Abstract
Depression is a chronic and debilitating condition that often emerges during adolescence, a period of significant brain maturation. Few studies, however, have examined how mechanisms of neuroplasticity, including myelination, are affected by adolescent-onset depression. Here, we used multimodal MR imaging to characterize myelin, indexed by R1, in white matter tracts previously associated with depression and compare 48 adolescents with lifetime depression (45 with current depression, 3 remitted) and 35 healthy controls in R1. Compared to healthy controls, R1 was higher in adolescents with lifetime depression in the uncinate fasciculus and corpus callosum genu (all βs > 0.42; all ps < 0.037). Sex significantly moderated the association between depression and R1 in the left uncinate fasciculus and corpus callosum genu (all βs > 0.86; all ps < 0.02), such that depressed female adolescents had significantly higher R1 in these tracts than did healthy female adolescents (all βs > 0.82; all ps < 0.0012). In contrast, depressed and non-depressed male adolescents did not differ in R1 in these tracts (all ps > 0.32). While fractional anisotropy (FA), a commonly examined measure of white matter organization based on diffusion-weighted MRI, in the left uncinate was positively associated with lifetime depression in our sample (β = 0.56; p = 0.016), we found no evidence of sex-specific effects of depression in FA. Our results suggest that R1 is more sensitive to sex-specific effects of depression than FA, particularly in female adolescents. Given evidence that myelin inhibits synapse formation and reduces brain plasticity, our findings implicate experience-driven regional myelination as a mechanism underlying depression during periods of significant neural maturation such as adolescence.
Similar content being viewed by others
Introduction
Depression is a chronic and debilitating condition that often emerges during adolescence [1]. Adolescent-onset depression increases the likelihood of experiencing depression, as well as other mental and physical health conditions, in adulthood [2, 3]. Given that adolescence is a period of significant brain maturation, understanding how dynamic processes related to the development of brain structure, such as myelination, are affected by adolescent-onset depression may provide important insights on the etiology of depression and on the optimization of treatments based on age or developmental stage. Optimizing adolescent-specific treatments for depression especially may provide maximal gains by preventing recurrent depressive episodes and other adverse health outcomes associated with this disorder later in life.
In this context, emerging evidence suggests that experience-dependent plasticity is not only a property of neurons, but also of oligodendrocytes, which are the primary myelin-forming cells in the brain [4,5,6]. To date, however, research conducted to elucidate the effects of adolescent depression on myelination has focused exclusively on metrics of white matter microstructure derived from diffusion MRI. While these metrics are a proxy for the degree of myelination, they are also sensitive to other related tissue properties, including axonal packing and fiber density [7]. Although these studies have demonstrated that depressed individuals have lower fractional anisotropy (FA) in frontolimbic and callosal tracts than do healthy controls ([8, 9], although see [10] for evidence of higher FA in medication-naïve depressed adolescents), the extent to which these depression-related effects of FA reflect abnormalities in myelination is unclear.
R1, which is the inverse of the T1 signal and quantifies the longitudinal relaxation rate of water hydrogen protons following excitation in a magnetic field, is a value that is tissue dependent and, therefore, can be used to infer tissue types such as myelin (which contains relatively high proportions of both lipids and water; see Fig. S1). As a quantitative MRI parameter, R1 is thus a promising alternative to diffusion MRI for non-invasively estimating myelin in MRI. Importantly, R1 has the additional benefit of being robust to variations in scan parameters and scanner hardware specifications [11], highlighting the potential for widespread clinical use of R1. R1 has been shown to be especially sensitive to myelin, with correlations between R1 and histological measures of myelin content in white matter range from 0.70 to 0.89 [12,13,14,15]. Because ~90% of the R1 signal in white matter is attributed to myelin (with the remaining 10% attributed to iron), investigators have begun using R1 as a robust in vivo assay for myelin in the brain [16].
No studies to date have examined R1 in adolescents with depression. One study with adults ages 19–58 years found that, compared to healthy controls, individuals with MDD had lower global values of R1 as well as lower values in the nucleus accumbens [17]. Other less sensitive MR-based measurements of myelin have also been found to be lower in the prefrontal cortex, cingulate cortex, occipital cortex, insula, and thalamus in adults older than 60 years with geriatric depression [18, 19]. All of these studies, however, mapped voxel-wise measures of myelin across the entire brain rather than in specific white matter tracts, in which these values are more likely to reflect myelin than they are iron (or other macromolecules). Moreover, myelin, assessed with R1, has been shown to increase steadily between the ages of 7 and 40 years, reaching a peak at 30–50 years before declining [20]. Because of these strong age-related changes in myelin, assessing the potentially enduring effects of depression on myelin is especially critical during significant periods of development, such as adolescence.
