Abstract
To investigate the association between intrauterine antidepressant exposure and offspring affective disorders over an 18-year follow-up period using Danish national registers. We included 42,988 singletons born during 1998–2011 and followed-up until 2016, death, emigration, or date of first affective disorder diagnosis. Children were categorised into two groups according to maternal antidepressant use within 2 years before and during pregnancy: continuation (use before and during pregnancy) or discontinuation (use before but not during pregnancy). The outcome was an affective disorders diagnosis in the offspring based on secondary/tertiary care records and primary care prescription data. Hazard ratios (HR) of affective disorders were estimated using Cox regression models. To consider confounding by shared environmental or genetic factors, we investigated the effect of paternal antidepressant use on the risk for affective disorders. Affective disorders were diagnosed in 1538 children. Children whose mothers continued antidepressants during pregnancy had an increased risk of affective disorders (HR = 1.20, 95% CI = 1.08–1.34), compared with children whose mothers discontinued before pregnancy. Similarly, continued paternal antidepressant use during pregnancy was associated with higher risk for offspring affective disorders (HR = 1.29, 95% CI = 1.12–1.49), compared to discontinuation. Based on data from primary and secondary/tertiary care, maternal antidepressant use during pregnancy was associated with an increased risk of affective disorders in the offspring. As similar associations were observed in children whose fathers continued antidepressant use across the pregnancy period, the observed association may be attributable to the underlying parental psychopathology, rather than the direct intrauterine exposure to antidepressants.
Similar content being viewed by others
Introduction
Major depressive disorder is highly prevalent, with one in five people experiencing an episode at some point in their life, and almost twice as common in women than in men [1]. Antidepressants are usually given as first-line treatment, including during pregnancy, either to prevent the recurrence of depression or as acute treatment in newly depressed patients [2]. Antidepressant use during pregnancy is widespread, with estimated prevalence rates ranging between 2 and 13% [3]. Since antidepressants cross the placenta and the blood-brain barrier [4], concern exists about sequelae of intrauterine antidepressant exposure on the unborn child.
A substantial number of studies have found associations between intrauterine antidepressant exposure and adverse neonatal outcomes [5], as well as poorer neurodevelopmental and psychiatric outcomes, including higher risk for autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) [6, 7]. However, research has demonstrated through sequential adjustment for confounding by indication (i.e., unadjusted, restricted to women with a depression diagnosis to control for the potential effect of the underlying illness or factors associated with it, restricted to women with a depression diagnosis, using propensity score stratification to further control for proxies of depression severity and other potential confounders), that the reported associations are, in large part, driven by the underlying maternal disorder [8, 9]. This is supported by observations that maternal psychopathology increases the risk of adverse outcomes in the child, including adverse birth outcomes, poorer long-term cognitive development, and the risk for psychopathology [10], via shared genetic and environmental factors [11].
Considering confounding by indication, our recent systematic review showed that affective disorders were the only offspring psychopathology significantly associated with prenatal exposure to antidepressants [8]. Mechanistically, it is conceivable that intrauterine antidepressant exposure renders the foetal brain specifically vulnerable for affective disorders: antidepressants target the serotonergic system [12], and serotonin mediates basic processes such as neurogenesis and synaptogenesis [13]. Prenatal exposure to antidepressants modulates this serotonin regulation at crucial neurodevelopmental stages, which may lead to an increased risk of affective psychopathology later in life [14].
Affective disorders are common in adolescence, their prevalence increasing with age [15]. Due to the limited follow-up duration of most longitudinal and register-based studies in existence today, affective disorders are currently under-investigated as long-term outcomes of intrauterine antidepressant exposure. Moreover, for outcome definitions, population-based studies of long-term outcomes have primarily relied on diagnoses by mental health care providers in secondary or tertiary care settings such as hospitals. However, this approach underestimates the number of individuals with affective disorders treated in primary care settings.
The aim of this study was to investigate the association between intrauterine antidepressant exposure and offspring affective disorders in the Danish national registers with a follow-up period of up to 18 years, the longest follow-up period to date. We focussed on affective disorders in this study because outcomes with childhood-onset, such as ASD and ADHD, have been investigated extensively. Affective disorders were defined based on records from secondary/tertiary care providers (e.g. hospital records) and prescription data (including from primary care settings) [16]. We compared children whose mothers continued antidepressant use during pregnancy to children whose mothers discontinued antidepressant use before pregnancy to limit confounding by the underlying maternal illness (confounding by indication). We further investigated the effect of paternal antidepressant use on the risk for an affective disorder in the offspring to quantify confounding by shared environmental or genetic factors.
