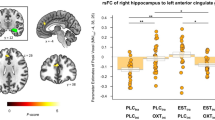
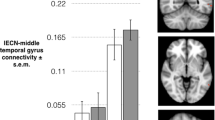
Abstract
Fluctuations of endogenous estrogen modulates fear extinction, but the influence of exogenous estradiol is less studied. Moreover, little focus has been placed on the impact of estradiol on broad network connectivity beyond the fear extinction circuit. Here, we examined the effect of acute exogenous estradiol administration on fear extinction-induced brain activation, whole-brain functional connectivity (FC) during the fear extinction task and post-extinction resting-state. Ninety healthy women (57 using oral contraceptives [OC], 33 naturally cycling [NC]) were fear conditioned on day 1. They ingested an estradiol or placebo pill prior to extinction learning on day 2 (double-blind design). Extinction memory was assessed on day 3. Task-based functional MRI data were ascertained on days 2 and 3 and resting-state data were collected post-extinction on day 2 and pre-recall on day 3. Estradiol administration significantly modulated the neural signature associated with fear extinction learning and memory, consistent with prior studies. Importantly, estradiol administration induced significant changes in FC within multiple networks, including the default mode and somatomotor networks during extinction learning, post-extinction, and during extinction memory recall. Exploratory analyses revealed that estradiol impacted ventromedial prefrontal cortex (vmPFC) activation and FC differently in the NC and OC women. The data implicate a more diffused and significant effect of acute estradiol administration on multiple networks. Such an effect might be beneficial to modulating attention and conscious processes in addition to engaging neural processes associated with emotional learning and memory consolidation.
Similar content being viewed by others
Introduction
Women are at higher risk of developing posttraumatic stress disorder (PTSD) and anxiety disorders compared to men [1, 2]. The pathophysiology of anxiety and fear-related disorders is associated with abnormal fear extinction learning and extinction memory retention [3,4,5,6,7]. Pavlovian fear conditioning and extinction is a widely used translational model that investigates the mechanisms underlying fear extinction and its memory consolidation [6, 8, 9]. Women and men exhibit different psychophysiological responses during fear conditioning and extinction [10,11,12], and fear conditioning and extinction appear to be impacted by the phase of the menstrual cycle in women (see [13] for a recent review). These findings are consistent with the rodent literature [14, 15] and with documented sex differences in humans across multiple emotional learning and memory paradigms [16,17,18].
Accumulating evidence suggests that sex hormones are likely to contribute to some of these differences [19,20,21,22,23]. Specifically, naturally cycling (NC) women undergoing extinction learning while in a naturally high-estrogen state showed facilitated extinction memory retention compared to women in low-estrogen state [11, 24]. In women with PTSD, estrogen levels were associated with extinction deficits [25, 26]. And higher estradiol levels protected against the negative impact of PTSD symptoms by enhancing habituation during fear conditioning and extinction [27]. A recent review highlighted the consistency of premenstrual exacerbation of anxiety symptoms and the protective effects of estradiol on recall of extinction learning in healthy women [13]. Collectively, these works suggest that there is potential for using estradiol as an adjunct to current PTSD treatments [5]. Currently, however, there is little evidence to show that exogenous administration of synthetic estradiol (whether acute or chronic) could impact the neural correlates of fear extinction and extinction memory recall. In a psychophysiological study, we have shown that administration of a estradiol pill before extinction learning significantly improved extinction memory in healthy women [28], supporting the idea that extinction learning and memory may be enhanced by exogenously manipulating estradiol.
The main objective of the current study was to examine how a single administration of estradiol affects the neural signature during and after fear extinction learning. Ninety healthy women were randomized to take placebo or estradiol pill (double-blind) and underwent a validated fear conditioning and extinction paradigm while skin conductance responses (SCR) and fMRI data were acquired [29,30,31,32]. In addition to studying the activation of key nodes of the fear extinction network, including the ventromedial prefrontal cortex (vmPFC), hippocampus, and amygdala [6, 33, 34], our analyses also focused on whole-brain functional connectivity (FC). This analytic approach is consistent with recent calls to focus on broadly distributed brain networks that underlie attention and conscious awareness in addition to emotional learning and memory [35,36,37]. Moreover, we analyzed data from resting-state immediately after extinction learning, given that animal [38,39,40,41] and human [42,43,44] studies have shown that the time window after extinction learning is important for extinction memory consolidation. We hypothesized that estradiol administration would modulate both brain activations and large-scale FC. We conducted secondary analyses to explore whether there is a difference between oral contraceptive (OC) users and NC women in the estradiol-induced activations and FC, and explored the potential impact of estradiol dose effects.
Materials and methods
Participants
Ninety healthy women (18–30 years old) were recruited at three sites in the study. Participants were either OC users (n = 57) or NC women (n = 33). For the OC users, we recruited women using monophasic, second and third generation OC pills with 20 mg or less of ethynyl estradiol. We did not obtain any additional specific information about the brand of type of OC being used. All women were required to have used OC for at least 3 months prior to their participation in our study. NC women underwent the paradigm within the first 10 days of menses. All procedures were approved by the Institute Review Board (IRB) of the three sites. Written informed consent was obtained from all participants. See Supplementary Material for more details.