To address these gaps, we conducted the first multimodal neuroimaging study to assess myelin in adolescents with lifetime depression. We acquired high-resolution anatomical, diffusion, and quantitative MRIs using a 3 Tesla scanner in 83 adolescents ages 13–18 years: 48 adolescents with lifetime depression (45 adolescents with current MDD, 3 adolescents with past MDD) and 35 healthy controls with no history of any psychiatric disorders. In all participants we performed deterministic tractography to trace individual-level white matter fibers in tracts that have been previously found to show robust effects of depression in adolescents and adults [8,9,10]: the corpus callosum genu and splenium, cingulate cingulum, and uncinate fasciculus. We hypothesized that the two groups of adolescents would differ in R1 and FA in these tracts, and that R1 would be predicted more strongly by clinical status and depression symptom severity than would FA. Finally, given evidence of sex differences in rates of depression during adolescence [1], as well as evidence from animal model research for sex differences in the regulation of myelination [21] and development of white matter [22], we examined whether sex moderated associations between depression and R1 as well as FA.
Methods
Participants and study design
Data from the present investigation were collected through two ongoing longitudinal neuroimaging studies at Stanford University: the Teen Inflammation Glutamate Emotion Research (TIGER) study [23] (NIH grant: K01MH117442) and the Early Life Stress (ELS) study (NIH grant: R37MH101495). Participants recruited for TIGER were either depressed adolescents who met criteria for clinical depression or were healthy controls with no psychiatric history [20] (see Supplement for more details). Participants recruited for ELS were enrolled into the study primarily based on age and pubertal development (based on Tanner staging) and not on the basis of psychiatric history (see Supplement for more details). To be included in the present study, participants were classified as having MDD (past or present) or CTL (no history of any psychiatric disorder (see Clinical assessments, below). Additional exclusion criteria were the same for both studies: presence of any medical, cognitive, or physical challenges that would limit adolescents’ ability to understand or complete study procedures, contraindications for MRI (e.g., braces, claustrophobia), and symptoms of concussion within the past 6 weeks or any loss of consciousness due to a concussion at any point in time. In accordance with the Declaration of Helsinki, all participants provided informed assent and their parent/legal guardian provided informed consent. All participants were recruited from the San Francisco Bay Area through media and online advertisements and were compensated for study participation. Both studies were approved by the Stanford University Institutional Review Board.
Of the 96 participants who met eligibility criteria and underwent MRI scanning, seven were excluded due to excessive motion during the diffusion MRI scan, four were excluded due to excessive motion during the quantitative MRI scans, one was excluded due to a coverage error during acquisition, and one was excluded to a brain anomaly observed in their anatomical scan, resulting in a total of 83 participants. Here we present analyses from 48 adolescents with lifetime MDD and 35 healthy controls, where 89% of adolescents with lifetime MDD and 54% of CTL were from the TIGER study (TIGER: 43 MDD, 19 CTL; ELS: 5 MDD, 16 CTL).
MRI acquisition
All MRI data were acquired using a 3T Discovery 750 MRI system (General Electric Healthcare, Milwaukee, WI, USA) with a 32-channel head coil (Nova Medical, Wilmington, MA, USA) housed at the Stanford University Center for Cognitive and Neurobiological Imaging. Both TIGER and ELS acquired a high-resolution T1-weighted anatomical scan using an SPGR sequence (TIGER: TR/TE/TI = 8.2/3.2/600 ms; flip angle = 12°; 156 axial slices; 1.0 mm isotropic voxels; ELS: TR/TE/TI = 6.24/2.34/450 ms; flip angle = 12°; 186 sagittal slices; 0.9 mm isotropic voxels). Participants from both studies completed the same diffusion-weighted MRI scan using an EPI sequence (TR/TE = 8500/93.5 ms; 64 axial slices; 2 mm isotropic voxels; 60 b = 2000 diffusion-weighted directions, and 6 b = 0 acquisitions at the beginning of the scan; anterior/posterior phase encoding direction). Participants from both studies completed the same quantitative MRI scan, a novel simultaneous multi-slice accelerated, inversion-recovery spin-echo echo planar imaging sequence that has been shown to produce T1 maps comparable to the gold-standard inversion-recovery pulse sequence (TR/TE = 3000/50 ms; 25 TIs were acquired with the shortest TI = 50 ms and an interval of 120 ms; flip angle = 77°; 25 axial slices; FOV = 23.2 cm; matrix = 80 mm × 80 mm; 2.9 mm isotropic voxels). Specifically, two slice-shuffled inversion-recovery simultaneous multi-slice EPI sequences with interleaved acquisition and in-plane acceleration were collected, with opposite phase encoding in order to correct for signal distortion [24]. More details can be found in our previous publication [25].
Diffusion MRI preprocessing and tractography
Diffusion MRI data were processed using the open source mrVista software distribution developed by the VISTA lab (https://vistalab.stanford.edu/) as outlined in previous work [26, 27]. Based on a recent harmonized meta-analysis of diffusivity-based white matter indicators of depression [9] and on prior investigations in adolescents with depression [8, 10], we focused our analyses on the following tracts of interest: corpus callosum genu and splenium, cingulum cingulate, and uncinate fasciculus. Diffusivity metrics including FA were estimated for 100 evenly spaced nodes along each respective tract and averaged across the entire tract for a more reliable estimate [27, 28]. See Supplement for more details on diffusion MRI preprocessing and tractography.