Methods
Sample
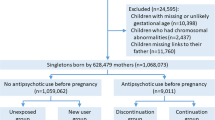
We carried out a population-based cohort study using data from Danish national registers. All live births and residents in Denmark are assigned a unique identification number in the Danish Civil Registration System [17], which permits linkage of individual-level data. We identified all liveborn singletons during 1998–2011 (n = 908,310) from the Danish Medical Birth Registry [18]. We excluded 7092 children who had missing or likely erroneous gestational age (<154 or >315 days); 2107 children with chromosomal abnormalities (ICD-10 [International Classification of Diseases, 10th revision] codes Q90–Q99) identified from the Danish National Patient Register [19]; and 7969 children missing links to their fathers. We included a total of 853,226 singletons born to 524,142 mothers. The study was approved by the Danish Data Protection Agency. By Danish law, no informed consent is required for a register-based study using anonymized data.
Antidepressant use
Information on maternal antidepressant use was obtained from the Danish National Prescription Registry [20]. This register includes a record of all prescriptions dispensed in Denmark since 1995. It contains the anatomical therapeutic chemical (ATC) classification codes, the number of defined daily doses per package, the number of packages dispensed, and the dispensing date. The detailed ATC codes are shown in Supplementary Table A, but in short, the ATC code for SSRIs was N06AB, and those for non-SSRI antidepressants were N06AA, N06AF, N06AG and N06AX. The start of antidepressant use was indicated by the dispensing date. We defined antidepressant use during pregnancy as a prescription dispensed on any date from one month before pregnancy until delivery. The first day of the mother’s last menstrual period was used to indicate the start of pregnancy. To capture recent episodes, we included antidepressant prescriptions dispensed from two years before pregnancy to delivery. Children were categorised into two mutually exclusive groups according to dispensing of an antidepressant prescription to the mother from 2 years to one month before pregnancy (referred to as ‘before pregnancy’) and during pregnancy: (i) discontinuation, with use before but not during pregnancy, (ii) continuation, with use both before and during pregnancy. We calculated the number of days exposed per prescription by multiplying the number of defined daily doses per package by the number of packages dispensed. We calculated the exact number of days exposed by adding the durations of all antidepressant prescriptions. To examine whether the associations seen were confounded by shared environmental or genetic variables, we repeated the analyses on paternal antidepressant use during the same window as the exposure [21]. We hypothesised that if any potential long-term effects on the child are due to intrauterine exposure, maternal antidepressant use during pregnancy should have a greater influence than paternal antidepressant use in the same period.
Primary and secondary outcomes
The primary outcome of interest was the occurrence of affective disorders in the offspring as defined by two approaches. Occurrence of affective disorders was ascertained (1) based on records from secondary/tertiary care providers, specifically, the first date of a relevant diagnosis: mood disorders (F30–F39), neurotic, stress-related and somatoform disorders (F40-F48), mixed disorders of conduct and emotions (F92), or emotional disorders with onset specific to childhood (F93), or (2) based on prescription data, after the child reached 5 years of age: redemption of prescriptions for antidepressants (ATC code N06A) and anxiolytics (N05B). We also considered each of these diagnoses and prescription types separately. Information on psychiatric diagnoses came from the Danish Psychiatric Central Research Register [22]. This register holds information on all inpatient and outpatient psychiatric treatment in Denmark. Information on antidepressant and anxiolytic prescriptions were obtained from the Danish National Prescription Registry.
Potential confounders
We identified potential confounders in our analyses using directed acyclic graphs. The majority related to maternal characteristics: history of a formal psychiatric diagnosis at delivery, retrieved from the Danish Psychiatric Central Research Register (ICD-8 codes 290–315; ICD-10 codes F00–F99); age at delivery (continuous variable); primiparity (yes/no); inpatient and outpatient psychiatric treatment from two years before pregnancy until delivery (yes/no); prescriptions for other psychotropic drugs (ATC codes N05 and N06 excluding N06A) during pregnancy (yes/no); prescriptions for antiepileptic drugs (ATC code N03) during pregnancy (yes/no); number of non-psychiatric hospital visits during pregnancy (0–1, 2–3, or ≥4); tobacco use during pregnancy (yes/no); marital status (married or cohabiting/single, divorced, or widowed); highest education (mandatory education/above mandatory education); and calendar year of delivery (1998–2002, 2003–07, or 2008–11). In addition, we included paternal psychiatric history at delivery. Data on these covariates came from the registers mentioned above as well as from Statistics Denmark’s registers on socioeconomic status [23].