Experimental design
Participants underwent a 3-day fear conditioning and extinction paradigm [29,30,31,32] (shown in Fig. 1A). On day 1, participants completed the fear conditioning phase outside of the scanner. They were presented with three cues (conditioned stimulus, CS), two of which were partially reinforced with a mild electric shock (CS+) while the other was not reinforced (CS−). On day 2, participants were randomized (double-blind) to take either a pill of placebo or estradiol (2 or 4 mg) at home. Approximately 5 h later, when estradiol levels were expected to be near peak (per manufacturer description), participants underwent the extinction learning phase in the fMRI scanner, during which they viewed one CS + (termed CS + extinguished [CS + E]) and one CS−, both presented without shock. About 10 min after extinction learning, participants underwent a 10-min resting-state scan. Twenty-four hours later (day 3), participants underwent the extinction retention test in the fMRI scanner. Participants were presented with all three cues: the CS + E, the CS+ that was not extinguished on day 2 (termed CS + U), and the CS−, without shock. Resting-state data were collected before the extinction retention test. On each experimental day, we collected blood samples from participants before the experiment to measure serum estradiol levels. SCR data were analyzed as described in prior studies [31, 32, 45]. For fear conditioning, we focused on the difference between both CS + s and the CS−, with the trials equally divided into two halves (early and late). For extinction learning, we conducted the analysis by calculating the differential SCRs (CS + minus CS−, 4 bins of 4 trials each). For the extinction retention test, we evaluated extinction retention using SCRs during the first 4 trials of the CS + E and CS + U. See Supplementary Material for more details.
A The 3-day fear conditioning and extinction paradigm. B Differential SCRs (CS+ minus CS−) in fear conditioning and extinction learning phases. In fear conditioning (day 1), the differential SCRs between women to receive estradiol pill (to-be E2) and women to receive the placebo pill (to-be Pbo) were comparable (main effect of group F(1,65) = 1.04, p = 0.31). In extinction learning, there was only a significant main effect of time (F(3,174) = 8.48, p < 0.001). C SCRs in extinction retention test. The women ingested the placebo pill showed significantly higher SCRs (CS + E minus CS + U) than women ingested the estradiol pill (t(57) = 2.0, p = 0.049). Error bars represent the SEM. Cond conditioning, Ext. extinction, Pbo placebo, E2 estradiol, *p < 0.05; **p < 0.01; ***p < 0.001.
Imaging data analyses
Task-based activation
For details regarding MRI data acquisition and processing, see Supplementary Material. For extinction learning, we created contrast maps for the early (the first 4 trials CS+ vs. CS−) and late (the last 4 trials CS+ vs. CS−) phase. For the extinction retention test, we focused on the early CS + E vs. CS + U (the first 4 trials) contrast map. The rationale for selecting these specific trials for each phase was based on prior fMRI and animal studies showing distinct neural activations for these trials within each examined phase [6, 31, 32, 46, 47]. Individuals’ contrast maps were used as the dependent variable for the second-level group analyses, with the estradiol levels at day 2 as the independent variable. For analyses of neural activations (and FC described below), we opted to use estradiol concentrations as a continuous measure rather than the placebo vs. estradiol groups. We did this for two reasons: First, this approach provides more robust statistical power in studying the impact of estradiol on the brain. Second, previous studies have shown that the inter-individual variance within groups was very high [43], likely reflecting individual differences pertaining to the pharmacodynamics and kinetics of estradiol absorption and metabolism. Therefore, examining estradiol levels in this manner could potentially capture inter-individual differences more effectively than a binary group approach. Study site was included as a covariate in our analyses to control for site effects. Group-level activation maps were thresholded with a voxel-level p < 0.001 and a cluster-level p < 0.05 (family-wise error rate [FWER] corrected). For significant activations, Montreal Neurological Institute (MNI) coordinates of the peaks were reported.
Task-based connectivity
We used a whole-brain generalized psychophysiological interaction method to examine task-based connectivity among brain regions [48, 49]. We divided the whole-brain into 432 functionally homogeneous regions, including 400 cortical regions [50], and 32 subcortical regions [51]. Each region was assigned to one of 8 canonical subnetworks according to previous studies [52], including the frontoparietal control network (CON), default mode network (DMN), dorsal attention network (DAN), limbic network (LIM), ventral attention network (VAN), somatomotor network (SMN), visual network (VIS), and subcortical network (SUB). For every two regions, we estimated their connections during early and late extinction learning and early extinction retention test, as defined above. We constructed a symmetrized 432 × 432 FC matrix for each condition, with each value representing an edge (connection) between paired regions. We used the Combat harmonization approach to correct site effects [53, 54]. We used the Network-Based Statistic (NBS) procedure—a well-validated method for controlling FWER [55]—to identify edges that showed significant correlations with estradiol levels on day 2 (for details, see Supplementary Material).
Resting-state FC
We extracted the mean time series of each brain region from the preprocessed resting-state fMRI data. We obtained a 432 × 432 FC matrix for each resting-state run by calculating Pearson correlations between paired regions. The NBS method was used to identify network components that significantly correlated with estradiol level on day 2.