Quantitative MRI model fitting and quality assurance
Quantitative MRI data were processed using an in-house Python script (see Code availability, below) to unshuffle slices in both scans, skullstrip the data at a fractional threshold of 0.5, correct for distortions, and fit a non-linear least squares model to estimate T1 per voxel. The resulting T1 maps were then registered to a skull-stripped SPGR scan aligned with the anterior and posterior commissures using FSL’s FLIRT [24]. In order to examine R1 within individual-specific white matter tracts of interest, we converted person-specific fiber tracts into binary masks using VISTA lab tools (https://vistalab.stanford.edu/). We then performed rigorous quality inspection, including visually checking each quantitative MRI scan and associated white matter tract masks for motion artifacts, registration accuracy, and anatomical accuracy for the location of each segmented white matter tract. Finally, we extracted mean T1 within each tract mask per participant and computed R1 (1/T1). See Fig. 1 for a visualization of the tracts of interest derived from the diffusion MRI scan overlaid on the quantitative MRI data from a representative subject. For each tract, we also computed the proportion of voxels that were outside of the boundaries of the quantitative MRI map and conservatively included these values as additional covariates in our statistical models involving R1 (see Fig. S2 for example tracts).
Clinical assessments
To determine MDD diagnosis based on DSM-IV criteria, all participants were interviewed using the Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime (K-SADS-PL; [29]). Every diagnostic code was reviewed by a subset of the authors to ensure consistency across all interviewers and to maximize reliability. Participants were classified as having MDD (past or present) or CTL (no history of any psychiatric disorder). See Supplement for more details.
Depression severity
To assess self-reported severity of current depression (past 2 weeks), all adolescents completed the Reynolds Adolescent Depression Scale (RADS-2), a 30-item scale that assesses symptoms of dysphoric mood, anhedonia/negative affect, negative self-evaluation, and somatic complaints [30]. The RADS-2 was developed and validated in adolescents ages 11–20 years and has been shown to have high internal consistency [30]. Here, the RADS-2 had excellent internal consistency across both study samples (TIGER: Cronbach’s α = 0.96; ELS: Cronbach’s α = 0.94; full sample: Cronbach’s α = 0.96).
Statistical approach
All statistical analyses and visualization plots were conducted using R version 3.6.1 (R Core Development Team), including functions from the following libraries: arsenal, easystats, heplots, lm, psych, tidyverse. Chi-square tests were conducted to test whether the distribution of individuals who identified as each sex (and gender) and race differed between the diagnostic groups. Multiple linear regression was conducted to examine effects of depression on R1 and FA.
Primary analyses
We examined whether diagnostic groups (MDD, CTL) differed in R1 (and FA, separately) across the entire sample, covarying for age at scan, biological sex, and tract length. For analyses involving R1, we also conservatively included the proportion of voxels from the tract fiber masks that extended beyond the quantitative MRI maps (see Fig. S2). We then examined whether the interaction effect of sex and diagnostic group was associated with R1 (and FA, separately). We also examined whether there was a main effect of RADS-2 scores, as well as an interaction effect with sex, on R1 (and FA, separately) using the same covariates as in our primary analyses.
Sensitivity analyses
We reran all models that yielded statistically significant effects with study group, psychotropic medication status, and Tanner stage as covariates to determine whether these variables influenced our findings. We also reran all models that yielded statistically significant effects when using depression diagnosis as a predictor by excluding the three participants with past MDD. Finally, we conducted logistic regression models “predicting” lifetime MDD and RADS-2 scores, respectively, from R1 and FA for a given tract to determine if R1 was significantly associated with depression diagnosis above and beyond the effect of FA.
Supplemental analyses
In post-hoc supplemental analyses, we examined whether age of initial depression onset and duration of current episode (in months) was related to R1 in any of our tracts of interest in the group with lifetime MDD. We also explored whether including age of menarche as a covariate impacted any of our results where sex was a significant moderator of MDD diagnosis or RADS-2 scores on R1 and where the effects of MDD diagnosis and RADS-2 scores were significant in female adolescents. We also reran all primary statistical models with axial diffusivity (AD), mean diffusivity (MD), and radial diffusivity (RD) as outcome variables. For consistency, all of these analyses used the same covariates as those in our primary statistical models. Finally, within the ELS sample only, we reran all significant models from our primary analyses covarying for a continuous measure of ELS severity and age at baseline (when the stress interview was conducted).