When producing estimates for paternal antidepressant use, we also adjusted for maternal antidepressant use during pregnancy (unexposed, discontinuation, continuation, or new user group), paternal age at delivery (continuous variable), and paternal inpatient or outpatient psychiatric treatment from two years before pregnancy to delivery (yes/no).
Statistical analysis
We used Stata 15 for statistical analyses. We followed children in the antidepressant discontinuation and continuation groups from birth until the first of the following events: end of the data (31st December 2016), death, emigration, or the date of first diagnosis of affective disorders/prescription of antidepressant or anxiolytic. We used Cox regression models to calculate hazard ratios (HRs) with 95% confidence intervals (CIs), with child’s age as the time scale. We evaluated proportionality by visually inspecting ‘log–log’ plots. To account for the dependence between siblings, we used the Huber sandwich estimator for the correction of standard errors. All p-values were based on Wald’s tests, and we considered p < 0.05 (two-sided test) to be statistically significant. For the covariates, tobacco use and education, 5.2% and 3.4% of values were missing, respectively; we applied 20 imputations using the Markov Chain Monte Carlo technique for imputing missing values [24]. To evaluate whether the underlying maternal psychiatric disorders were associated with offspring psychiatric disorders, we calculated the HRs with the children whose mothers discontinued antidepressant use prior to pregnancy as the reference group.
Comparisons between antidepressant continuation and discontinuation groups
To control for how the underlying maternal psychiatric disorders influence risk of our outcome (occurrence of affective disorders in the offspring), we carried out detailed analyses comparing the antidepressant continuation group to the discontinuation group, adjusting for the above-mentioned covariates. To examine whether the associations between antidepressant exposure and affective disorders depended on the timing of exposure, we divided the exposure window into three groups based on the last menstrual period: first trimester only (one month before pregnancy to 90 days after last menstrual period); second or third trimester only (91–180 days after last menstrual period or 181 days after last menstrual period to childbirth); and more than one trimester. We considered a child to be exposed to antidepressants in a specific trimester if the dispensing date fell within the trimester or if the number of days prescribed overlapped that trimester. To study whether the associations varied with different classes of antidepressants, we categorised antidepressant treatment into SSRI monotherapy, non-SSRI antidepressant monotherapy, and both SSRI and non-SSRI antidepressant treatment. To determine whether the associations were modified by the duration of use, we divided the duration of antidepressant use during pregnancy into 90 days or less, 91–180 days, and 181 days or more. We also included the duration as a continuous variable, per 30-day increase, in the models to see whether duration response relations existed.
Sensitivity analyses
We carried out three sensitivity analyses to test the robustness of our results. First, to reduce the misclassification of antidepressant exposure, we restricted our analyses to mothers who filled at least two prescriptions for antidepressants during pregnancy. This aimed to exclude mothers who decided not to take antidepressants in pregnancy and, therefore, did not renew their prescriptions. Second, to reduce misclassification of antidepressant discontinuation, we redefined the exposure windows. For children to be included in the discontinuation group, mother had to use antidepressants from two years to three months before pregnancy, but not from three months before pregnancy until delivery. Third, as mothers who had inpatient psychiatric treatment may have more severe symptoms, we repeated our analyses, excluding the 4304 pregnancies of mothers with inpatient psychiatric treatment from 2 years before pregnancy until delivery.
Results
Table 1 lists the psychiatric and demographic characteristics of the study population. Of the 853,226 children, 42,988 children born to mothers who used antidepressants two years before pregnancy were included in the final analyses. Among them, 15,892 (1.9%) were born to mothers who used antidepressants during pregnancy. Approximately 1.7% (n = 14,818) of mothers used SSRI monotherapy, 0.3% (n = 2915) used non-SSRI antidepressant monotherapy, and 0.2% (n = 1452) used both SSRI and non-SSRI antidepressants. The children were followed for a maximum of 18 years. The mean age at first affective disorder was 12.0 (SD = 3.8) years. The children in the antidepressant discontinuation and continuation groups contributed a total of 4.3 × 105 person–years at risk. Overall, 1,538 children were identified as having an affective disorder based on records from secondary/tertiary care providers or prescription data from primary care settings. Of the 1538 children with affective disorder, 1074 had a diagnosis from secondary/tertiary care providers, while 464 were identified using prescription data from primary care settings.
