Abstract
Autism spectrum disorder (ASD) comprises a heterogeneous group of neurodevelopmental outcomes in children with a commonality in deficits in social communication and language combined with repetitive behaviors and interests. The etiology of ASD is heterogeneous, as several hundred genes have been implicated as well as multiple in utero environmental exposures. Over the past two decades, epigenetic investigations, including DNA methylation, have emerged as a novel way to capture the complex interface of multivariate ASD etiologies. More recently, epigenome-wide association studies using human brain and surrogate accessible tissues have revealed some convergent genes that are epigenetically altered in ASD, many of which overlap with known genetic risk factors. Unlike transcriptomes, epigenomic signatures defined by DNA methylation from surrogate tissues such as placenta and cord blood can reflect past differences in fetal brain gene transcription, transcription factor binding, and chromatin. For example, the discovery of NHIP (neuronal hypoxia inducible, placenta associated) through an epigenome-wide association in placenta, identified a common genetic risk for ASD that was modified by prenatal vitamin use. While epigenomic signatures are distinct between different genetic syndromic causes of ASD, bivalent chromatin and some convergent gene pathways are consistently epigenetically altered in both syndromic and idiopathic ASD, as well as some environmental exposures. Together, these epigenomic signatures hold promising clues towards improved early prediction and prevention of ASD as well genes and gene pathways to target for pharmacological interventions. Future advancements in single cell and multi-omic technologies, machine learning, as well as non-invasive screening of epigenomic signatures during pregnancy or newborn periods are expected to continue to impact the translatability of the recent discoveries in epigenomics to precision public health.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) refers to a complex group of neurodevelopmental disorders characterized by deficits in social communication and language and gains in repetitive and restrictive behaviors and interests. The prevalence of ASD has been steadily increasing over the past 20 years, from US child estimates of 0.66% in 2002 [1], 1.13% in 2008 [2], 1.85 in 2016 [3], and 2.27% in 2018 [4]. Changes over this period in the rate of ASD is in part due to increased awareness and changing diagnoses [5,6,7,8]. However, even estimates that account for diagnostic changes still leave an apparent increase that cannot likely be explained by genetics alone [9]. Furthermore, ASD heritability estimates have been discordant in different twin studies, depending on the number of subjects, geographical, and demographic differences [10,11,12,13]. While there has been much progress in the discovery of new ASD genes by detection of rare de novo mutations from exome sequencing studies, no single ASD gene can account for more than 1% of ASD [14,15,16,17,18]. Approaches to identify common genetic variants for ASD using genome-wide association studies have revealed a shared genetic architecture with other disorders and traits [19]. Together, these finding have demonstrated that ASD etiology is decidedly complex, involving hundreds of genes and interactions with environmental factors.
Epigenetics, literally meaning “on top of genetics” is a field that investigates additional layers of relevant biological information for interpreting phenotypes that do not alter the genetic code. Poised at the interface of genetic and environmental influences, the investigation of epigenetic modifications such as DNA methylation can reveal novel insights that are not apparent in the DNA sequence. See Box 1 for more details of epigenetic definitions and terms. However, the field of epigenetics is inherently integrated with genetics, as genetic variation frequently influences epigenetic variation [20]. Since the most common heritable variability exists outside of protein coding exons, these variants can be more difficult to interpret without the important context of epigenetics. Epigenetic layers of information have been used to functionally annotate the human genome with activity levels of promoters and enhancers as well as chromatin loops and domains of similar DNA methylation levels [21,22,23,24]. Since environmental factors act by altering responsive gene expression patterns, these can leave distinctive “signatures” at the level of epigenetic modifications that leave long-lasting effects on gene expression, particularly when the exposures occur in utero or early postnatal life [25, 26].
The field of autism epigenetics began initially through genetics, specifically the investigation of known genetic neurodevelopmental disorders with ASD comorbidity that were epigenetic in their inheritance pattern and/or gene function [27, 28]. These included the disorders of the parentally imprinted gene cluster at 15q11-q13, in which large maternal deletions cause Angelman syndrome but the same deletion inherited paternally cause Prader-Willi syndrome [29,30,31,32]. Also of early interest in this field was Rett syndrome, an X-linked dominant disorder affecting females, caused by mutation in MECP2, a gene encoding a known epigenetic player, methyl CpG binding protein 2 [33, 34]. Candidate gene approaches to investigate epigenetic differences were also based on expectations from both genetics and neuroscience and include genes such as oxytocin receptor [35]. More recent genome-wide investigations of epigenetic differences in ASD have included epidemiology-based epigenome-wide association studies (EWAS) that most frequently utilize commercial array-based platforms. However, sequencing-based approaches such as whole genome bisulfite sequencing (WGBS) and those using some degree of reduced representation are becoming more common. See Box 2 for an explanation of platform differences in EWAS.