Results
Descriptive statistics
A summary of the participant demographic and white matter characteristics is presented in Table 1 with additional clinical information on the lifetime MDD sample in Table S1. Participants were between the ages of 13 and 18 years and predominately female at birth (n = 53, 64%); two participants (both in the lifetime MDD group) also reported identifying as non-binary. The MDD and CTL groups did not differ significantly in demographic characteristics, with the exception of age (the MDD group was older than the CTL group by a mean of 0.573 years) and Tanner stage (the MDD group was on average between 4.5 and 5 and the CTL group was on average between 4 and 4.5). Importantly, participants did not differ in global R1, tract length, or motion during the scan (Table S1). A correlation matrix of the primary variables of interest is presented in Fig. 2 (see also Fig. S3 which presents associations with other diffusivity metrics). Interestingly, bivariate correlations between R1 and FA were mostly non-significant within our tracts of interest, suggesting that these metrics index orthogonal characteristics of white matter.
Greater R1 is associated with depression
Across the entire sample, we tested whether diagnostic groups differed in R1 and FA. Compared to CTL, the MDD group had significantly greater R1 in the left uncinate fasciculus (β = 0.53; p = 0.022), right uncinate fasciculus (β = 0.42; p = 0.037), and in the corpus callosum genu (β = 0.44; p = 0.025). Similarly, the MDD group had significantly higher FA in the left uncinate fasciculus (β = 0.56; p = 0.016). See Fig. S4 and Table 2A for more details. The MDD group did not differ from the CTLs in R1 of the cingulum cingulate or corpus callosum splenium (all ps > 0.135) or in FA of the other tracts of interest (all ps > 0.167). Finally, neither R1 (all ps > 0.156) nor FA (all ps > 0.107) of our tracts of interest were associated with RADS-2 scores.
With respect to sensitivity analyses, for all models exhibiting a significant effect of depression, including study group and medication status reduced the significance of the effect of depression diagnosis in all models (left uncinate: p = 0.18 and p = 0.213, respectively; right uncinate: p = 0.103 and p = 0.213, respectively; corpus callosum genu: p = 0.175 and p = 0.126, respectively). While including Tanner stage did not affect the significance of the effect of depression on R1 in the left uncinate fasciculus (p < 0.05), it did reduce the significance of the effect of depression in our remaining models (right uncinate: p = 0.062; corpus callosum genu: p = 0.068). While excluding the three participants with past MDD reduced the significance of the effect of depression diagnosis in the uncinate (left uncinate: p = 0.054; right uncinate: p = 0.074) it did not change the significance of the effect of depression diagnosis in the corpus callosum genu (p = 0.022).
When including both R1 and FA as predictors of depression diagnosis in a logistic regression model, R1 in the uncinate was associated with an increased but non-significant odds of MDD (left uncinate: β = 0.48, p = 0.089; right uncinate: β = 0.57, p = 0.071), while R1 in the corpus callosum genu was associated with a statistically significant increased odds of MDD (β = 0.65, p = 0.047). In none of these models was FA significantly associated with odds of MDD (all ps > 0.144).
Sex moderates the association between R1 and depression
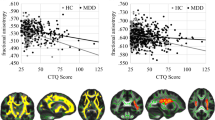
Sex moderated the association between R1 in the left uncinate fasciculus and diagnostic group (interaction effect: β = 1.13; p = 0.010), such that female adolescents with a history of MDD had greater R1 than did female healthy adolescents (β = 1.05; p < 0.001); there was no such depression-related difference among the male adolescents (p = 0.317). Sex moderated the association between R1 in the corpus callosum genu and MDD diagnosis (interaction effect: β = 0.86; p = 0.020), such that female adolescents with history of MDD had greater R1 than did healthy female adolescents (β = 0.82; p < 0.001), but there was no such difference based on diagnosis among the male adolescents (p = 0.648). See Fig. 3 and Table 2B for more details.
Compared to psychiatrically healthy controls, adolescents with a diagnosis of MDD exhibit higher R1 in left uncinate fasciculus (A) and corpus callosum genu (B). Current depression severity scores are positively with higher R1 in the left uncinate fasciculus (C). See Table 2 and Table S3 for more details.
With respect to sensitivity analyses, adding study as a covariate did not change the significance of the interaction effect of sex and depression diagnosis (left uncinate: p = 0.0057; corpus callosum genu: p = 0.011) or the significance of the effect of depression diagnosis within female adolescents only (left uncinate: p = 0.0034; corpus callosum genu: p = 0.0006). Similarly, adding medication as a covariate did not change the significance of the interaction effect of sex and depression diagnosis (left uncinate: p = 0.007; corpus callosum genu: p = 0.018) or the significance of the effect of depression diagnosis within female adolescents only (left uncinate: p = 0.0038; corpus callosum genu: p = 0.0006). Adding Tanner as a covariate did not change the significance either of the interaction effect of sex and depression diagnosis (left uncinate: p = 0.015; corpus callosum genu: p = 0.048) or of the effect of depression diagnosis within female adolescents only (left uncinate: p = 0.0008; corpus callosum genu: p = 0.0006). Excluding the three participants with past MDD did not change the significance of the interaction effect of sex and depression diagnosis on R1 (left uncinate: p = 0.017; corpus callosum genu: p = 0.016) or the significance of the effect of depression diagnosis in female adolescents only (left uncinate: p = 0.0017; corpus callosum genu: p = 0.0136).