Comparisons between antidepressant continuation and discontinuation groups
The risk for affective disorders among offspring in the continuation group was higher than that in the discontinuation group (adjusted HR = 1.20, 95% CI = 1.08–1.34) (Table 2).
Children exposed to antidepressants during more than one trimester had the highest risk of affective disorders compared to the discontinuation group, while the children exposed during the first trimester only, or the second or third trimester only did not have a significantly different risk for affective disorders (Table 2). The risk of affective disorders did not differ between children exposed to SSRI monotherapy, non-SSRI antidepressant monotherapy, or SSRI and non-SSRI use (Table 2). For different durations of antidepressant use, a higher risk was seen among children whose mothers were in the continuation group and took antidepressants for more than 180 days during the pregnancy and pre-pregnancy periods (adjusted HR = 1.33, 95% CI = 1.16–1.53) (Table 2).
When examining the risks of prescription and disorder subcategories (Table 3), we found increased risks in the continuation group compared with the discontinuation group for antidepressant use (adjusted HR = 1.47, 95% CI = 1.18–1.83), mood disorder (adjusted HR = 1.46, 95% CI = 1.05–2.03), neurotic, stress related and somatoform disorder (adjusted HR = 1.18, 95% CI = 1.01–1.39), and emotional disorder with onset specific to childhood (adjusted HR = 1.57, 95% CI = 1.18–2.10), but not prescription of anxiolytics (adjusted HR = 1.07, 95% CI = 0.85–1.34), or a diagnosis of mixed disorders of conduct and emotions (adjusted HR = 0.90, 95% CI = 0.62–1.32).
To consider effects of the underlying maternal illness on our defined outcomes, as well as effects of more general susceptibility to affective disorders through genes and environment, we investigated paternal antidepressant use during the pregnancy period. We found that paternal antidepressant use during pregnancy was similarly associated with higher risk for affective disorders with an adjusted hazard ratio in the continuation group of 1.29 (95%CI = 1.12–1.49), compared with the discontinuation group. The associations between paternal antidepressant use and affective disorders in offspring were slightly but not significantly higher than the associations between maternal antidepressant use and affective disorders in offspring (Fig. 1).
Sensitivity analyses
To address potential misclassification of antidepressant exposure, we restricted our analyses to mothers who filled at least two prescriptions for antidepressants during pregnancy. Among mothers who continued antidepressant use during pregnancy, 74.9% (n = 11,900) received two or more prescriptions. Redefining antidepressant exposure as two prescriptions gave an adjusted hazard ratio for affective disorders in the continuation group of 1.27 (95%CI = 1.12–1.43), compared with the discontinuation group. To reduce misclassification of antidepressant discontinuation, we redefined the exposure windows. Sensitivity analyses showed similar results when we redefined antidepressant discontinuation as use of antidepressants before pregnancy with no use from three months before pregnancy until delivery or excluded pregnancies in mothers with inpatient psychiatric treatment from two years before pregnancy until delivery (Supplementary Table B).
Discussion
In this prospective Danish population-based study of 42,988 children born in 1998–2011, and followed up until 2016, we examined the effects of intrauterine antidepressant exposure on the risk for affective disorders. We found an increased risk for affective disorders among children born to mothers who continued antidepressants during pregnancy, compared with mothers who discontinued antidepressant use before pregnancy, and a similar pattern in fathers.
Previous research using Finnish national register data found an association between prenatal SSRI and increased rates of depression, but not anxiety diagnoses by age 14.9 years [25]. However, the follow-up period in the current study is longer than in previous research, thus capturing individuals with a later onset of illness. Moreover, unlike previous studies, we broadened the definition of affective disorders to include both diagnoses based on records from secondary/tertiary care providers and on prescription data from primary care settings, providing a more complete estimate of the prevalence of affective disorders in the offspring. We found that continued maternal use of antidepressants during pregnancy was linked to an increased risk for diagnoses of affective disorders in the offspring, based on secondary/tertiary care records and based on antidepressant prescription by primary care providers. Consequently, this is one of the first studies to show a link between maternal antidepressant use during pregnancy and antidepressant use in the offspring.
