Abstract
Tianeptine is an atypical antidepressant used in Europe to treat patients who respond poorly to selective serotonin reuptake inhibitors (SSRIs). The recent discovery that tianeptine is a mu opioid receptor (MOR) agonist has provided a potential avenue for expanding our understanding of antidepressant treatment beyond the monoamine hypothesis. Thus, our studies aim to understand the neural circuits underlying tianeptine’s antidepressant effects. We show that tianeptine induces rapid antidepressant-like effects in mice after as little as one week of treatment. Critically, we also demonstrate that tianeptine’s mechanism of action is distinct from fluoxetine in two important aspects: (1) tianeptine requires MORs for its chronic antidepressant-like effect, while fluoxetine does not, and (2) unlike fluoxetine, tianeptine does not promote hippocampal neurogenesis. Using cell-type specific MOR knockouts we further show that MOR expression on GABAergic cells—specifically somatostatin-positive neurons—is necessary for the acute and chronic antidepressant-like responses to tianeptine. Using central infusion of tianeptine, we also implicate the ventral hippocampus as a potential site of antidepressant action. Moreover, we show a dissociation between the antidepressant-like phenotype and other opioid-like phenotypes resulting from acute tianeptine administration such as analgesia, conditioned place preference, and hyperlocomotion. Taken together, these results suggest a novel entry point for understanding what circuit dysregulations may occur in depression, as well as possible targets for the development of new classes of antidepressant drugs.
Similar content being viewed by others
Introduction
The monoamine hypothesis has long been the dominant theoretical framework guiding depression research and drug development. It suggestst that depression arises from a deficiency in the monoaminergic neurotransmitters serotonin and norepinephrine, and that antidepressants function by increasing extracellular availability of these monoamines in the brain [1]. However, this hypothesis cannot fully explain the pathophysiology of depression nor the mechanisms of antidepressant action, given the absence of robust mood changes following serotonin depletion—especially in patients with untreated depression [2] and the mismatch in time-course between the chemical and therapeutic effects of most antidepressants [3]. Moreover, monoaminergic antidepressants have limited efficacy: about a third of depressed patients do not remit following treatment with monoaminergic drugs [4], and those who do often experience cumbersome side effects such as nausea and sexual dysfunction in the case of selective serotonin reuptake inhibitors (SSRIs) [5]. Consequently, over the last few years, there has been a pronounced shift in research focus toward other systems, such as the glutamatergic [6] and opioid [7] systems, that may also be dysregulated in depression.
Opioids have already shown promise in clinical trials for depression. Buprenorphine (a partial mu opioid receptor agonist and kappa opioid antagonist) effectively reduces depression in patients who are resistant to treatment with SSRIs and tricyclic antidepressants [8,9,10] or patients with comorbid substance use disorder [11]. Similarly, small clinical studies of individuals with treatment-resistant depression have shown that the synthetic opioid tramadol (a weak MOR agonist) appears to have antidepressant/anti-suicidal effects [12, 13]. In rodent models for depression, acute pharmacological activation of delta (DOR) and mu opioid receptors (MOR) produces antidepressant-like effects, whereas kappa opioid receptor (KOR) agonists and antagonists elicit depressant- and antidepressant-like effects, respectively [7].
A particularly exciting example of a clinically-effective opioid antidepressant is tianeptine. Tianeptine has been used as an effective antidepressant for several decades in Europe, Asia, and South America [14,15,16], and has distinct advantages over standard treatments, causing fewer side effects than SSRIs and tricyclics [14], and rapidly alleviating the cognitive and anxiety symptoms of depression. One clinical study reports therapeutic onset within one week of treatment, rather than after the several weeks required for SSRIs [17]. Tianeptine may also be effective in patients underserved by existing treatments, such as the elderly [18], those refractory to SSRI monotherapy [19], those who experience increased suicidal ideation during the first weeks of antidepressant treatment [20], and those experiencing depression comorbid with other conditions such as Parkinson’s disease [21] or post-traumatic stress disorder [22].
Although it was originally believed to be a selective serotonin reuptake enhancer [23, 24], it was recently shown that tianeptine does not interact with serotonin transporters or receptors at all, but is rather a full agonist for MOR and—to a lesser extent—for DOR [25]. Subsequently, we demonstrated that MOR expression is required for the acute and chronic antidepressant-like effects of tianeptine, and that MOR antagonists block these effects [26]. Given that MOR is expressed widely throughout the nervous system on a number of different cell types [27], the present work aims to identify the population(s) of MORs required for tianeptine’s antidepressant-like effect in order to elucidate potential cellular- and circuit-level mechanisms.
Materials and methods
For further details please see Supplementary Methods.
Mice
All mouse protocols were approved by the New York State Psychiatric Institute Institutional Animal Care and Use Committee at Columbia University, and conform to the NIH Guide for the Care and Use of Laboratory Mice. Experiments were designed to minimize the suffering and number of animals used. Animals were group-housed with free access to food and water (except during the novelty suppressed feeding and sucrose preference tests) and maintained on a 12-h light-dark cycle. Testing was performed during the light period.
Drugs
Tianeptine sodium salt was provided by Servier or purchased from Nyles7.com. The drug’s identity and purity were independently verified using NMR spectroscopy. Fluoxetine hydrochloride was purchased from Anawa Trading (Zurich, Switzerland). Both tianeptine and fluoxetine were dissolved in sterile 0.9% saline for administration. For acute behavioral tests, tianeptine was administered via intraperitoneal (i.p.) injection at a dose of 30 mg/kg at a volume of 10 ml/kg, given 15 min (for hot plate) or 1 hour (all other tests) prior to behavioral testing. For chronic experiments, corticosterone (CAT #: C2505, Sigma, St Louis, MO) was dissolved in 0.45% hydroxypropyl-β-cyclodextrin (β-CD; CAT #: 297561000, Fisher Scientific, Waltham, MA) at 35 ug/ml. It was provided in opaque bottles to shield it from light and available ad libitum to animals in their drinking water, as described previously [26]. After 4 weeks of corticosterone treatment, mice were also given twice daily i.p. injections of tianeptine (30 mg/kg; 10 ml/kg) for 3–28 days (as specified in the figures/legends), after which behavioral testing commenced. For chronic fluoxetine experiments, corticosterone-treated mice were administered 18 mg/kg/day of fluoxetine via oral gavage. The vehicle of 0.9% saline was used as a control for both drugs. For chronic treatment studies, all behavioral tests were conducted at least 18 h after the last drug administration to avoid any acute effects.