Mediation analyses
Mediation analysis tests whether the effect of an independent variable (IV) on a dependent variable (DV) is transmitted by a mediator [56]. In this study, we specifically tested two models. The first (learning model) was a standard mediation model, which tested whether FC during extinction learning (mediator) mediates the effects of estradiol administration (IV) on post-extinction learning resting-state FC (rsFC, DV). The second (memory model) was a parallel mediation model, which tested whether extinction learning (mediator 1) and post-extinction learning rsFC (mediator 2) mediate the effects of estradiol administration (IV) on extinction retention connectivity (DV). Bootstrap tests (10,000 iterations) were used for testing the significance of mediation effects. For the mediation analyses, we focused on FC patterns during early extinction learning. This choice was made for two reasons. First, prior fMRI and animal studies have shown distinct neural activations for the early and late extinction learning [6, 31, 32, 46, 47]; Second, we observed robust effects of estradiol on FC during early extinction learning for CS+ vs. CS− (Fig. 3A–C).
Secondary analyses
First, we examined neural activation and FC for the OC and NC subgroups separately to explore whether there is a difference between OC and NC in the estradiol-induced activations and FC. To ensure that the observed modulation differences were not due to sample size, we also conducted the same analyses on a subset of OC (i.e., all OC from one site), which had a comparable sample size to NC. Moreover, we used general linear models with interaction terms (contraceptive status [OC vs. NC] × estradiol concentrations) to assess how different patterns of estradiol might affect NC and OC. The second exploratory analyses were done to examine the potential impact of estradiol dose. We divided all participants into three groups (placebo, 2 mg estradiol, or 4 mg estradiol) and then compared the brain activations or FCs between these 3 groups (Supplementary Fig. S5).
Results
Estradiol and psychophysiological measures
The estradiol concentrations obtained across the 3 experimental days are presented in Supplementary Fig. S1. On day 1, as expected, there were no differences in serum estradiol levels (t(88) = 0.12, p = 0.90) between participants (those to receive estradiol on day 2 vs. those to receive placebo pill). On day 2, estradiol levels were significantly higher for women that ingested the estradiol pill compared to those that ingested the placebo pill (t(88) = 6.86, p < 0.001), confirming the effectiveness of estradiol administration in elevating serum estradiol levels. For the women ingesting the estradiol pill, the impact of dose (2 vs. 4 mg) on estradiol levels was not significant (t(51) = 1.85, p = 0.07), suggesting the high inter-individual variance in response to estradiol administration. On day 3, estradiol levels remained significantly higher in women that had ingested the estradiol pill (t(88) = 3.98, p < 0.001), suggesting that the impact of estradiol might have lingered onto the recall test.
With regards to psychophysiological measures, fear conditioning on day 1 (indexed by SCR) was achieved and did not differ between women to receive the estradiol on day 2 and those to receive the placebo pill. Time (early and late) × Group (placebo and estradiol administration) mixed model ANOVA revealed no significant results (ps > 0.3 for main and interaction effects) (Fig. 1B). Extinction learning was also achieved with no differences between women ingesting the estradiol vs. placebo pills. There was a significant effect of Time, indicating that SCRs significantly declined across fear extinction learning (F(3,174) = 8.48, p < 0.001), but main effects of Group (F(1,58) = 0.05, p = 0.82) and the Time × Group interaction were not significant (F(3,174) = 0.82, p = 0.31) (Fig. 1B). During extinction retention test, estradiol-treated women exhibited significantly less recovery of fear compared to placebo-treated women, indexed by SCR (CS + E vs. CS + U) (Fig. 1C, t(57) = 2.0, p = 0.049). We conducted exploratory analyses on the SCR data for the OC and NC groups, see Supplementary Material for details.
Impact of estradiol on task-induced activations
We examined the overall impact of estradiol administration on brain activation across participants (Fig. 2A). We found that individuals with higher estradiol levels exhibited increased activation in the cuneus gyrus (MNIxyz = [−10, −82, 26], t = 4.62, pcluster-FWE < 0.001) and vmPFC (MNIxyz = [−10, 42, 6], t = 4.39, pcluster-FWE = 0.001), during early and late extinction learning, respectively. During the extinction retention test, activation of the dorsal anterior cingulate cortex (dACC, MNIxyz = [2, 0, 40], t = 3.83, pcluster-FWE < 0.001) was positively correlated with estradiol levels. Using a less conservative threshold (voxel-level p < 0.005), we observed an impact of estradiol on hippocampal activation (Supplementary Fig. S2).
A Significant brain regions identified based on all participants. B Significant brain regions identified based on only oral contraceptives users. C Significant brain regions identified based on only naturally cycling women. All regions were identified at a voxel-level threshold of p < 0.001 and a family-wise error corrected cluster threshold of p < 0.05. vmPFC ventromedial prefrontal cortex, dACC dorsal anterior cingulate cortex, Ext. extinction, OC oral contraceptives users, NC naturally cycling women, n.s. not significant.
Results of exploratory analyses: OC and NC
The OC subgroup exhibited similar associations between estradiol levels and activations (Fig. 2B). However, NC exhibited different patterns of neural modulation (Fig. 2C). Specifically, in NC women we found a positive correlation between estradiol level and vmPFC activation (MNIxyz = [0, 36, 10], t = 4.16, p < 0.001, uncorrected) in early but not late extinction learning. The null result in late extinction learning is not likely due to reduced power, since we identified significant association between the vmPFC activations and estradiol levels in OC with a similar sample size (Supplementary Fig. S3). During extinction retention test, the vmPFC activation (MNIxyz = [−8, 48, 0], t = 5.44, pcluster-FWE < 0.001) of NC women was positively correlated with estradiol levels, which is consistent with our previous study [24]. To statistically compare the two subgroups, we examined the interaction effects of contraceptive status (OC vs. NC) and estradiol concentrations on brain activations (Supplementary Fig. S4). We observed significant interaction effects in the vmPFC activation during extinction retention test. For the extinction learning phase, we also found significant interactions in the vmPFC activations with a less conservative threshold. Note that the variance of estradiol concentrations on day 2 (which was used for correlation analyses) was similar for the OC and NC groups (Levene’s test, F(1, 88) = 2.8, p = 0.10). Additionally, there was no significant difference between the OC and NC groups regarding the mean estradiol concentrations on day 2 (t(88) = 1.26, p = 0.21). Therefore, the observed differences based on the correlation analyses (for activation analyses and connectivity analyses shown below) were not likely due to differences in measurements of estradiol between the OC and NC group.