Comparing prenatally antidepressant-exposed individuals to individuals born to mothers who discontinued antidepressants before pregnancy may not sufficiently address confounding by indication because women who continue antidepressants throughout pregnancy may be fundamentally different from women who discontinue [26]. Previous research has attempted to circumvent this issue via innovative study designs deemed to mitigate the problem of confounding by indication, for example by using sequential adjustment for confounding by indication or unexposed siblings, without ever fully removing the uncertainty [8, 9]. The problem emerges because register-based studies lack information about the severity of the underlying maternal disorder. Yet, mothers with severe symptoms may be more likely to continue treatment during pregnancy compared to mothers with mild to moderate symptoms or compared to a less severe episode, for example, during another pregnancy [27]. We attempted to take the underlying maternal disorder into account by adjusting for various psychiatric variables, including dispensing of prescriptions for antiepileptic and other psychotropic drugs during pregnancy, and inpatient and outpatient psychiatric treatment before pregnancy. Following adjustment, the magnitude of the association was slightly attenuated, but the increased risk of affective disorders in offspring remained. Nevertheless, future research is required to understand how the severity of the underlying maternal disorder impacts the risk for affective disorders in the offspring.
The highest point estimates for affective disorders were observed for children whose mothers took antidepressants for more than 180 days and whose mothers took antidepressants in more than one trimester. Although only approaching statistical significance, the highest risk for affective disorders was observed among children exposed to both SSRI and non-SSRI antidepressants, indicating that these mothers were treated with polypharmacy or switching drugs. A longer treatment duration, polypharmacy or drug switching may reflect persistent symptoms and limited or no treatment response in the mothers [28], consistent with greater severity of the disorder. Consequently, we specultate that the observed associations may be attributable to the severity of the underlying maternal disorders, rather than intrauterine effects of the medication.
Repeating the analysis in fathers revealed an increased risk for affective disorders in children whose fathers continued antidepressant use during pregnancy, compared with fathers who discontinued, similar in magnitude to maternal antidepressant continuation. These findings suggest that the associations observed for maternal use of antidepressants are likely driven by the underlying maternal psychopathology, which may be confounding the relation between intrauterine antidepressant exposure and offspring psychopathology, and may be transmitted to the child via shared genetic and/or environmental factors. Consequently, when comparing the effects of maternal versus paternal antidepressant use during the pregnancy period, our findings do not support the idea of a causal intrauterine effect of antidepressants on long-term mental illness in the child. Instead, our findings indicate that the observed increased risk of affective disorders in children presented in Fig. 1 are driven by the underlying parental disorder (confounding by indication) via shared environmental/genetic factors.
Strengths and limitations of the study
In a large population-based sample, we examined affective disorders as defined by records from secondary/tertiary care providers and prescription data from primary care settings. This approach may provide more accurate estimates of the prevalence of affective disorders in the offspring than records from secondary/tertiary care providers alone. Moreover, the independent and prospective collection of exposure and outcome data reduced the chances of ascertainment and recall bias. Lastly, this study has a follow-up period of up to 18 years, the longest to date, allowing us to investigate mental health outcomes with a later onset. Future studies should also investigate other outcomes, such as ASD and ADHD, using this long follow-up period.
A number of limitations should be considered alongside these results. Firstly, register-based designs such as this cannot assess symptoms severity [26] and it is conceivable that mothers who continue antidepressant use throughout pregnancy have a more severe disorder than those who discontinue [27]. While we aimed to control for effects of the underlying indication by comparing women with antidepressant use before but not during pregnancy to women who used antidepressants throughout pregnancy, confounding by severity may still be present. Secondly, we employed prescription data to define antidepressant exposure. Exposure misclassification may result from certain patients filling prescriptions without actually taking the antidepressants, a limitation present in all observational pharmaco-epidemiological studies. Yet, the extent of misclassification is most likely small because in Denmark antidepressant treatment compliance during pregnancy is high [29]. Moreover, results remained the same when we redefined antidepressant exposure on the basis of two prescriptions. Based on a measure defined by the World Health Organization, we estimated the duration of antidepressant treatment as the number of defined daily doses, relating to the average amount of a drug needed for long-term therapeutic use. However, prescribed daily doses might differ [30], which also may lead to misclassification of treatment duration. The assumption may have biased our estimate of the effect of the treatment durations on affective disorders. Thirdly, mothers who have been treated for psychopathology may be more likely to seek treatment for their children, introducing detection bias. However, we examined affective disorders with onset specific to childhood and those with later onset [31]. Similar associations were observed irrespective of age at onset, suggesting that detection bias is unlikely. Fourthly, like in other observational studies, unmeasured confounding cannot be ruled out. Fifthly, it is currently unclear how generalisable our findings are to populations that differ from the Danish population, for example, with regards to cultural diversity or genetic makeup. Lastly, while our sample size is large, some subgroup analyses are based on a small number of cases.