BrdU and Doublecortin (DCX) immunohistochemistry
BrdU and DCX immunohistochemistry was performed on brain sections of tianeptine-, fluoxetine-, and saline-treated mice as has been described previously (for details, see supplementary methods). BrdU+ cells were counted manually. Because DCX labeling was so intense in fluoxetine-treated brains, individual cells could not be counted. Instead, images were converted to black and white using Otsu thresholding, and the number of black pixels in each image were quantified using ImageJ.
Behavioral testing
A battery of behavioral tests were performed in each group of mice, ordered from least to most stressful: open field, feeding, novelty suppressed feeding, forced swim test, and hot plate. Mice were allowed at least a day to recover between testing sessions. Detailed methods are included in Supplementary Materials.
Cannulations
Mice underwent stereotaxic surgery in which a bilateral guide cannula was implanted into the ventral hippocampus (Bregma −3.6, ML ± 2.8, DV −3.5). The animals were handled daily and habituated to having injectors taken in and out of their guide cannulas. Caps inserted into the cannulae ensured that no particulate matter entered when infusions were not taking place. After one week or more of recovery time, animals were given acute central infusions of either tianeptine or saline, then subjected to FST 15 min later. Following FST, mice were placed in an open field arena to assess locomotor effects. To inject tianeptine or saline, mice were briefly restrained by scruffing while a 26 ga infusion needle was inserted through the surgically implanted guide cannula in each brain hemisphere. The infusion needles were attached by polyethylene (PE50) tubing to 10 μl Hamilton syringes, which were controlled by a microinfusion pump (Harvard Apparatus). The infusion needles extended 200 μm beyond the cannula. A volume of 0.5 μl of the tianeptine solution was delivered at a rate of 0.5 μl/min bilaterally.
RNAscope
To confirm targeted knockdown of MOR expression in the various Cre lines, mRNA in situ hybridization (ISH) was performed on fresh frozen brain tissue using RNAscope© (Advanced Cell Diagnostics, ACD, Hayward, CA) technology. Gene expression was visualized using the RNAscope Fluorescent Multiplex Assay (cat. no. 320850) according to the manufacturer’s protocol. We used commercial probes for MOR (Mm-Oprm1, #315841; Mm-Oprm1-O4-C2, #544731-C2), VGAT (Mm-Slc32a1, #319191), and SST (Mm-Sst-C3, #404631-C3) in this procedure. RNAscope images were acquired using Leica confocal microscopy (405 laser for DAPI, 488 or 552 for MOR, 552 for VGAT, and 638 for SST). Two to three sections from each mouse were selected for manual quantification, and cells containing more than five puncta were considered positive. Five animals were included per genotype.
Results
Tianeptine shows antidepressant-like effects after as little as 7 days of treatment
We previously reported that tianeptine has chronic antidepressant-like effects [26], and now wanted to investigate whether these effects could emerge after a shorter treatment duration than is required for SSRIs. To do so, we first induced a depressive-like phenotype in mice by exposing them to chronic glucocorticoids. Following 28 days of corticosterone (35 ug/ml) administration via drinking water, mice were given twice daily i.p. injections of tianeptine (30 mg/kg) or saline for 3, 7, or 21 days, then assessed using the novelty suppressed feeding (NSF) test, an anxiety- and depression-related behavioral assay sensitive to both acute benzodiazepines and chronic antidepressants [28, 29] (Fig. 1). Tianeptine treatment did not affect latency to feed in the novel arena following 3 days of treatment (Fig. 1A), but did produce significant antidepressant-like effects after both 7 (Fig. 1C) and 21 days (Fig. 1E) of treatment. Home cage feeding was not influenced (Fig. 1B, D, F), indicating that these arena results were not impacted by any effects of tianeptine on hunger.
A, B NSF results after 3 days of tianeptine treatment (30 mg/kg twice daily) in corticosterone-treated mice. n = 10 per group. A Latency to feed in the novel arena is expressed both as a bar graph (left) and survival curves (right). Logrank (Mantel–Cox Survival): p = 0.1099. B A control measure of latency to feed in the home cage was measured following the arena test. Logrank (Mantel–Cox Survival): p = 0.3509. C, D NSF results after 7 days of tianeptine treatment. n = 29–30 per group. C Logrank (Mantel–Cox Survival) for arena results: ****p = 0.0006. D Logrank (Mantel–Cox Survival) for home cage controls: p = 0.2934. E, F NSF results after 21 days of tianeptine treatment. n = 21–22 per group (E) Logrank (Mantel–Cox Survival): **p = 0.0020. F Logrank (Mantel–Cox Survival) for home cage controls: p = 0.6504. All behavioral assays were conducted at least 18 h post injection. Each dot represents an individual mouse. All bar graphs indicate mean ± SEM.
We also assessed the time course of tianeptine’s antidepressant-like effects in another depression-related paradigm: chronic varied odor restraint stress (CVORS). CVORS pairs traditional chronic restraint stress with various odors in order to prevent animals from habituating to the stressor [30], and is more naturalistic than the corticosterone model in that mice experience chronic stress rather than having their glucocorticoid levels pharmacologically manipulated. After 3 weeks of CVORS, mice were administered tianeptine for 7 or 28 days before testing in the NSF assay (Supplementary Fig. S1A, D). As observed with the corticosterone-treated mice, tianeptine significantly reduced NSF arena latencies following both 7 (Supplementary Fig. S1B) and 28 days (Supplementary Fig. S1E) of treatment. Once again, this difference was only present in the novel enclosure, and not in the home cage, suggesting that these results were also not confounded by hunger (Supplementary Figs. S1C, 1F). Taken together, these findings demonstrate—using two separate depression-related paradigms—a faster onset of antidepressant-like efficacy for tianeptine than would be expected from SSRI treatment.
Tianeptine has a distinct mechanism of action from fluoxetine
To assess whether MOR is necessary for the efficacy of SSRIs, we measured the behavioral response to fluoxetine in corticosterone-treated MOR KO and WT mice in the NSF test (Fig. 2A). As we previously showed [26], the antidepressant-like response to chronic tianeptine was absent in corticosterone-treated MOR KO mice (Fig. 2B). Home cage controls confirm that these results are not confounded by hunger (Supplementary Fig. S2A). In contrast, chronic fluoxetine treatment decreased the latency to feed for both WT and MOR KO mice (Fig. 2C). Here, however, the home cage controls showed a trend in which fluoxetine tended to decrease latency to feed even in a familiar home cage environment (Supplementary Fig. S2B). Although this difference was not statistically significant, it still somewhat confounds interpretation of the NSF result by introducing hunger, rather than attenuated anxiety- and depression-like states, as a possible driver for decreased feeding latencies in fluoxetine-treated mice.