Impact of estradiol on task-based FC
Next, we explored how estradiol modulates FC across experimental phases. In early extinction learning, the NBS analysis revealed an association of estradiol level and a network component (p < 0.05). The component contained 264 edges between 155 regions, mainly involving the DMN, SMN, and SUB (Fig. 3A, B). The mean connectivity of this component showed a significant negative correlation with estradiol levels (r = −0.63, p < 0.001). We did not observe any significant components during the late extinction learning phase with the CS+ vs. CS− contrast. However, we observed two significant components during late extinction CS+ processing, one negatively correlated with estradiol levels; the other showed a marginally positive association with estradiol levels (Supplementary Fig. S5). During extinction retention test (day 3), we identified another network component that showed a significant negative correlation with estradiol levels (r = −0.64, p < 0.001). The component contained 351 edges between 209 regions, mainly involving the DMN and SMN (Fig. 3D, E). There were no overlapping edges between this component and the significant component in early extinction learning, suggesting different patterns of modulation by estradiol across experimental phases.
A The identified network in early extinction learning. B The identified network mainly involved the default mode network, somatomotor network, and subcortical network. C The correlations between functional connectivity and estradiol level were significant for naturally cycling women (r = −0.53, p = 0.003) and oral contraceptives users (r = −0.69, p < 0.001). D The identified network in extinction retention test. E The identified network mainly involved the default mode network and somatomotor network. F The correlation between functional connectivity and estradiol level was significant for oral contraceptives users (r = −0.71, p < 0.001), but not for naturally cycling women (r = −0.31, p = 0.089). CON frontoparietal control network, DMN default mode network, DAN dorsal attention network, LIM limbic network, VAN ventral attention network, SMN somatomotor network, SUB subcortical network, VIS visual network.
Results of exploratory analyses: OC and NC
We examined the association of estradiol level and the mean connectivity of the identified network components, separately within the OC and NC subgroups. In early extinction learning (Fig. 3C), we observed similar patterns of negative correlation within the OC (r = −0.69, p < 0.001) and NC groups (r = −0.51, p = 0.003). However, during the extinction retention test (Fig. 3F), the OC and NC groups exhibited dissociated patterns. We observed a significant negative correlation in the OC (r = −0.71, p < 0.001) but not in the NC group (r = −0.31, p = 0.089). Bootstrap analysis indicated that the difference between the two correlation coefficients was significant (Δr = −0.40, 95% CI: [−0.70, −0.13], p < 0.005). This difference was not likely because of sample size, since we observed a similar pattern of a significantly robust negative correlation on a subset of OC (on site 1, r = −0.67, p < 0.001).
Impact of estradiol on resting-state FC
We examined whether estradiol modulated rsFC after extinction learning and before extinction memory retrieval. We first examined the post-extinction rsFC, where memory consolidation is likely to occur. When including all participants, we identified a network component with 464 edges between 281 regions (r = 0.76, p < 0.001), mainly involving the DMN, SMN, and VIS (Fig. 4A, B). We examined the relationship between estradiol and rsFC on day 3, before the extinction retention test, but did not find significant network components.
A The identified network in post-extinction learning resting state. B The identified network mainly involved the default mode network, somatomotor network, and visual network. C The correlations between functional connectivity and estradiol level were significant for oral contraceptives users (r = 0.84, p < 0.001) and naturally cycling women (r = 0.44, p = 0.015). The difference of the two correlation coefficients was significant (Δr = 0.40, p < 0.005).
Results of exploratory analyses: OC and NC
For the significant component identified in post-extinction learning rsFC (Fig. 4C), we observed a stronger positive correlation with estradiol in the OC (r = 0.84, p < 0.001) compared to the NC group (r = 0.44, p = 0.015). Bootstrap analysis confirmed the significant difference between the two correlation coefficients (Δr = 0.40, 95% CI: [−0.75, −0.11], p < 0.005). On a subset of OC participants, we observed similar pattern of positive correlation (r = 0.86, p < 0.001), suggesting the difference between OC and NC is not likely due to sample size. These results suggest a different impact of estradiol on post-extinction learning rsFC for OC and NC groups.
Mediation analysis
The analyses conducted above revealed a significant impact of estradiol on task-based FC (tbFC) and rsFC. Is there a link or an association between changes in FC during the task and changes in functional activity after learning and before recall? To answer this question, we conducted mediation analyses to test two models—a learning model and a memory model. Within the learning model, we explored the mediator effect of early extinction learning tbFC on the role of estradiol administration on post-extinction learning rsFC. In the memory model, we explored the mediator effect of both early extinction learning rsFC (mediator 1) and post-extinction learning rsFC (mediator 2) on the influence of estradiol on extinction memory retention tbFC. For the learning model (Fig. 5A), we found that the connectivity in early extinction learning partially mediated the effects of estradiol on the post-extinction rsFC (β = −0.02, p = 0.016). For the memory model (Fig. 5B), the post-extinction rsFC (β = −0.19, p = 0.007), but not the tbFC during extinction learning (β = −0.05, p = 0.24), partially mediated the effects of estradiol on the connectivity during follow-up extinction retention test. These results suggest an interaction between estradiol-induced changes during extinction learning, post-extinction learning memory-consolidation phase, and the memory retrieval phase.