Similarly, sex moderated the associations between R1 of the left uncinate fasciculus and RADS-2 scores (β = 0.45; p = 0.046), such that greater R1 in the left uncinate fasciculus was associated with more severe depression symptoms in female adolescents (β = 0.33; p = 0.012), whereas there was no significant association between R1 in the left uncinate fasciculus and severity of depression symptoms in male adolescents (p = 0.150). See Fig. 3 and Table 2B for more details. Sex did not moderate any of associations between FA and MDD diagnosis (all ps > 0.156) or depression symptoms (all ps > 0.333) for any of our tracts of interest.
With respect to sensitivity analyses, adding study as a covariate did not change the significance of the interaction effect of sex and RADS-2 on R1 in the left uncinate fasciculus (p = 0.037) but did reduce the significance of the effect of RADS-2 on R1 in the left uncinate fasciculus in female adolescents (p = 0.096). Adding medication status and Tanner as covariates, however, reduced the significance of the interaction effect of sex and RADS-2 on R1 in the left uncinate fasciculus (p = 0.143 and p = 0.051 within female adolescents, respectively).
Results of supplemental analyses
Age of first MDD onset and duration of current episode was not significantly associated with R1 in any of our tracts of interest (all ps > 0.122 and ps > 0.190, respectively). Including age of menarche did not change the significance of the effect of depression diagnosis in female adolescents (all ps < 0.001) but did reduce the significance of the effect of RADS-2 on R1 in the left uncinate in female adolescents (p = 0.056). See Supplement for more details. Regarding analyses with other diffusivity metrics, the lifetime MDD group exhibited significantly lower RD of the left uncinate only (β = −0.59, p = 0.009) whereas greater RADS-2 scores were significantly associated with lower AD in the corpus callosum genu (β = −0.27, p = 0.018) and the left cingulate (β = −0.34, p = 0.003). The interaction effect of sex and depression did not significantly explain diffusivity metrics in any other tracts of interest (all ps > 0.05). See Table S3 for more details. See Supplement for a summary of the results within the ELS sample only covarying for ELS severity.
Discussion
This multimodal neuroimaging study is the first to characterize myelin in white matter tracts in adolescents with lifetime history of depression. Importantly, we leveraged information from both quantitative T1 relaxometry and diffusion MRI scans to examine myelin content in specific white matter tracts relevant to affective functioning. We show that myelin, indexed by R1, in the uncinate fasciculus and corpus callosum genu is higher in adolescents with a diagnosis of depression than it is in psychiatrically healthy adolescents. We also found that sex moderated the association between depression diagnosis and R1 in the left uncinate fasciculus and corpus callosum genu, such that female adolescents with a lifetime diagnosis of depression had significantly higher R1 in these tracts than did female adolescents without any psychiatric diagnosis. Finally, while depressed adolescents had higher FA (a commonly examined measure of white matter organization based on diffusion-weighted MRI) than did psychiatrically healthy adolescents, there were no sex-specific effects of depression on FA.
Overall, our results indicate that R1 may be a more sensitive sex-specific white matter marker of adolescent depression than is FA. Our finding of higher FA in the left uncinate fasciculus is consistent with results from a previous study of medication-naïve adolescents with depression [10] but stands in contrast both to an older study in depressed adolescents [8] and to a more recent multi-site harmonized meta-analytic investigation in depressed individuals [9], in which depression was associated with lower FA in the uncinate fasciculus and the corpus callosum genu and splenium in adults only. Differences in scanner acquisition, including non-isotropic voxels, which is suboptimal for tractography (as in the case of [8]), as well as heterogeneity of clinical and demographic characteristics (e.g., age and developmental stage) among the sites participating in the harmonized meta-analysis [9] may contribute to important differences in directionality between our findings and these previous results. It is important to note that the harmonized meta-analysis in fact did not report robust differences in FA between depressed and non-depressed adolescents nor did they report significant effects of sex as a moderator in their adolescent-specific analyses. Thus, consistent with the recent harmonized meta-analysis, we did not find that sex moderated the association between FA and depression. These null results are surprising given evidence of sex-associated differences in these depression-relevant white matter tracts [31] and of sex differences in rates of adolescent depression [1]. We did, however, find evidence that R1 in the left uncinate fasciculus and corpus callosum minor reflects the effects of depression more strongly in female than in male adolescents, suggesting that R1 is a more robust sex-specific indicator of depression than is FA.