A Timeline for B and C. n = 8–10 per group for B and 7–10 for C. B NSF results previously published in [26]. Latency to feed in the novel arena following chronic treatment with tianeptine (30 mg/kg twice daily for 3 weeks) in chronic corticosterone-treated mice (35 mg/ml in drinking water). Logrank (Mantel–Cox Survival): p = 0.0262. Post hoc logrank test, saline vs. tianeptine: *p = 0.033 for WT; p = 0.930 for MOR KO. C NSF results. Latency to feed in the novel arena following chronic treatment with fluoxetine (14 mg/kg daily for 3 weeks) in chronic corticosterone-treated mice. Logrank (Mantel–Cox Survival): p = 0.001. Post hoc logrank test, saline vs. tianeptine: p = 0.050 for WT; ***p = 0.001 for MOR KO. D Neurogenesis in the dentate gyrus of the hippocampus was assessed by staining for BrdU (top) and DCX (bottom) following chronic antidepressant treatment. Mice were treated with 0.9% saline (left), 30 mg/kg tianeptine (middle), or 14 mg/kg fluoxetine (right) for 4 weeks, injected with BrdU (4 × 75 mg/kg) on the final day of treatment, and killed 24 h later. E BrdU positive cells were counted in the dentate (both sides) for every 6th section of the hippocampus. n = 3–4 mice per group. One-way ANOVA: p < 0.0001. **p = 0.005, tianeptine relative to saline; ****p < 0.0001, fluoxetine relative to saline (unpaired t-test). F Doublecortin expression was quantified by thresholding each image (Otsu) and determining the number of black pixels (DCX stain) in the dentate (both sides) for every sixth section of the hippocampus. n = 2–3 mice per group. One-way ANOVA: p < 0.001. **p = 0.005, fluoxetine relative to saline (unpaired t-test). All behavioral assays were conducted at least 18 h post injection. Each dot represents an individual mouse. All bar graphs indicate mean ± SEM. TIA tianeptine, FLX fluoxetine, BX behavior.
As such, the behavioral effects of chronic fluoxetine were also assessed using the FST, which robustly detects antidepressant-like effects for SSRIs. Here, we again observe that fluoxetine is effective in MOR-deficient mice (Supplementary Fig. S2C), suggesting that MOR expression is not required for the chronic antidepressant-like effects of SSRIs. Although tianeptine is an agonist at DOR as well as MOR, we found that tianeptine continues to produce acute antidepressant- and opioid-like effects in a battery of behavioral tests (FST, home cage feeding, hot plate, open field) in DOR KO mice (Supplementary Fig. S3). Taken together, these data indicate that tianeptine requires MOR, but not DOR, for its antidepressant-like effects, whereas fluoxetine does not require MOR. It therefore appears that tianeptine and fluoxetine exert antidepressant-like effects via distinct mechanisms.
Given that increased hippocampal neurogenesis following chronic fluoxetine treatment contributes to some of its antidepressant-like effects [31], we also examined the effect of tianeptine on cell proliferation (BrdU) and maturation (DCX) (Fig. 2D). We observed small but significant increases in the number of BrdU+ cells following tianeptine treatment, though these were much smaller than those seen following fluoxetine (Fig. 2E). Unlike the almost fourfold increase in DCX staining following fluoxetine treatment, there was no effect of tianeptine on DCX expression (Fig. 2F). Thus, tianeptine’s effect on brain and behavior differs from that of fluoxetine in two important aspects: (1) it requires MORs, while fluoxetine does not, and (2) it is likely hippocampal neurogenesis-independent.
The acute and chronic antidepressant-like effects of tianeptine require MOR expression on GABAergic neurons
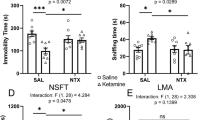
Because MOR is widely expressed throughout the brain [27], understanding tianeptine’s molecular- and circuit-level mechanisms requires identification of the necessary specific subpopulations of MOR-expressing cells. In several brain regions, MORs are primarily expressed on GABAergic interneurons [32, 33], so that was the first population we examined. By crossing a floxed-MOR line to mice expressing Cre under the VGAT promoter (Fig. 3A), we selectively deleted MOR from GABAergic cells and measured the behavioral response to acute and chronic tianeptine. In the forced swim test (FST), a classic predictor of antidepressant efficacy [28], tianeptine decreased immobility time in Cre− but not in Cre+ mice, suggesting that MOR expression on GABAergic neurons is necessary for tianeptine’s acute antidepressant-like action (Fig. 3B).
A MOR was selectively deleted from GABAergic cells by crossing MOR-floxed mice, which have exon 2/3 of their MOR gene flanked by LoxP sites, to mice expressing Cre recombinase driven by the VGAT promotor. B FST results. (Left) bar graph shows combined immobility results of last 4 min. Two-way ANOVA: p = 0.022 for treatment × genotype. Post hoc t-test, saline vs. tianeptine: ****p < 0.0001 for VGAT Cre−; p = 0.584 for VGAT Cre+. (Right) Line graph shows immobility per minute over the 6-min test. Three-way ANOVA (Time × genotype × treatment): p = 0.031 for treatment × genotype. Post hoc repeated measures two-way ANOVA, saline vs. tianeptine: ****p < 0.0001 for VGAT Cre− and p = 0.269 for VGAT Cre+. C The conditioned place preference paradigm was used to test the rewarding effects of tianeptine. The preference score (time spent in drug-paired side – time spent in control side) after 8 days of context pairings with tianeptine (30 mg/kg) or saline is shown. Two-way ANOVA: main effect of treatment: p = 0.013. Planned comparison, saline vs. tianeptine: *p = 0.041 for VGAT Cre−; p = 0.129 for VGAT Cre+ (unpaired t-test). D Analgesia was assessed using latency to lick hind paw after being placed on the hot plate (15 min post injection with 30 mg/kg tianeptine, i.p). Two-way ANOVA: main effect of treatment, p < 0.0001; p = 0.027 for treatment × genotype. Post hoc t-test, saline vs. tianeptine: ****p < 0.000001 for VGAT Cre−; ***p < 0.001 for VGAT Cre+. E Hyperactivity was assessed using total distance traveled in an open field box over 30 min. Two-way ANOVA: main effect of treatment: p < 0.0001. Planned comparisons, saline vs tianeptine: **p = 0.001 for VGAT Cre−; ***p < 0.001 for VGAT Cre+. n = 28–33 per group for B, D, and F and 15–22 per group for C and E. All acute behavioral assays except hot plate were conducted 1 h after an acute i.p. injection of tianeptine. F Home cage feeding over 5 min after an 18-h deprivation period was assessed as a measure of hypophagia. Two-way ANOVA: main effect of treatment: ****p < 0.0001; p = 0.004 for treatment × genotype. Post hoc t-test, saline vs. tianeptine: ****p < 0.000001 for VGAT cre−; p = 0.200 for VGAT cre+. G Timeline for H. Tianeptine (30 mg/kg, i.p.) was administered twice daily for 3 weeks to chronic corticosterone-treated mice. H NSF results. Latency to feed in the novel arena (18 h post injection) for male mice expressed as a bar graph (left) and survival curves (right). Logrank (Mantel–Cox Survival): p = 0.072. Post-hoc logrank test, saline vs. tianeptine: *p = 0.035 for VGAT Cre−; p = 0.632 for VGAT Cre+. Each dot represents an individual mouse. All bar graphs indicate mean ± SEM. BX Behavior.