A The network component in early extinction learning significantly mediated the impact of estradiol administration on post-extinction learning resting-state functional connectivity. B The parallel mediation model revealed that only the post-extinction learning resting-state functional connectivity, but not the network in early extinction learning, significantly mediated the impact of estradiol administration on the functional connectivity during extinction retention test. Path coefficients are listed for each path with bootstrapped 95% confidence intervals in parentheses. Pbo placebo, E2 estradiol; *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
We examined the impact of acute exogenous estradiol administration on task-based and resting-state activations and connectivity during and after fear extinction learning. We found that estradiol administration significantly affected brain activations and FC across different phases of the experiment. Our data also showed that the impact of estradiol during and post fear extinction learning is far more diffused across multiple brain regions and networks. Our exploratory analyses revealed that neural modulation patterns differed between NC women and OC users.
We observed effects of exogenous estradiol administration on extinction learning at the neurobiological level (Fig. 2), mainly on the vmPFC. Studies in rodents [57] and humans [31, 58, 59] have shown the involvement of the vmPFC in mediating extinction. Women with higher endogenous estradiol showed increased vmPFC activation during extinction learning [24]. The results herein replicate and extend previous studies by showing the impact of exogenous estradiol on vmPFC activation during extinction learning. During the memory retention test, we found an impact of estradiol on the dACC, which has been broadly implicated in cognitive and emotional processes [60,61,62], including fear extinction and its memory recall [34, 61]. Overall, these results suggest that exogenous estradiol modulates activations of regions involved in fear extinction learning and memory consolidation.
Beyond the vmPFC and dACC, estradiol administration modulated large-scale FC across experimental phases (Fig. 3 and 4). Resting-state studies have shown that endogenous estradiol modulates FC with the amygdala [63] and connectivity across distributed brain regions [64, 65]. Our results provide evidence that exogenous estradiol not only modulates rsFC, but also connectivity during extinction learning and its memory retention test. Rodent [38,39,40,41] and human studies [42,43,44] have consistently shown that the time window after extinction learning is critical for memory consolidation. Administration of the estrogen-receptor beta agonist within this time window facilitated extinction recall [24]. Therefore, the effects of estradiol on resting-state connectivity during this period may explain estradiol’s influence on improving extinction memory consolidation, a hypothesis that is supported by the results of our mediation (Fig. 5B).
The estradiol-modulated connections were distributed across functionally distinct systems including the DMN and SMN. Many of the ‘resident’ brain regions within the DMN, like the prefrontal cortex, are areas containing high levels of estrogen receptors [66, 67]. FC of DMN and SMN is modulated by endogenous estradiol across the menstrual cycle [64, 65, 68]. The DMN is implicated in conscious awareness, affective learning, and memory consolidation [69,70,71]. Dysfunction of the DMN is associated with impaired fear extinction in PTSD [72]. The SMN is not only associated with motor function, but also involved in the pathophysiology of psychiatric disorders [73], including PTSD [74,75,76] and anxiety disorders [77]. Activations in the SMN are correlated with flashbacks in PTSD [78], suggesting a possible function in preparing for reacting to a potential threat [74]. Consistent with recent study showing distributed brain network modulations of endogenous estradiol [65], we also observed effects of estradiol on other networks, such as the VIS and SUB. These findings align with view that emotional learning and regulation should engage systems involved in attention control, conscious awareness, and sensory motor functioning [36]. Together, these results suggest that estradiol administration improves extinction memory retention via modulating neural systems implicated in attention, motor and sensory systems, and memory encoding and retrieval.
The exploratory analyses we conducted in the current study revealed different patterns of activation and connectivity across experimental phases for OC users and NC women. In a previous psychophysiological study [28], we observed impaired extinction retention in OC users compared to NC women in a high-estrogen state. OC users and NC women that underwent extinction in a low-estrogen state showed comparable extinction retention. Hwang et al. also showed comparable brain activations within the neural circuits underlying fear extinction in OC women compared to NC women in low-estrogen state [79]. In the current study, the NC women underwent fear extinction in a low-estrogen state, like those OC users. Based on these results and our prior studies, we speculate that the differential impact of exogenous estrogen administration on the two subgroups (OC and NC) observed here is likely due to the chronic use of OC and its impact on the brain. This speculative interpretation is consistent with studies showing that the use of OC changes brain structures and FC underlying affective and cognitive processing [80, 81], both during rest [68, 82] and during tasks [79, 83]. There is also evidence that the use of OC is associated with mental health outcomes [81, 84, 85], though these findings have not been consistently reported [86]. Moreover, estrogens are locally synthesized within numerous brain regions including prefrontal cortical areas and the hippocampus [87,88,89]. There could be a potential interaction between locally produced estrogens within the brain and those exogenously administered. Yet, it is unclear how long-lasting this interaction would persist and the potential impact of such an interaction given our very acute manipulation setting within the current experimental design. It is important to note that our preliminary results require replication and more refined experimental parameters. Future studies focusing on examining interactions between local estrogen production and exogenous administration of estradiol (acute and/or chronic) could contribute towards enhancing our understanding of the functionality and expression of estrogen-receptor expression. Prospective studies are needed with emphasis on (1) larger sample sizes in each group, (2) the interrogation of various types of OCs, and (3) the duration of the utilization of OCs on neural correlates of fear extinction. Such studies could provide mechanistic advances to understanding the potential differences in neural responses between the NC and OC women.