Our findings that depression is associated with higher R1 (and FA) in specific frontolimbic tracts implicated in affective and cognitive processing is broadly consistent with the formulation that these regions are more sensitive or more engaged in response to environmental influences in adolescents who have experienced depression. Indeed, basic science research has demonstrated that the production of myelin is driven by the electrical activity of neurons and, specifically, by the release of adenosine, which appears to stimulate differentiation of oligodendrocyte precursor cells and the formation of myelin [32], and ATP, which then leads to a release of leukemia inhibitory factor from astrocytes, prompting myelination by mature oligodendroctyes [33]. Immobilization stress in adult rats has been shown to increase oligodendrogenesis [34], and humans with PTSD have been shown to have greater hippocampal myelin content (using a less sensitive MR-based measurement than that used in the current study) than do healthy controls [35]. Together, these results suggest that more activation in frontolimbic circuits in response to stress promotes increased regional myelination through the action of oligodendrocytes and, further, that this may be a key mechanism for understanding both maladaptive brain responses to psychosocial stress and conditions characterized by pathological responses to stress, including depression. In light of basic science work suggesting that myelin inhibits synapse formation, arrests axon growth [36, 37], and reduces brain plasticity [37,38,39], it is critical that we understand how depression impacts myelination and mechanisms of brain plasticity during the sensitive period of adolescence.
Recent work has also implicated sex steroids in processes related to myelination. For example, in vitro studies have highlighted that estradiol enhances membrane sheet formation while progesterone increases cellular branching [40]. Similarly, in vivo studies in rodents have found that compared to sham control females, prepubertal ovariectomy in females resulted in a higher number of neurons and glia and a larger volume of white matter in the medial prefrontal cortex; in contrast, castrated males did not differ from sham males on any of the neuronal, glial, or volume measures in the study [41]. In another study, ovariectomized female rodents differed from sham control females in the number of myelinated axons [22]. Together, these studies suggest that the production of sex steroids during puberty have a significant impact on processes related to myelination. Human neuroimaging studies have also related concentrations of sex steroids with white matter microstructure in various tracts (for a review, see [42]), including increases in testosterone from early to mid-puberty being associated with increases in FA in the corpus callosum and cingulate, and increases in estradiol from early to mid-puberty being associated with increases in FA in the left uncinate fasciculus in female adolescents only [27]. While the data thus far suggest stronger effects of sex steroids in the brains of female animals, it will be important for future work to delineate both how sex steroids, particularly during the pubertal transition, affect myelination and white matter development, and the extent to which these processes may be sex-specific.
Given emerging evidence that myelination increases throughout adolescence [14], our finding of higher R1 in specific white matter tracts in adolescents with a diagnosis of depression than in psychiatrically healthy controls is also broadly consistent with accelerated biological aging models of depression [43]. In this formulation, depression is characterized by accelerated aging across multiple phenotypes, including cellular markers [44] and brain morphometry [45]. It is clear that longitudinal studies are required to examine whether depression is marked by an accelerated aging process regardless of individuals’ developmental stage when they experience depression, or whether there are particular points in development during which depression has a more enduring impact on white matter development in the brain.
Limitations and future directions
Despite the novelty and importance of the present investigation, we should note several limitations that constrain interpretation of our findings. First, this study was conducted in a moderately-sized sample recruited from a single geographic location. To assess the generalizability of our results, it will be crucial that our analyses be replicated with a larger sample that is more representative of the general population with respect to key demographic features. Second, we assessed an adolescent sample within a relatively limited age (13–18 years), which precluded us from examining whether greater myelin content in the uncinate fasciculus and corpus callosum genu is found only at certain points in development. Although there have been studies examining effects on R1 across the lifespan using a cross-sectional design [46], it is critical for the field to characterize longitudinal trajectories of myelination from childhood through adulthood; such research is also necessary for us to better situate our findings within the broader literature of neurodevelopment and depression. Third, trauma exposure, which has been linked with regional increases in myelin in animals [35], and with accelerated biological aging in humans [47], may be another critical factor that, at least partially, is driving our effects. A major limitation of our current study is the absence of a common measure of childhood trauma or adversity, and insufficient information about lifetime depression burden (i.e., total duration of depression). While we did not find evidence that age of first onset of depression or duration of current episode were significantly associated with R1 in any of our tracts of interest, future studies seeking to understand the association between depression and myelination should account for the possible effects of trauma exposure and illness burden. Finally, it is possible that there are genetic contributions to white matter tract organization and myelin production and synthesis that we did not assess in this study. Identifying and controlling for genetic markers of myelination might provide greater insight concerning changes in myelination that are uniquely associated with depression and that are due to modifiable environmental factors.
Conclusions
In summary, this study provides novel evidence of sex differences in depression-related patterns of myelin within the uncinate fasciculus and corpus callosum. Moreover, our results suggest that regional increases in myelin across several frontolimbic tracts that have previously been implicated in depression may be especially pronounced in female adolescents. This study lays the groundwork for future examinations of myelination in adolescent depression, possibly including translational research aimed at generating developmentally informed treatments and interventions for this disorder.