In addition to tianeptine’s antidepressant-like effects, we also assessed the classic opioid-like responses to tianeptine which we previously found to be dependent on MOR expression [26]. The rewarding properties of tianeptine were assessed using the conditioned place preference (CPP) paradigm. We found that tianeptine induced a preference for the tianeptine-paired side in both genotypes (main effect of treatment: p = 0.0134), although planned comparisons were only significant in the Cre− mice (Fig. 3C). Similarly, in both genotypes, tianeptine produced acute analgesic effects in the hot plate test, as evidenced by a significant increase in the latency to jump when placed on a heated surface (Fig. 3D), and induced hyperlocomotion in the open field test, indicated by an increase in total distance traveled (Fig. 3E). Interestingly, tianeptine decreased home cage feeding in Cre− but not in Cre+ mice, suggesting that the opioid-like acute hypophagic response to tianeptine requires MORs on GABAergic cells (Fig. 3F). Overall these data show that while the acute antidepressant-like effect of tianeptine clearly requires GABAergic MOR expression, the analgesic and locomotor effects do not—at least not in a way captured by our current bigenic line—suggesting a dissociation in the mechanisms of action.
We next investigated whether this population of MOR also mediates the chronic antidepressant-like effect of tianeptine using the chronic corticosterone model (Fig. 3G). We analyzed the data separated by sex and found that in males, tianeptine decreased latency to feed in Cre− but not in Cre+ mice (Fig. 3H, Supplementary Fig. S4A). In females, however, tianeptine did not have an effect in either genotype (Supplementary Fig. S4B), likely because females are less susceptible to corticosterone administration, as reported previously [29]. This is supported by the observation that baseline latency to feed in the arena is much lower for female Cre− mice treated with saline than for male Cre− mice treated with saline, suggesting that tianeptine’s chronic effect in female Cre− mice may have been masked by a floor effect (Supplementary Fig. S4A, B). In the home cage, tianeptine did not influence latency to feed regardless of sex or genotype, ruling out hunger as a confounding factor in these behavioral studies (Supplementary Fig. S4C, D). Overall, at least for male mice, GABAergic MORs appear to be necessary for both the acute and chronic antidepressant-like effects of tianeptine.
The acute and chronic antidepressant-like effects of tianeptine may require MORs on SST cells
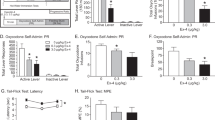
To identify a specific population of GABAergic cells involved in mediating tianeptine’s antidepressant effects, we targeted subsets of GABAergic neurons using additional Cre mouse lines. Many GABAergic cells are locally projecting interneurons, but there are a few classes of long-range GABAergic cells, most notably the medium spiny neurons (MSNs) of the striatum [34]. Given that altered reward processing is a hallmark of depression [35], and that MOR expression on D1 MSNs has previously been shown to mediate the rewarding properties of morphine [36], we targeted these neurons using the Drd1-Cre line (Supplementary Fig. S5A). We found that in mice lacking MOR on D1+ cells, tianeptine still induced substantially reduced immobility in the FST (Supplementary Fig. S5B). The majority of tianeptine’s opioid-like effects were also still intact, including the acutely rewarding effects assessed by CPP (Supplementary Fig. S5C), hypophagia (Supplementary Fig. S5D), and analgesia (Supplementary Fig. S5E). Interestingly, tianeptine did not induce hyperlocomotion in the open field in D1 Cre+ mice (Supplementary Fig. S5F).
Next, we considered MORs on somatostatin (SST) and parvalbumin (PV) cells, both of which are major, non-overlapping classes of GABAergic interneurons that express MOR [37,38,39]. To specifically knock down MOR on SST cells, we crossed the MOR-floxed line to mice expressing Cre recombinase driven by the SST promoter (Fig. 4A). In the FST, tianeptine significantly reduced immobility time for the SST Cre− but not the SST Cre+ mice, suggesting that MOR expression on SST cells may play a role in mediating tianeptine’s acute antidepressant-like effect (Fig. 4B). Notably, a baseline genotype difference was also observed between the SST Cre− and Cre+ groups for this test, raising the possibility that floor effect prevented us from observing tianeptine’s acute antidepressant-like efficacy in the SST Cre+ mice. Classic opioid-like effects including analgesia (Fig. 4C), hyperactivity (Fig. 4D), and hypophagia (Fig. 4E), were intact, suggesting that antidepressant-like effects of tianeptine can be dissociated from the classic opioid effects, and likely have different mechanisms of action.