The present study has limitations that should be considered. First, the data of NC and OC were collected in different sites. However, it is unlikely that the observed effects were caused by site or sample-size differences, because similar results were observed with an equalized sample, and for our extinction learning FC analysis, we have found similar patterns for the NC and OC groups. Second, we did not collect subjective ratings of fear from participants. It would be important for future studies to examine the link between the neural signature changes, such as observed in this study, and the subjective reports of participants. Third, we only recruited women in this study, thus the impact of estradiol on men should be examined in future studies.
In summary, our findings are consistent with prior studies showing that estradiol administration facilitates extinction memory retention and modulates brain activation and network connectivity during and after extinction learning. There is growing evidence supporting the important role of estradiol on anxiety and fear-related disorders [13, 25,26,27]. Some studies have shown the impact of endogenous estradiol on treatment efficacy [90, 91]. The results here showed that a single dose of estradiol could engage numerous key nodes within and beyond the fear extinction network while subjects underwent fear extinction. The data we report raise an intriguing and exciting possibility about the use of estradiol as a possible adjunct to exposure therapies for PTSD and, perhaps, other anxiety and mood disorders [5, 92]. Future studies are needed to explore if estrogen levels and natural cycling status in women at time of trauma exposure might be linked to the etiology of PTSD and other psychiatric disorders. In addition, our results may also be relevant to major depressive disorder (MDD). For example, there is evidence showing that patients diagnosed with MDD exhibit abnormal patterns during fear conditioning and extinction [93, 94], and that there is association between estradiol levels and depression symptoms [85]. Further studies should examine this prospect and the relevance of these findings to MDD as well as other psychiatric disorders.
References
Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the world health organization collaborative study on psychological problems in general health care. Arch Gen Psychiatr. 1998;55:405–13.
McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–35.
Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, et al. Anxiety disorders [no. 1]. Nat Rev Dis Prim. 2017;3:1–19.
Fenster RJ, Lebois LAM, Ressler KJ, Suh J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man [no. 9]. Nat Rev Neurosci. 2018;19:535–51.
Hammoud MZ, Foa EB, Milad MR. Oestradiol, threat conditioning and extinction, post-traumatic stress disorder, and prolonged exposure therapy: a common link. J Neuroendocrinol. 2020;32:e12800.
Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–51.
Ressler KJ. Translating across circuits and genetics toward progress in fear- and anxiety-related disorders. AJP. 2020;177:214–22.
Greco JA, Liberzon I. Neuroimaging of fear-associated learning [no. 1]. Neuropsychopharmacology. 2016;41:320–34.
Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, et al. Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci Biobehav Rev. 2017;77:247–85.
Lonsdorf TB, Haaker J, Schümann D, Sommer T, Bayer J, Brassen S, et al. Sex differences in conditioned stimulus discrimination during context-dependent fear learning and its retrieval in humans: the role of biological sex, contraceptives and menstrual cycle phases. J Psychiatr Neurosci. 2015;40:368–75.
Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–8.
Velasco ER, Florido A, Milad MR, Andero R. Sex differences in fear extinction. Neurosci Biobehav Rev. 2019;103:81–108.
Nillni YI, Rasmusson AM, Paul EL, Pineles SL. The impact of the menstrual cycle and underlying hormones in anxiety and PTSD: what do we know and where do we go from here? Curr Psychiatr Rep. 2021;23:8.
Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34.
Markus EJ, Zecevic M. Sex differences and estrous cycle changes in hippocampus-dependent fear conditioning. Psychobiology. 1997;25:246–52.
Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–66.
Cahill L. Why sex matters for neuroscience [no. 6]. Nat Rev Neurosci. 2006;7:477–84.
Merz CJ, Kinner VL, Wolf OT. Let’s talk about sex … differences in human fear conditioning. Curr Opin Behav Sci. 2018;23:7–12.
Andreano JM, Touroutoglou A, Dickerson B, Barrett LF. Hormonal cycles, brain network connectivity, and windows of vulnerability to affective disorder. Trends Neurosci. 2018;41:660–76.
Cover KK, Maeng LY, Lebrón-Milad K, Milad MR. Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology [no. 8]. Transl Psychiatr. 2014;4:e422–e422.
Li SH, Graham BM. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatr. 2017;4:73–82.
Pineles SL, Arditte Hall KA, Rasmusson AM. Gender and PTSD: different pathways to a similar phenotype. Curr Opin Psychol. 2017;14:44–48.
Taxier LR, Gross KS, Frick KM. Oestradiol as a neuromodulator of learning and memory [no. 10]. Nat Rev Neurosci. 2020;21:535–50.
Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatr. 2011;70:920–7.
Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatr. 2012;72:19–24.
Pineles SL, Nillni YI, King MW, Patton SC, Bauer MR, Mostoufi SM, et al. Extinction retention and the menstrual cycle: different associations for women with posttraumatic stress disorder. J Abnorm Psychol. 2016;125:349–55.