Code availability
Code for preprocessing the quantitative MRI data and estimating T1 per voxel can be found here: https://github.com/cni/t1fit (t1fit_unwarp.py). Code for quality assurance, qT1 data extraction, and visualization of quantitative MRI data can be found here: https://github.com/lucindasisk/TIGER_qT1_Tracts. Code for statistical modeling can be found here: https://github.com/tiffanycheingho/TIGER.
References
Breslau J, Gilman SE, Stein BD, Ruder T, Gmelin T, Miller E. Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Transl Psychiatry. 2017;7:e1139.
Leone M, Kuja-Halkola R, Leval A, D’Onofrio BM, Larsson H, Lichtenstein P, et al. Association of youth depression with subsequent somatic diseases and premature death. JAMA Psychiatry. 2021;78:302–10.
Naicker K, Galambos NL, Zeng Y, Senthilselvan A, Colman I. Social, demographic, and health outcomes in the 10 years following adolescent depression. J Adolesc Health. 2013;52:533–8.
Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–85.
Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:6183.
Long P, Corfas G. Dynamic regulation of myelination in health and disease. JAMA Psychiatry. 2014;71:1296–7.
Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–54.
LeWinn KZ, Connolly CG, Wu J, Drahos M, Hoeft F, Ho TC, et al. White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J Am Acad Child Adolesc Psychiatry. 2014;53:899–909.
Van Velzen LS, Kelly S, Isaev D, Aleman A, Aftanas LI, Bauer J, et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry. 2019;25:1511–25.
Aghajani M, Veer IM, van Lang ND, Meens PH, van den Bulk BG, Rombouts SA, et al. Altered white-matter architecture in treatment-naive adolescents with clinical depression. Psychological Med. 2014;44:2287–98.
Heath F, Hurley SA, Johansen-Berg H, Sampaio-Baptista C. Advances in noninvasive myelin imaging. Dev Neurobiol. 2017;78:136–51.
Stüber C, Morawski M, Schäfer A, Labadie C, Wähnert M, Leuze C. Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. Neuroimage. 2014;93:95–106.
Mottershead JP, Schmierer K, Clemence M, Thornton JS, Scaravilli F, Barker GJ. High field MRI correlates of myelin content and axonal density in multiple sclerosis–a post-mortem study of the spinal cord. J Neurol. 2003;250:1293–301.
Schmierer K, Tozer DJ, Scaravilli F, Altmann DR, Barker GJ, Tofts PS, et al. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J Magn Reson Imaging. 2007;26:41–51.
Schmierer K, Wheeler-Kingshott CA, Tozer DJ, Boulby PA, Parkes HG, Yousry TA, et al. Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magn Reson Med. 2008;59:268–77.
Lutti A, Dick F, Sereno MI, Weiskopf N. Using high-resolution quantitative mapping of R1 as an index of cortical myelination. Neuroimage. 2013;93:176–88.
Sacchet MD, Gotlib IH. Myelination of the brain in Major Depressive Disorder: an in vivo quantitative magnetic resonance imaging study. Sci Rep. 2017;7:2200.
Kumar A, Gupta RC, Albert Thomas M, Alger J, Wyckoff N, Hwang S. Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res. 2004;130:131–40.
Gunning-Dixon FM, Hoptman MJ, Lim KO, Murphy CF, Klimstra S, Latoussakis V. Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. Am J Geriatr Psychiatry. 2008;16:255–62.
Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nat Commun. 2014;5:4932.
Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS. Proliferation and Death of Oligodendrocytes and Myelin Proteins Are Differentially Regulated in Male and Female Rodents. J Neurosci. 2006;26:1439–47.
Yates MA, Juraska JM. Pubertal ovarian hormone exposure reduces the number of myelinated axons in the splenium of the rat corpus callosum. Exp Neurol. 2008;209:284–7.
Walker JC, Teresi GI, Weisenburger RL, Segarra JR, Ojha A, Kulla A. Study Protocol for Teen Inflammation Glutamate Emotion Research (TIGER). Front Hum Neurosci. 2020;14:585512.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;208:19.
Wu H, Dougherty RF, Kerr AB, Zhu K, Middione MJ. Fast T1 mapping using slice‐shuffled simultaneous multi‐slice inversion recovery EPI. Int Soc Magn Reson Med. 2015;1057:440.
Ho TC, King LS, Leong JK, Colich NL, Humphreys KL, Ordaz SJ, et al. Effects of sensitivity to life stress on uncinate fasciculus segments in early adolescence. Soc Cogn Affect Neurosci. 2017;12:1460–9.
Ho TC, Colich NL, Sisk LM, Oskirko K, Jo B, Gotlib IH. Sex differences in the effects of gonadal hormones on white matter microstructure development in adolescence. Dev Cogn Neurosci. 2020;42:100773.