A MOR was selectively deleted from SST cells by crossing MOR-floxed mice to mice expressing Cre recombinase driven by the SST promotor. B FST day 1 results. (Left) bar graph shows combined immobility results of last 4 min. Planned comparisons, saline vs. tianeptine: *p = 0.011 for SST Cre−; p = 0.231 for SST Cre+ (unpaired t-test). (Right) Line graph shows immobility per minute over the 6-min test. Planned comparisons, saline vs. tianeptine: *p = 0.021 for SST Cre− and p = 0.256 for SST Cre+ (repeated measures two-way ANOVA). C Analgesia was assessed using latency to jump after being placed on the hot plate (15 min post injection). Two-way ANOVA: main effect of treatment: p < 0.0001. Planned comparisons, saline vs. tianeptine: ****p < 0.000001 for SST Cre−; ****p < 0.000001 for SST Cre+ (unpaired t-test). D Open field hyperlocomotion results. Two-way ANOVA: main effect of treatment: p = 0.041. Planned comparisons, saline vs. tianeptine: p = 0.336 for SST Cre−; *p = 0.034 for SST Cre+.15–21 per group for B–E. All acute behavioral assays except hot plate were conducted 1 h after an acute i.p. injection of tianeptine. E Home cage hypophagia results. Two-way ANOVA: main effect of treatment: p < 0.0001. Planned comparisons, saline vs. tianeptine: ****p < 0.000001 for SST Cre−; p = 0.200 for SST Cre+ (unpaired t-test). F Timeline for G. n = 8–12 per group. Tianeptine (30 mg/kg, i.p.) was administered twice daily for 3 weeks to chronic corticosterone-treated mice. G FST results. (Left) bar graph shows combined immobility results of last 4 min. Two-way ANOVA: p = 0.048 for treatment × genotype. Planned comparisons, saline vs. tianeptine: *p = 0.011 for SST Cre−; p = 0.231 for SST Cre+ (unpaired t-test). (Right) Line graph shows immobility per minute over the 6-min test. Planned comparisons, saline vs. tianeptine: *p = 0.021 for SST Cre− and p = 0.811 for SST Cre+ (repeated measures two-way ANOVA). Each dot represents an individual mouse. All bar graphs indicate mean ± SEM. BX behavior.
In chronic corticosterone-treated mice (Fig. 4F), chronic tianeptine significantly reduced forced swim immobility in SST−Cre− but not SST−Cre+ mice, suggesting that MOR expression on SST cells may also be required for the chronic antidepressant-like effects of tianeptine (Fig. 4G). Here, the FST was used in place of NSF as a chronic test because tianeptine did not exhibit an antidepressant effect even in control mice for the NSF test. This is likely due to differences in genetic background between the different mixed strains we have been using. Different tests and test conditions are effective in certain strains of mice and not others, and forced swim turned out to be more sensitive to the chronic effects of tianeptine in the genetic background of the SST−Cre mice.
We also assessed the necessity of MOR expression on another subset of interneurons, namely those that are PV+ (Supplementary Fig. S6A). Unlike SST+ cells, MOR expression on PV cells was not necessary for the antidepressant or opioid-like effects of tianeptine (Supplementary Fig. S6B–E).
MORs in the ventral hippocampus, but not the habenula, may be involved the antidepressant effects of tianeptine
In addition to cell-type specificity, we also investigated which brain regions are engaged by tianeptine. The hippocampus has been extensively implicated in depression and has high expression of MORs on GABAergic cells, making it a compelling candidate for tianeptine’s site of action [15, 40,41,42]. Because the hippocampus is thought to be functionally heterogeneous along its longitudinal axis, with the dorsal hippocampus involved in learning and spatial memory and the ventral hippocampus associated with regulating emotional and motivated behaviors, we considered the ventral hippocampus, in particular, as a potential site of action for tianeptine.
We assessed the sufficiency of hippocampal MORs for tianeptine’s acute antidepressant-like effects using central infusions of tianeptine. WT C57BL/6 mice had bilateral cannulae surgically implanted into the ventral hippocampus, through which tianeptine was administered 15 min prior to behavioral testing in the forced swim and open field assays (Fig. 5A). We found that central infusion of tianeptine significantly decreased FST immobility compared to saline controls (Fig. 5B), and that this difference could not be attributed to increased tianeptine-induced hyperlocomotion, as there was no statistically significant difference in open field locomotion (Fig. 5C). This suggests that hippocampal MORs alone may be enough to mediate tianeptine’s acute antidepressant-like effect, and that tianeptine’s opioid-like hyperactivity effect may be mediated by MORs in another brain region.
A Schematic of cannula placement into the ventral hippocampus (Bregma-3.6, ML ± 2.8, DV-3.5). B FST results. (Left) Bar graph shows combined immobility results of last 4 min. Unpaired t-test, saline vs. tianeptine: *p = 0.0453. (Right) Line graph shows immobility per minute over the 6-min test. Repeated measures two-way ANOVA, effect of treatment: p = 0.0749, effect of time: p < 0.0001, time x treatment: p = 0.0005. C Open field locomotion results. Unpaired t-test, saline vs. tianeptine: p = 0.5017. D Confocal imaging of RNAscope (ACDbio®) probes targeting Oprm1 (green) and Slc32a1 (red) mRNAs in addition to DAPI staining (blue) shows colocalization of the two transcripts in ventral hippocampus sections. Oprm1 expression on VGAT cells is strongly reduced in VGAT Cre+ mice compared to VGAT Cre− mice. E Representative confocal images of Oprm1 (green) and Sst (red) mRNA transcript colocalization shows reduced Oprm1 expression in SST cells in SST Cre+ mice compared to SST Cre− controls. F Quantification of double Oprm1/Slc32a-positive cells in the ventral hippocampus of VGAT Cre+ (pink) mice and VGAT Cre− (black) controls. Both the number of Slc32a-positive cells that express Oprm1 (left) and the percent of Slc32a-positive cells that express Oprm1 (right) within a section of hippocampus are dramatically lower in VGAT Cre+ mice compared to VGAT Cre− mice (unpaired t-test, ****p < 0.0001 for both). G Quantification of double Oprm1/Sst-positive cells in the ventral hippocampus of SST Cre+ (cyan) and SST Cre− (purple) mice. Both the number of Sst-positive cells that express Oprm1 (left) and the percent of Sst-positive cells that express Oprm1 (right) are significantly lower in SST Cre+ mice compared to SST Cre− controls (unpaired t-test, **p < 0.01 and *p = 0.01, respectively).
Moreover, because tianeptine is no longer effective in MOR-floxed VGAT Cre+ and SST Cre+ mice, the brain regions involved should exhibit a marked reduction of MOR expression in Cre+ mice from both crosses. Using RNAscope ISH, we found that Oprm1(MOR) and Slc32a1 (VGAT) mRNAs are highly expressed and colocalized in ventral hippocampus sections of VGAT Cre− mice, and that Oprm1 expression on VGAT cells is strongly reduced in VGAT Cre+ mice compared to controls (Fig. 5D). Similarly, we observed reduced Oprm1 transcript expression in SST cells in SST Cre+ mice compared to SST Cre− mice (Fig. 5E). Quantification of ISH signals revealed significant reduction of both double Oprm1/Slc32a-positive neurons in the ventral hippocampus of VGAT Cre+ mice (Fig. 5F), and double Oprm1/Sst-positive cells in the ventral hippocampus of SST Cre+ mice (Fig. 5G), indicating a selective loss of Oprm1 mRNA in GABAergic and SST-expressing neurons, respectively, within the ventral hippocampus. These results are consistent with our behavioral data implicating ventral hippocampal MORs in the antidepressant-like effects of tianeptine.