Conclusion
Maternal antidepressant use during pregnancy was associated with an increased risk of affective disorders in offspring based on data from primary and secondary/tertiary care settings. As similar associations were observed in children whose fathers continued antidepressant use across the pregnancy period, the observed association may be attributable to the underlying parental psychopathology, rather than the direct exposure to antidepressants in utero.
Funding and disclosure
ASR, NCM, NMM, XL, TMO and VB are supported by the National Institute of Mental Health (NIMH) (R01MH122869). XL is also supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 891079. TMO is supported by iPSYCH, the Lundbeck Foundation Initiative for Integrative Psychiatric Research (R155-2014-1724), The Lundbeck Foundation (R313-2019-569), AUFF NOVA (AUFF-E 2016-9-25), and Fabrikant Vilhelm Pedersen og Hustrus Legat. VB has received funding from the Netherlands Organization for Scientific Research (clinical fellow and VENI incentive). The investigators conducted the research independently. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: support for the submitted work as described above; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–312. https://doi.org/10.1016/S0140-6736(18)31948-2.
Charlton RA, Jordan S, Pierini A, Garne E, Neville AJ, Hansen AV, et al. Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: a population-based study in six European regions. BJOG Int J Obstet Gynaecol. 2015;122:1010–20. https://doi.org/10.1111/1471-0528.13143.
Molenaar NMNM, Bais B, Lambregtse-van den Berg MPMP, Mulder CLCL, Howell EAEA, Fox NSNS, et al. The international prevalence of antidepressant use before, during, and after pregnancy: a systematic review and meta-analysis of timing, type of prescriptions and geographical variability. vol. 264. 2020. https://doi.org/10.1016/j.jad.2019.12.014.
Ewing G, Tatarchuk Y, Appleby D, Schwartz N, Kim D. Placental transfer of antidepressant medications: implications for postnatal adaptation syndrome. Clin Pharmacokinet. 2015;54:359–70. https://doi.org/10.1007/s40262-014-0233-3.
Prady SL, Hanlon I, Fraser LK, Mikocka-Walus A. A systematic review of maternal antidepressant use in pregnancy and short- and long-term offspring’s outcomes. vol. 21. 2018. https://doi.org/10.1007/s00737-017-0780-3.
Quinlan DM, Brown TE. Assessment of short-term verbal memory impairments in adolescents and adults with ADHD. J Atten Disord. 2003;6:143–52. https://doi.org/10.1177/108705470300600401.
Liu X, Agerbo E, Ingstrup KG, Musliner K, Meltzer-Brody S, Bergink V, et al. Antidepressant use during pregnancy and psychiatric disorders in offspring: Danish nationwide register based cohort study. BMJ. 2017;358:j3668.
Rommel AS, Bergink V, Liu X, Munk-Olsen T, Molenaar NM. Long-term effects of intrauterine exposure to antidepressants on physical, neurodevelopmental, and psychiatric outcomes: a systematic review. J Clin Psychiatry. 2020;81. https://doi.org/10.4088/JCP.19r12965.
Huybrechts KF, Palmsten K, Avorn J, Cohen LS, Holmes LB, Franklin JM, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397–407.
Jarde A, Morais M, Kingston D, Giallo R, MacQueen GM, Giglia L, et al. Neonatal outcomes in women with untreated antenatal depression compared with women without depression: a systematic review and meta-analysis. JAMA Psychiatry. 2016;73:826–37. https://doi.org/10.1001/jamapsychiatry.2016.0934.