Sartin-Tarm A, Ross MC, Privatsky AA, Cisler JM. Estradiol modulates neural and behavioral arousal in women with posttraumatic stress disorder during a fear learning and extinction task. Biol. Psychiatr Cogn Neurosci Neuroimaging. 2020;5:1114–22.
Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatr. 2013;73:371–8.
Marin M-F, Hammoud MZ, Klumpp H, Simon NM, Milad MR. Multimodal categorical and dimensional approaches to understanding threat conditioning and its extinction in individuals with anxiety disorders. JAMA Psychiatr. 2020;77:618–27.
Marin M-F, Zsido RG, Song H, Lasko NB, Killgore WDS, Rauch SL, et al. Skin conductance responses and neural activations during fear conditioning and extinction recall across anxiety disorders. JAMA Psychiatr. 2017;74:622.
Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatr. 2007;62:446–54.
Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatr. 2009;66:1075–82.
Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatr. 2007;164:1476–88.
Fullana MA, Albajes-Eizagirre A, Soriano-Mas C, Vervliet B, Cardoner N, Benet O, et al. Fear extinction in the human brain: a meta-analysis of fMRI studies in healthy participants. Neurosci Biobehav Rev. 2018;88:16–25.
Fullana MA, Albajes-Eizagirre A, Soriano-Mas C, Vervliet B, Cardoner N, Benet O, et al. Fear extinction in the human brain: a meta-analysis of fMRI studies in healthy participants. Neurosci Biobehav Rev. 2018;88:16–25.
LeDoux JE, Pine DS. Using neuroscience to help understand fear and anxiety: a two-system framework. AJP. 2016;173:1083–93.
Shalev A, Liberzon I, Marmar C. Post-traumatic stress disorder. N. Engl J Med. 2017;376:2459–69.
Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–80.
Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14:147–53.
Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval [no. 1]. Neuropsychopharmacology. 2008;33:56–72.
Wu C-T, Haggerty D, Kemere C, Ji D. Hippocampal awake replay in fear memory retrieval [no. 4]. Nat Neurosci. 2017;20:571–80.
de Voogd LD, Fernández G, Hermans EJ. Awake reactivation of emotional memory traces through hippocampal–neocortical interactions. NeuroImage. 2016;134:563–72.
Gerlicher AMV, Tüscher O, Kalisch R. Dopamine-dependent prefrontal reactivations explain long-term benefit of fear extinction [no. 1]. Nat Commun. 2018;9:1–9.
Hermans EJ, Kanen JW, Tambini A, Fernández G, Davachi L, Phelps EA. Persistence of amygdala–hippocampal connectivity and multi-voxel correlation structures during awake rest after fear learning predicts long-term expression of fear. Cereb Cortex. 2017;27:3028–41.
Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–64.
Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–82.
Quirk GJ, Repa JC, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–39.
McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage. 2012;61:1277–86.
Tompson SH, Kahn AE, Falk EB, Vettel JM, Bassett DS. Functional brain network architecture supporting the learning of social networks in humans. NeuroImage. 2020;210:116498.
Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018;28:3095–114.
Tian Y, Margulies DS, Breakspear M, Zalesky A. Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat. Neurosci. 2020;23:1421–32.
Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Yu M, Linn KA, Cook PA, Phillips ML, McInnis M, Fava M, et al. Statistical harmonization corrects site effects in functional connectivity measurements from multi-site fMRI data. Hum Brain Mapp. 2018;39:4213–27.
Fortin J-P, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. NeuroImage. 2017;161:149–70.
Zalesky A, Fornito A, Bullmore ET. Network-based statistic: Identifying differences in brain networks. NeuroImage. 2010;53:1197–207.
MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2006;58:593–614.
Do-Monte FH, Manzano-Nieves G, Quiñones-Laracuente K, Ramos-Medina L, Quirk GJ. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J Neurosci. 2015;35:3607–15.
Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value [no. 10]. Nat Neurosci. 2004;7:1144–52.
Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905.
Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93.
Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. AJP. 2011;168:1255–65.
Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex [no. 3]. Nat Rev Neurosci. 2011;12:154–67.
Engman J, Linnman C, Van Dijk KRA, Milad MR. Amygdala subnuclei resting-state functional connectivity sex and estrogen differences. Psychoneuroendocrinology. 2016;63:34–42.
Arélin K, Mueller K, Barth C, Rekkas PV, Kratzsch J, Burmann I, et al. Progesterone mediates brain functional connectivity changes during the menstrual cycle—a pilot resting state MRI study. Front Neurosci. 2015;9:44.
Pritschet L, Santander T, Taylor CM, Layher E, Yu S, Miller MB, et al. Functional reorganization of brain networks across the human menstrual cycle. NeuroImage. 2020;220:117091.
Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Horn JDV, Esposito G, et al. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. PNAS. 1997;94:8836–41.
Wang ACJ, Hara Y, Janssen WGM, Rapp PR, Morrison JH. Synaptic estrogen receptor-α levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 2010;30:12770–6.
Petersen N, Kilpatrick LA, Goharzad A, Cahill L. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. NeuroImage. 2014;90:24–32.
Higgins C, Liu Y, Vidaurre D, Kurth-Nelson Z, Dolan R, Behrens T, et al. Replay bursts in humans coincide with activation of the default mode and parietal alpha networks. Neuron. 2021;109:882–93.