One initially promising candidate for tianeptine’s site of action was the medial habenula (MHb), a structure with a high density of MOR expression [43, 44]. In order to achieve habenula-specific knockdown of MOR, we used the B4 subunit of the nicotinic acetylcholine receptor (Chrnb4, henceforth abbreviated as B4, which is mainly localized to the medial habenula) [45]. We crossed B4-Cre mice with MOR-floxed mice (Supplementary Fig. S7A) and used confocal imaging of RNAscope® probes targeting Oprm1 (the gene for MOR) and Chrnb4 mRNAs confirmed that B4 Cre+ mice exhibit habenula-specific reduction of MOR expression, restricted to B4-neurons (Supplementary Fig. S7C) [43]. Nevertheless, in the FST, tianeptine significantly reduced immobility time for both the B4 Cre− and B4 Cre+ mice, suggesting that MORs in the habenula are likely not responsible for the acute antidepressant effects of tianeptine (Supplementary Fig. S7B). However, while habenular expression of MOR is markedly diminished in B4 Cre+ mice compared to controls, some expression still remains (Supplementary Fig. S7C, right); consequently, we cannot entirely exclude the possibility that these residual MOR-positive cells are sufficient to mediate the acute antidepressant-like response to tianeptine.
Despite these caveats, our RNAscope results are largely congruous with both the existing literature and our own behavioral data. The MHb is known to contain mainly glutamatergic neurons [46]; accordingly, RNAscope ISH showed a complete absence of Slc32a1 mRNA (encoding VGAT) within the MHb, and habenular Oprm1 expression remained unchanged in VGAT Cre+ mice compared to Cre− controls (Supplementary Fig. S7D). Additionally, Oprm1 and Sst mRNAs appear to be expressed in largely non-overlapping cell populations within the MHb, and MOR expression was comparable in both SST Cre− and Cre+ mice (Supplementary Fig. S7E). The lack of MOR deletion in the MHb in the two lines of Cre mice in which we observed abolition of tianeptine’s antidepressant effects is consistent with the notion that the MHb is not tianeptine’s acute site of action.
Discussion
This study is the first to our knowledge that draws a direct distinction between the antidepressant mechanisms of tianeptine and fluoxetine. Notably, we show that tianeptine produces a rapid antidepressant-like phenotype in mice after just 7 days of treatment, which is consistent with at least one clinical study reporting initial therapeutic benefits after one week of tianeptine treatment, rather than several weeks as required for SSRIs [17]. Using cell-type specific MOR knockout, we not only establish that MOR expression on GABA and SST cells are involved in mediating tianeptine’s acute and chronic antidepressant-like effects, we also demonstrate a double dissociation of the antidepressant-like phenotype from other opioid-like phenotypes resulting from acute tianeptine administration. Mice lacking MOR expression on GABAergic neurons failed to show the antidepressant-like effect, but still showed acute hyperlocomotion, analgesia, and conditioned place preference. Conversely, knockdown of MOR expression on D1 receptor-expressing neurons resulted in the absence of typical opioid-induced hyperlocomotion, with an intact antidepressant phenotype.
We focused on MOR on GABAergic cells because of the large proportion of MORs on GABA neurons, including within the hippocampus. GABAergic cells that co-express SST are perhaps the most interesting interneuron subtype in the context of depression. Evidence from human postmortem and animal studies suggests a selective vulnerability of SST neurons in MDD [47,48,49]. Moreover, work in mice suggests a causal role for reduced SST cell function in mood disorders. SST knockout mice have been shown to exhibit elevated depressive- and anxiety- like behaviors, and disinhibiting interneurons co-expressing SST and GABA has an anxiolytic and antidepressant-like effect [40]. The neuropeptide SST itself also produces anxiolytic- and antidepressant-like effects when infused into rodent corticolimbic brain regions [41, 42].
In addition to cell type mechanisms, we also investigated brain region specificity. We demonstrate that the medial habenula, one of the strongest expression sites for MORs [44], is not a site of action for tianeptine, and instead show that direct actions on ventral hippocampal neurons may be responsible for tianeptine’s antidepressant-like effects. This is consistent with a large body of literature linking the hippocampus with depression. The hippocampus undergoes dramatic changes during depression, including dendritic atrophy, decreased volume, reduced levels of cerebral metabolites, and decreased adult neurogenesis [15, 40,41,42]. Connectivity studies have identified the hippocampus as one of several regions in a network for emotional regulation that is dysregulated in MDD [50], and when various domains of cognitive function are assessed in depressed patients, the most significant impairment is observed in memory measures that are heavily hippocampus-dependent [51]. Strikingly, many of the morphological changes to the hippocampus observed in depressed/chronically stressed subjects (e.g., reduction in dendritic length and complexity in CA3 pyramidal neurons) can be specifically reversed by tianeptine [52, 53].
The opioid system likely plays a role in hippocampal plasticity and function, as the hippocampus is an opioid-rich structure that expresses all three major opioid receptors and their associated ligands [54]. Thus, hippocampal function may be crucially dysregulated in depression and normalized by antidepressant treatment. Studies have shown that MORs can modulate activity-dependent synaptic transmission in various hippocampal pathways regulating aspects of learning and memory [55]; MOR antagonists have been found to impair the induction of long term potentiation [56] and both MOR agonists and antagonists have been shown to modify dendritic spines, whose morphology is correlated with synaptic plasticity [57,58,59].
Broadly, this work has intriguing implications about the nature of opioid antidepressants. Two overarching hypotheses that have been used to justify the use of opioids as a treatment for depression are euphoria (i.e., that the rewarding effects of opioids counteract anhedonia) [60] and mental pain (i.e., that the putative overlap between the neural circuits underlying physical and mental pain means analgesics can also help alleviate aversive emotional states) [61,62,63]. However, our results do not directly support either notion, as both conditioned place preference and hot plate analgesia have been dissociated from acute antidepressant-like effects for tianeptine. This does not mean that the reward and pain systems are irrelevant to depression, but it does suggest that these two circuits are not the ones responding to tianeptine in a manner captured by our current depression assays. Instead of restoring reward or producing euphoria, tianeptine might instead rectify dysregulation of the corticolimbic network of mood regulation by engaging structures such as the prefrontal cortex, anterior cingulate, hippocampus, and amygdala, all of which are interconnected and have been shown to exhibit morphological and functional abnormalities in depressed patients [64].