Dean K, Stevens H, Mortensen PB, Murray RM, Walsh E, Pedersen CB. Full spectrum of psychiatric outcomes among offspring with parental history of mental disorder. Arch Gen Psychiatry. 2010;67:822–9. https://doi.org/10.1001/archgenpsychiatry.2010.86.
Pawluski JL. Perinatal selective serotonin reuptake inhibitor exposure: impact on brain development and neural plasticity. Neuroendocrinology. 2012; 95. https://doi.org/10.1159/000329293.
Teissier A, Soiza-Reilly M, Gaspar P. Refining the role of 5-HT in postnatal development of brain circuits. Front Cell Neurosci. 2017;11:139. https://doi.org/10.3389/fncel.2017.00139.
Brummelte S, Mc Glanaghy E, Bonnin A, Oberlander TF. Developmental changes in serotonin signaling: implications for early brain function, behavior and adaptation. Neuroscience. 2017;342:212–31. https://doi.org/10.1016/j.neuroscience.2016.02.037.
Ghandour RM, Sherman LJ, Vladutiu CJ, Ali MM, Lynch SE, Bitsko RH, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr. 2019;206:256–67.e3. https://doi.org/10.1016/j.jpeds.2018.09.021.
Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90. https://doi.org/10.2147/CLEP.S91125.
Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39:22–5. https://doi.org/10.1177/1403494810387965.
Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45:320–3.
Andersen TF, Madsen M, Jørgensen J, Mellemkjær L, Olsen JH. The Danish National Hospital Register: a valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–8.
Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39:38–41. https://doi.org/10.1177/1403494810394717.
Smith GD. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin Pharmacol Toxicol. 2008;102:245–56. https://doi.org/10.1111/j.1742-7843.2007.00191.x. Basic Clin Pharmacol Toxicol.
Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39:54–7. https://doi.org/10.1177/1403494810395825.
Petersson F, Baadsgaard M, Thygesen LC. Danish registers on personal labour market affiliation. Scand J Public Health. 2011;39:95–8. https://doi.org/10.1177/1403494811408483.
Royston P, White IR. Multiple imputation by chained equations (MICE): implementation in Stata. J Stat Softw. 2011;45:1–20. https://doi.org/10.18637/jss.v045.i04.
Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, et al. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry. 2016;55:359–66. https://doi.org/10.1016/j.jaac.2016.02.013.
Palmsten K, Hernández-Díaz S. Can nonrandomized studies on the safety of antidepressants during pregnancy convincingly beat confounding, chance, and prior beliefs? Epidemiology. 2012;23:686–8. https://doi.org/10.1097/EDE.0b013e318258fbb2.
Patten SB. Confounding by severity and indication in observational studies of antidepressant effectiveness. Can J Clin Pharm. 2008;15:e367–71.
Vigod SN, Wilson CA, Howard LM. Depression in pregnancy. BMJ. 2016;352. https://doi.org/10.1136/bmj.i1547.
Olesen C, Søndergaard C, Thrane N, Lauge Nielsen G, De Jong-Van Den Berg L, Olsen J. Do pregnant women report use of dispensed medications? Epidemiology. 2001;12:497–501. https://doi.org/10.1097/00001648-200109000-00006.
Lahon K, Shetty HM, Paramel A, Sharma G. Sexual dysfunction with the use of antidepressants in a tertiary care mental health setting—a retrospective case series. J Pharm Pharmacother. 2011;2:128–31. https://doi.org/10.4103/0976-500X.81913.
Pedersen CB, Mors O, Bertelsen A, LindumWaltoft B, Agerbo E, McGrath JJ, et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry. 2014;71:573–81. https://doi.org/10.1001/jamapsychiatry.2014.16.
Acknowledgements
The lead author (the manuscript’s guarantor) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Author information
Authors and Affiliations
Contributions
ASR, NCM, NMM, XL, TM-O, and VB conceived and designed the study. ASR drafted the manuscript. NCM, and XL had full access to the data, analysed the data, and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors interpreted the data and revised the manuscript critically.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rommel, AS., Momen, N.C., Molenaar, N.M. et al. Long-term prenatal effects of antidepressant use on the risk of affective disorders in the offspring: a register-based cohort study. Neuropsychopharmacol. 46, 1518–1525 (2021). https://doi.org/10.1038/s41386-021-01005-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01005-6
This article is cited by
-
Prenatal antidepressant exposure and emotional disorders until age 22: a danish register study
Child and Adolescent Psychiatry and Mental Health (2023)