DNA methylation is the most frequently studied epigenetic modification in genome-wide ASD studies for both practical and biological reasons. On a practical level, DNA is much easier to obtain from a variety of limited clinical samples and DNA methylation is a relatively stable mark in archived frozen tissues [36], relative to RNA or histone modifications. But also biologically, DNA methylation in the human genome is highly abundant, correlates with other epigenetic layers including open chromatin and histone modifications, and is the layer closest to and most influenced by DNA sequence [37]. Therefore, this review will focus on investigations of DNA methylation and the signatures of DNA methylation patterns that are emerging and converging between different etiologies of ASD. Starting with a review of the evidence that DNA methylation signatures reflect predominantly gene by environment interactions in general, I will then move to the evidence for individual genetic versus environmental etiologies.
Evidence that DNA methylation predominantly reflects gene by environment interactions in early life
Genetic and environmental factors may act independently on DNA methylation, or in additive or multiplicative interactions. At the simplest level, some genetic variants including single nucleotide polymorphisms (SNPs) can directly determine DNA methylation, for instance, if they cause a gain or loss of a CpG. These are expected to be rare (<1%) based on a study of neonatal genotype-methylation comparisons from cord blood [38]. In this array-based study, only 25% of variably methylated regions were estimated to be explained by genotype alone, and 75% were explained by genotype x in utero environment interactions [38]. A sequencing-based study identified a slightly higher 32% of methylated CpGs as being genetically regulated and enriched in enhancers, compared to ~14% that were not dependent on genotype and enriched in repressed regions and near transcription start sites [39]. A more recent and larger array-based investigation of newborn cord blood determined the best models to explain DNA methylation, concluding that genetic (G), gene plus environment (G+E), and gene by environment interaction (GxE) explained roughly equal proportions of variably methylated regions, and was consistent with previous studies in showing no evidence for environment alone [40]. In this study, variants with best models G, G + E or GxE all showed significant enrichment within GWAS signals for complex disorders beyond the enrichment of the functional variants themselves. ASD strikingly stood out among other neuropsychiatric disorders as having the highest enrichment of GWAS loci enriched in methylation patterns best explained by GxE (Odds ratio > 2) [40].
Variably methylated regions (VMRs) have been investigated in more detail across different human tissues and environmental conditions, revealing some interesting insights. An array-based study examined polymorphic human methylation patterns across five cell types and ages, identifying both unique and common VMRs [41]. Interestingly, these VMRs were found to form co-methylated networks that were enriched for genes and transcription factor binding sites with cell-type relevant functions. In neurons, the top enriched gene function was for “synapse assembly” while in glia it was “negative regulation of neurogenesis” [41], suggesting the influence of VMRs on gene functions relevant to ASD. A sequencing-based approach mapped VMRs across the human genome, estimating that they make up 11% of the genome and are enriched in histone modifications indicative of enhancers, transcription factor binding sites and GWAS variants, including those for ASD [42]. A sequencing-based study identified that the most interindividual differences in DNA methylation between humans occurred in defined correlated regions of systemic interindividual variation (CoRSIVs), defined as having similar DNA methylation patterns across tissue types [43]. While CoRSIVs include genes associated with human disease phenotypes, conserved across diverse human ethnic groups, and sensitive to periconceptional environment, they unfortunately are very sparsely covered on commercial methylation arrays.
Twin studies have been critical to understanding the heritability of DNA methylation patterns in humans. Array-based comparisons of monozygotic (MZ) to dizygotic twins (DZ) using 27k and 450k platforms (Box 2) have estimated heritability of DNA methylation ranging from 3–20% depending on the age of the subjects. A more recent 450k study examined both genetic and environmental contribution of multiple twin cohorts of different ages (0–92 years) by calculating familial correlations of DNA methylation compared to time of twin cohabitation [44]. In this study, familial correlations for 6.6% of CpG sites assayed changed with twin pair cohabitation history and these “cohabitation” sites were enriched for regions with the highest high heritability (31% versus 19% heritability for all sites). These “cohabitation” sites are therefore consistent with the high degree of methylation explained by GxE in prior studies [38, 40], and were similarly enriched for locations of genes involved in nervous system development and genetic associations for cognitive traits [44]. Importantly, the life course design of this twin study provided evidence that early life could affect later life health through DNA methylation. Furthermore, the studies of both twins and unrelated subjects have demonstrated that the CpG sites with the strongest heritability are also those that are also most influenced by environmental factors.