Our work also highlights potentially promising future clinical applications of tianeptine. Due to its distinct mechanism from SSRIs, tianeptine might be effective in specific subsets of patients for whom current treatments are suboptimal [18,19,20,21,22]. In particular, depressed patients with high rejection sensitivity (sometimes called atypical depression) may be uniquely suited to benefit from mu opioid-based antidepressant treatments, as rejection sensitivity has been recently associated with opioid deficits [65]. Depressed patients display reduced MOR activation in brain regions regulating stress, mood, and motivation during social rejection compared to healthy controls [66], and a functional variation of the MOR gene has been linked to dispositional and neural sensitivity to social rejection in humans [67].
Tianeptine’s opioid-based mechanism may raise concerns about its potential abuse liability, and rightly so. According to the CDC, tianeptine exposure calls to U.S. poison control centers increased during 2014–2017, and the associated health effects included neurologic, cardiovascular, and gastrointestinal symptoms reminiscent of opioid toxicity and withdrawal. However, case reports of tianeptine dependence and withdrawal predominantly feature individuals with a prior history of substance use disorder (63%) who had been taking far more than the recommended therapeutic dose (an average of ~1924 mg/day as opposed to 25 or 50 mg/day) [68]. Moreover, we have shown previously that tianeptine has a short half-life and displays a reduced withdrawal/tolerance profile compared to other mu-opioid agonists like morphine [26]. As such, while tianeptine certainly comes with a fair share of risks, given proper medical supervision, it may still be a suitable treatment option for select populations of treatment-resistant depressed patients.
In conclusion, we have demonstrated that tianeptine is mechanistically distinct from SSRIs, both in its direct engagement of the mu opioid rather than the monoaminergic system, and in the hippocampal neurogenesis-independent nature of its chronic antidepressant effects. Furthermore, we have identified MORs on GABAergic—and more specifically SST expressing—neurons in the ventral hippocampus as functionally relevant targets for tianeptine’s acute and chronic effects. In doing so we have illuminated a new avenue for understanding what circuit dysregulations may occur in depression, and identified an entry point for the development of new classes of antidepressant drugs.
References
Castren E. Is mood chemistry? Nat Rev Neurosci. 2005;6:241–6.
Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–59.
Nestler EJ. Antidepressant treatments in the 21st century. Biol Psychiatry. 1998;44:526–33.
Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449–59.
Atmaca M, Kuloglu M, Tezcan E, Buyukbayram A. Switching to tianeptine in patients with antidepressant-induced sexual dysfunction. Hum Psychopharmacol. 2003;18:277–80.
Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–41.
Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206.
Machado-Vieira R, Zarate CA Jr. Proof of concept trials in bipolar disorder and major depressive disorder: a translational perspective in the search for improved treatments. Depress Anxiety. 2011;28:267–81.
Fava M, Thase ME, Trivedi MH, Ehrich E, Martin WF, Memisoglu A, et al. Opioid system modulation with buprenorphine/samidorphan combination for major depressive disorder: two randomized controlled studies. Mol Psychiatry. 2020;25:1580–91.
Stanciu CN, Glass OM, Penders TM. Use of Buprenorphine in treatment of refractory depression-A review of current literature. Asian J Psychiatr. 2017;26:94–98.
Gerra G, Leonardi C, D’Amore A, Strepparola G, Fagetti R, Assi C, et al. Buprenorphine treatment outcome in dually diagnosed heroin dependent patients: A retrospective study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:265–72.
Shapira NA, Verduin ML, DeGraw JD. Treatment of refractory major depression with tramadol monotherapy. J Clin Psychiatry. 2001;62:205–6.
Spencer C. The efficacy of intramuscular tramadol as a rapid-onset antidepressant. Aust N. Z J Psychiatry. 2000;34:1032–3.
Wagstaff AJ, Ormrod D, Spencer CM. Tianeptine: a review of its use in depressive disorders. CNS Drugs. 2001;15:231–59.
Kasper S, McEwen BS. Neurobiological and clinical effects of the antidepressant tianeptine. CNS Drugs. 2008;22:15–26.
McEwen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P, et al. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry. 2010;15:237–49.
Novotny V, Faltus F. First signs of improvement with tianeptine in the treatment of depression: an analysis of a double-blind study versus fluoxetine. Eur Neuropsychopharmacol. 2003;13:S230.
Karpukhin IB. Use of Coaxil (tianeptine) in elderly patients with combined mild cognitive and depressive-anxiety disorders. Neurosci Behav Physiol. 2009;39:53–6.
Woo YS, Bahk WM, Jeong JH, Lee SH, Sung HM, Pae CU, et al. Tianeptine combination for partial or non-response to selective serotonin re-uptake inhibitor monotherapy. Psychiatry Clin Neurosci. 2013;67:219–27.
Nobile B, Jaussent I, Gorwood P, Lopez Castroman J, Olie E, Guillaume S, et al. Tianeptine is associated with lower risk of suicidal ideation worsening during the first weeks of treatment onset compared with other antidepressants: a naturalistic study. J Psychiatr Res. 2018;96:167–70.
Levin OS. Coaxil (tianeptine) in the treatment of depression in Parkinson’s disease. Zh Nevrol Psikhiatr Im S S Korsakova. 2006;106:20–5.
Onder E, Tural U, Aker T. A comparative study of fluoxetine, moclobemide, and tianeptine in the treatment of posttraumatic stress disorder following an earthquake. Eur Psychiatry. 2006;21:174–9.
Mennini T, Mocaer E. Garattini S. Tianeptine, a selective enhancer of serotonin uptake in rat brain. Naunyn Schmiedebergs Arch Pharm. 1987;336:478–82.
Fattaccini CM, Bolanos-Jimenez F, Gozlan H, Hamon M. Tianeptine stimulates uptake of 5-hydroxytryptamine in vivo in the rat brain. Neuropharmacology 1990;29:1–8.
Gassaway MM, Rives ML, Kruegel AC, Javitch JA, Sames D. The atypical antidepressant and neurorestorative agent tianeptine is a mu-opioid receptor agonist. Transl Psychiatry. 2014;4:e411.
Samuels BA, Nautiyal KM, Kruegel AC, Levinstein MR, Magalong VM, Gassaway MM, et al. The behavioral effects of the antidepressant tianeptine require the Mu-opioid receptor. Neuropsychopharmacology. 2017;42:2052–63.
Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, et al. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct. 2015;220:677–702.
Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36.