Kaplan R, Adhikari MH, Hindriks R, Mantini D, Murayama Y, Logothetis NK, et al. Hippocampal sharp-wave Ripples Influence Selective Activation of the Default Mode Network. Curr Biol. 2016;26:686–91.
Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–47.
Miller DR, Hayes SM, Hayes JP, Spielberg JM, Lafleche G, Verfaellie M. Default mode network subsystems are differentially disrupted in posttraumatic stress disorder. Biol Psychiatr Cogn Neurosci Neuroimag. 2017;2:363–71.
Northoff G, Hirjak D, Wolf RC, Magioncalda P, Martino M. All roads lead to the motor cortex: psychomotor mechanisms and their biochemical modulation in psychiatric disorders. Mol Psychiatr. 2021;26:92–102.
Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. AJP. 1999;156:1787–95.
Shang J, Lui S, Meng Y, Zhu H, Qiu C, Gong Q, et al. Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: a resting-state fMRI study. PLOS ONE. 2014;9:e96834.
Zhu H, Yuan M, Qiu C, Ren Z, Li Y, Wang J, et al. Multivariate classification of earthquake survivors with post-traumatic stress disorder based on large-scale brain networks. Acta Psychiatr Scandinavica. 2020;141:285–98.
Northoff G. Anxiety Disorders and the Brain’s Resting State Networks: From Altered Spatiotemporal Synchronization to Psychopathological Symptoms. Anxiety Disorders. 2020;71–90.
Whalley MG, Kroes MCW, Huntley Z, Rugg MD, Davis SW, Brewin CR. An fMRI investigation of posttraumatic flashbacks. Brain Cognition. 2013;81:151–9.
Hwang MJ, Zsido RG, Song H, Pace-Schott EF, Miller KK, Lebron-Milad K, et al. Contribution of estradiol levels and hormonal contraceptives to sex differences within the fear network during fear conditioning and extinction. BMC Psychiatr. 2015;15:295.
Brønnick MK, Økland I, Graugaard C, Brønnick KK. The effects of hormonal contraceptives on the brain: a systematic review of neuroimaging studies. Front Psychol. 2020;11.
Montoya ER, Bos PA. How oral contraceptives impact social-emotional behavior and brain function. Trends Cogn Sci. 2017;21:125–36.
Lisofsky N, Riediger M, Gallinat J, Lindenberger U, Kühn S. Hormonal contraceptive use is associated with neural and affective changes in healthy young women. NeuroImage. 2016;134:597–606.
Miedl SF, Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH. Neural activity during traumatic film viewing is linked to endogenous estradiol and hormonal contraception. Psychoneuroendocrinology. 2018;87:20–26.
Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347–55.
Skovlund CW, Mørch LS, Kessing LV, Lidegaard Ø. Association of hormonal contraception with depression. JAMA Psychiatr. 2016;73:1154–62.
Robakis T, Williams KE, Nutkiewicz L, Rasgon NL. Hormonal contraceptives and mood: review of the literature and implications for future research. Curr Psychiatr Rep. 2019;21:57.
Rossetti MF, Cambiasso MJ, Holschbach MA, Cabrera R. Oestrogens and progestagens: synthesis and action in the brain. J Neuroendocrinol. 2016;28.
Fester L, Prange-Kiel J, Jarry H, Rune GM. Estrogen synthesis in the hippocampus. Cell Tissue Res. 2011;345:285.
Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45:S116–S124.
Graham BM, Li SH, Black MJ, Öst L-G. The association between estradiol levels, hormonal contraceptive use, and responsiveness to one-session-treatment for spider phobia in women. Psychoneuroendocrinology. 2018;90:134–40.
Graham BM, Ash C, Den ML. High endogenous estradiol is associated with enhanced cognitive emotion regulation of physiological conditioned fear responses in women. Psychoneuroendocrinology. 2017;80:7–14.
Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Hormones Behav. 2015;76:106–17.
Nissen C, Holz J, Blechert J, Feige B, Riemann D, Voderholzer U, et al. Learning as a model for neural plasticity in major depression. Biol Psychiatry. 2010;68:544–52.
Kuhn M, Höger N, Feige B, Blechert J, Normann C, Nissen C. Fear extinction as a model for synaptic plasticity in major depressive disorder. PLOS ONE. 2014;9:e115280.
Funding
This work was funded by National Institute of Mental Health grants R61-MH126090-01 (MRM), R01-MH097880-01 (MRM).
Author information
Authors and Affiliations
Contributions
Conceptualization: EBF, MRM; Funding acquisition: EBF, MRM; Methodology: MRM; Validation: JCS, RCG, MRM; Project administration: MZH, JCS, LB, MFM, AA, EBF, MRM; Supervision: LB, MFM, AA, RCG, EBF; Investigation: MZH, JCS, JJ, MFM; Data curation: ZW, MZH, JJ; Statistical analyses: ZW; Software: ZW; Visualization: ZW; Writing-original draft: ZW, MRM; Writing-review and editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wen, Z., Hammoud, M.Z., Scott, J.C. et al. Impact of exogenous estradiol on task-based and resting-state neural signature during and after fear extinction in healthy women. Neuropsychopharmacol. 46, 2278–2287 (2021). https://doi.org/10.1038/s41386-021-01158-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01158-4
This article is cited by
-
Effects of exogenous oxytocin and estradiol on resting-state functional connectivity in women and men
Scientific Reports (2023)