Kircanski K, Sisk LM, Ho TC, Humphreys KL, King LS, Colich NL, et al. Early life stress, cortisol, frontolimbic connectivity, and depressive symptoms during puberty. Dev Psychopathol. 2019;31:1011–22.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8.
Reynolds WM. Reynolds Adolescent Depression Scale. In: Hersen M, Segal DM, Hilsenroth M, eds. Comprehensive handbook of psychological assessment, Volume 2: personality assessment and psychopathology. 2nd ed. New York, NY: John Wiley & Sons; 2004. p. 224–36.
Seitz J, Cetin-Karayumak S, Lyall A, Pasternak O, Baxi M, Vangel M, et al. Investigating Sexual Dimorphism of Human White Matter in a Harmonized, Multisite Diffusion Magnetic Resonance Imaging Study. Cereb Cortex. 2021;31:201–12.
Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–68.
Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–32.
Chetty S, Friedman AR, Taravosh-Lahn K, Kirby ED, Mirescu C, Guo F, et al. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol Psychiatry. 2014;19:1275–83.
Chao LL, Tosun D, Woodward SH, Kaufer D, Neylan TC. Preliminary Evidence of Increased Hippocampal Myelin Content in Veterans with Posttraumatic Stress Disorder. Front Behav Neurosci. 2015;9:333.
Bandtlow C, Zachleder T, Schwab ME. Oligodendrocytes arrest neurite growth by contact inhibition. J Neurosci. 1990;10:3837–48.
Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–9.
McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–6.
McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–8.
Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci. 2004;26:245–54.
Koss WA, Lloyd MM, Sadowski RN, Wise LM, Juraska JM. Gonadectomy before puberty increases the number of neurons and glia in the medial prefrontal cortex of female, but not male, rats. Dev Psychobiol. 2015;57:305–12.
Vijayakumar N, Op de Macks Z, Shirtcliff EA, Pfeifer JH. Puberty and the human brain: insights into adolescent development. Neurosci Biobehav Rev. 2018;92:417–36.
Lindqvist D, Simon NM, Wolkowitz OM. Is Depression Associated With Accelerated Aging? Mechanisms and Implications. In: Quevedo J, Carvalho A, Zarate CA, eds. Neurobiology of Depression, 1st ed. Cambridge, MA: Academic Press; 2019. p. 207–29.
Humphreys KL, Sisk LM, Manczak EM, Lin J, Gotlib IH. Depressive Symptoms Predict Change in Telomere Length and Mitochondrial DNA Copy Number Across Adolescence. J Am Acad Child Adolesc Psychiatry. 2020;59:1364–70.
Han LKM, Dinga R, Hahn T, Ching CRK, Eyler LT, Aftanas L, et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol Psychiatry. 2020. https://doi.org/10.1038/s41380-020-0754-0.
Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nat Commun. 2014;5:4932.
Colich NL, Rosen ML, Williams ES, McLaughlin KA. Biological aging in childhood and adolescence following experiences of threat and deprivation: a systematic review and meta-analysis. Psychol Bull. 2020;146:721–64.
Acknowledgements
We thank Alexess Sosa, Anna Cichocki, Amar Ojha, Holly Pham, and Kira Oskirko for assistance with data collection and organization. We also thank Lucy King and Jaclyn Schwartz for helpful comments during the preparation of this paper. Finally, we wish to thank the participants and their families for contributing to this study.
Funding
This research was supported in part by the National Institutes of Health (K01MH117442 to TCH and R37MH101495 to IHG), the Ray and Dagmar Dolby Family Fund (TCH), Stanford Maternal & Child Health Research Institute (TCH), Stanford Center for Cognitive and Neurobiological Imaging University (TCH), Stanford Precision Health and Integrated Diagnostics (IHG and TCH), the Klingenstein Third Generation Foundation (Child and Adolescent Depression Award to TCH), the National Science Foundation (Graduate Research Fellowship Award NSF DGE-1752134 to LMS), and Stanford Bio-X (Undergraduate Summer Research Program to AK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Author information
Authors and Affiliations
Contributions
TCH conceived and obtained funding for the study; TCH and LMS designed the study and drafted the first version of the paper; TCH, LMS, AK, GT, MMH, and HW contributed to the acquisition and analysis of the data; TCH, LMS, and IHG interpreted the data; all authors reviewed the paper and critically revised it for important intellectual content. All authors agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
All authors report no biomedical competing interest. The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ho, T.C., Sisk, L.M., Kulla, A. et al. Sex differences in myelin content of white matter tracts in adolescents with depression. Neuropsychopharmacol. 46, 2295–2303 (2021). https://doi.org/10.1038/s41386-021-01078-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01078-3
This article is cited by
-
Understanding the phenotypic variability in Niemann-Pick disease type C (NPC): a need for precision medicine
npj Genomic Medicine (2023)
-
Brain-based Sex Differences in Depression: A Systematic Review of Neuroimaging Studies
Brain Imaging and Behavior (2023)