Mekiri M, Gardier AM, David DJ, Guilloux JP. Chronic corticosterone administration effects on behavioral emotionality in female c57bl6 mice. Exp Clin Psychopharmacol. 2017;25:94–104.
Grissom N, Iyer V, Vining C, Bhatnagar S. The physical context of previous stress exposure modifies hypothalamic-pituitary-adrenal responses to a subsequent homotypic stress. Horm Behav. 2007;51:95–103.
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–93.
Simmons ML, Chavkin C. Endogenous opioid regulation of hippocampal function. Int Rev Neurobiol. 1996;39:145–96.
Lau BK, Ambrose BP, Thomas CS, Qiao M, Borgland SL. Mu-Opioids Suppress GABAergic Synaptic Transmission onto Orbitofrontal Cortex Pyramidal Neurons with Subregional Selectivity. J Neurosci. 2020;40:5894–5907.
Caputi A, Melzer S, Michael M, Monyer H. The long and short of GABAergic neurons. Curr Opin Neurobiol. 2013;23:179–86.
Admon R, Pizzagalli DA. Dysfunctional reward processing in depression. Curr Opin Psychol. 2015;4:114–18.
Cui Y, Ostlund SB, James AS, Park CS, Ge W, Roberts KW, et al. Targeted expression of mu-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward. Nat Neurosci. 2014;17:254–61.
Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron. 2016;91:260–92.
Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61.
Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12:119–36.
Fuchs T, Jefferson SJ, Hooper A, Yee PH, Maguire J, Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry. 2017;22:920–30.
Engin E, Stellbrink J, Treit D, Dickson CT. Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: behavioral and neurophysiological evidence. Neuroscience. 2008;157:666–76.
Prevot TD, Gastambide F, Viollet C, Henkous N, Martel G, Epelbaum J, et al. Roles of hippocampal somatostatin receptor subtypes in stress response and emotionality. Neuropsychopharmacology. 2017;42:1647–56.
Boulos LJ, Ben Hamida S, Bailly J, Maitra M, Ehrlich AT, Gaveriaux-Ruff C, et al. Mu opioid receptors in the medial habenula contribute to naloxone aversion. Neuropsychopharmacology. 2020;45:247–55.
Gardon O, Faget L, Chu Sin Chung P, Matifas A, Massotte D, Kieffer BL. Expression of mu opioid receptor in dorsal diencephalic conduction system: new insights for the medial habenula. Neuroscience. 2014;277:595–609.
Shih PY, Engle SE, Oh G, Deshpande P, Puskar NL, Lester HA, et al. Differential expression and function of nicotinic acetylcholine receptors in subdivisions of medial habenula. J Neurosci. 2014;34:9789–802.
Boulos LJ, Darcq E, Kieffer BL. Translating the Habenula-from rodents to humans. Biol Psychiatry. 2017;81:296–305.
Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130–42.
Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:1194–202.
Douillard-Guilloux G, Lewis D, Seney ML, Sibille E. Decrease in somatostatin-positive cell density in the amygdala of females with major depression. Depress Anxiety. 2017;34:68–78.
Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res. 2010;44:799–807.
Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11:111–9.
Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharm. 1992;222:157–62.
McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22.
Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, et al. Mu, Delta, and Kappa Opioid Receptor mRNA expression in the Rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–38.
Puryear CB, Brooks J, Tan L, Smith K, Li Y, Cunningham J, et al. Opioid receptor modulation of neural circuits in depression: what can be learned from preclinical data? Neurosci Biobehav Rev. 2020;108:658–78.
Xie CW, Lewis DV. Opioid-mediated facilitation of long-term potentiation at the lateral perforant path-dentate granule cell synapse. J Pharm Exp Ther. 1991;256:289–96.
Liao D, Lin H, Law PY, Loh HH. Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci USA. 2005;102:1725–30.
Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–9.
Hauser KF, McLaughlin PJ, Zagon IS. Endogenous opioid systems and the regulation of dendritic growth and spine formation. J Comp Neurol. 1989;281:13–22.
Tenore PL. Psychotherapeutic benefits of opioid agonist therapy. J Addict Dis. 2008;27:49–65.
Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1264–80.
Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psychiatry Rep. 2013;15:421.
Mee S, Bunney BG, Reist C, Potkin SG, Bunney WE. Psychological pain: a review of evidence. J Psychiatr Res. 2006;40:680–90.
Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–74.
Hsu DT, Jarcho JM. “Next up for psychiatry: rejection sensitivity and the social brain”. Neuropsychopharmacology. 2021;46:239–40.
Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Walker SJ, et al. It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol Psychiatry. 2015;20:193–200.
Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc Natl Acad Sci USA. 2009;106:15079–84.
Lauhan R, Hsu A, Alam A, Beizai K. Tianeptine abuse and dependence: case report and literature review. Psychosomatics. 2018;59:547–53.
Funding
This work was supported by the Hope for Depression Research Foundation. (RH/JH), NIH Grant MH068542 (RH), NIMH K99/R00 106731 (KMN), NIH Grant MH116462 (JP/RH), NIH Grant DA05010 (BLK), NIMH K08 MH109735 (AZH), NARSAD Young Investigator Awards from the Brain Behavior Research Foundation (to AZH and KMN).
Author information
Authors and Affiliations
Contributions
JH, VA, VMM, and KMN performed behavioral experiments and RNAscope ISH. JH, KMN, and RH wrote the manuscript. CL, EAP, SGG, and JAJ conducted the time course studies. FA and BLK performed the habenula-specific experiments, AZH developed the CVORS paradigm, JP contributed mouse lines and helped with experimental design, and RH and KMN were supervising PIs.
Corresponding authors
Ethics declarations
Competing interests
RH is a consultant for Psychogenics. RH and JAJ are co-founders of Kures Inc and co-inventors on patents on tianeptine analogs held by Columbia University and licensed to Kures. All other authors have no competing interests to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Han, J., Andreu, V., Langreck, C. et al. Mu opioid receptors on hippocampal GABAergic interneurons are critical for the antidepressant effects of tianeptine. Neuropsychopharmacol. 47, 1387–1397 (2022). https://doi.org/10.1038/s41386-021-01192-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01192-2
This article is cited by
-
The mu opioid receptor and the orphan receptor GPR151 contribute to social reward in the habenula
Scientific Reports (2022)
-
Coadministration of tianeptine alters behavioral parameters and levels of neurotrophins in a chronic model of Maple Syrup Urine disease
Metabolic Brain Disease (2022)
-
Tianeptine, but not fluoxetine, decreases avoidant behavior in a mouse model of early developmental exposure to fluoxetine
Scientific Reports (2021)