJ, Knopman DS, Vemuri P, Mielke MM, Weigand SD, et al. Rates of -amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82:1605–12.
Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: Immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000.
Kreutzmann JC, Havekes R, Abel T, Meerlo P. Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience. 2015;309:173–90.
Acknowledgements
The MEMENTO cohort is sponsored by Bordeaux University Hospital (coordination: CIC1401-EC, Bordeaux) and was funded through research grants from the Fondation Plan Alzheimer (Alzheimer Plan 2008–2012), the French ministry of research,higher education and innovation (Plan Maladies Neurodégénératives (2016–2019)). The MEMENTO cohort has received funding support from AVID, GE Healthcare, and FUJIREBIO through private-public partnerships. The Insight-PreAD sub-study was promoted by INSERM in collaboration with the Institut du Cerveau et de la Moelle épinière, Institut Hospitalo-Universitaire, and Pfizer and has received support within the “Investissement d’Avenir” (ANR-10-AIHU-06) program. Sponsor and funders were not involved in the study conduct, analysis and interpretation of data. This work was undertaken using resources on the Dementias Platform UK (DPUK) Data Portal; the Medical Research Council supports DPUK through grant MR/L023784/2.” The study was supported in part by the French National Agency for Research (“Investissements d’Avenir” no. ANR-11-LABX-0018-01), IRON.
Author information
Authors and Affiliations
Consortia
Contributions
QG, VB, and TD gathered data, which were analyzed and interpreted by QG, TD, and VB. ML and MC performed neuroimaging post-treatment and analyses. MOH, ACB, AS, CB, NA, MJR, LB, FA, JPC, VG, have participated substantial contributions to the conception, QG and TD wrote the article. All authors (QG, VB, ML, MOH, MC, JD, TL, GR, RD, SB, ACB, AS, CB, NA, MJR, LB, FA, JPC, VG, WE, VC, BG, TD) participated in drafting, editing, and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
TD reports personal fees from Lundbeck, Otsuka and Eisai. GR reports personal fees from Janssen & Janssen and Ostuka. RD reports personal fees from Janssen & Janssen, lundbeck, Lilly, BMS, Servier, Eisai and Biogen. WE reports personal fees from Eisai, Janssen, Lundbeck, Otsuka, UCB, Roche and Chugai. VC reports personal fees from Janssen and Bristol Meyers Squibb. All other authors declare no competing interests. As far as we are aware of, among the pharmaceutical companies mentioned here, only Roche is involved in the production of benzodiazepines (namely diazepam, in its marketed form of Valium in France). Neither Roche nor any of the other pharmaceutical companies mentioned here were consulted regarding the planning or analysis of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gallet, Q., Bouteloup, V., Locatelli, M. et al. Benzodiazepine use and neuroimaging markers of Alzheimer’s disease in nondemented older individuals: an MRI and 18F Florbetapir PET study in the MEMENTO cohort. Neuropsychopharmacol. 47, 1114–1120 (2022). https://doi.org/10.1038/s41386-021-01246-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01246-5
This article is cited by
-
Benzodiazepines in Alzheimer’s disease: beneficial or detrimental effects
Inflammopharmacology (2023)
-
Large multi-ethnic genetic analyses of amyloid imaging identify new genes for Alzheimer disease
Acta Neuropathologica Communications (2023)