Evidence for epigenomic signatures of human syndromic neurodevelopmental disorders with ASD
Consistent with distinguishable genetic effects on DNA methylation, syndromic human neurodevelopmental disorders are also emerging as having distinct epigenomic signatures. While these syndromes have known genetic etiologies, many of these involve mutations to modifiers of chromatin [45], so differences in DNA methylation are likely reflecting genome-wide changes in chromatin compared to controls. The discovery of syndrome-specific epigenomic signatures has direct relevance to clinical diagnosis because they offer the possibility of using a single test platform, such as the Illumina Infinium 450k or EPIC array, using blood-derived DNA, to distinguish multiple neurodevelopmental syndromes from each other. But perhaps more importantly, the epigenomic signature in both brain and appropriate surrogate tissues provides specific clues throughout the genome about the downstream effects on genes, transcription factors, and pathways in the molecular pathogenesis of each syndromic disorder.
DNA methylation patterns have been used for decades to diagnose imprinted and X-linked disorders such as Prader-Willi/Angelman syndromes (PWS/AS) and fragile X syndrome because they offered specific tests that would encompass different genetic and epigenetic causes of gene loss or repression. However, a recent study demonstrated that four different imprinted syndromes (PWS, AS, Beckwith-Wiedemann, Silver-Russell) could be distinguished from each other and 364 reference controls with 100% specificity and sensitivity using the 450k array platform from blood DNA [46]. What is new in recent years is the number of genetic syndromes not arising from mutations in epigenetically regulated chromosomal loci that can be predicted based on DNA methylation signatures. In 2019, a clinical DNA methylation assay, “EpiSign,” was introduced for the screening of 14 syndromes using supervised and unsupervised classification algorithms [47]. In 2020, the same group utilized a similar strategy to identify distinct epigenomic signatures in 34 out of 42 neurodevelopmental syndromes [48]. Most of these epigenomically defined syndromes are caused by known ASD candidate genes involved in chromatin regulation (ADNP, ATRX, CHD8, KDM6A, KTM2B) or DNA methylation (DNMT1, DNMT3A, DNMT3B). Approximately 50 different syndromes caused by genes encoding epigenetic regulators, dubbed “chromatinopathies”, have been clinically defined and the clinical utility of using methylation data and EpiSign for improved diagnosis has been validated by independent teams [49, 50]. While these epigenomic signatures are secondary events in the disease pathogenesis downstream of the causative mutation, they offer insight into disease pathogenesis and well as applications in clinical diagnosis.
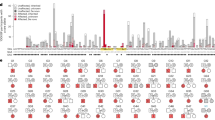
In addition to the syndromic forms of ASD caused by rare mutations in single genes, epigenomic signatures have also provided some novel insights into the molecular pathogenesis into syndromes caused by copy number variants (CNVs), such as 15q11-q13 duplication [51, 52] or 16p11.2 deletion [53] syndromes, or aneuploidy, specifically Down syndrome (trisomy 21). These investigations, particularly those that have investigated brain and cell culture models, have provided important clues as to the genes within the large chromosomal regions that are responsible for the epigenetic changes observed (Fig. 1). While the specific mechanisms predicted to cause the epigenome-wide dysregulation observed in these different chromosomal syndromes are distinct, there are commonalities in the chromatin features, genes, and gene pathways impacted across disorders. Importantly, these patterns revealed from human genetics are beginning to complement the emerging evidence from animal models that neuronal maturation involves a cross-talk between de novo DNA methylation and histone modifications that mediate developmental plasticity through multiple mechanisms [54,55,56]. In zebrafish embryos, developmentally plastic “placeholder” nucleosomes, containing H2A.Z and H3K4me1, are anti-correlated with DNA methylation and their removal results in accumulation of DNA methylation [57]. In mammals, DNA hypomethylation is observed over developmentally poised “bivalent” chromatin containing histone H2A or H2A.Z that are both acetylated and ubiquitinated, and histone H3 that is methylated at both K4 and K27 residues [58, 59]. Specifically, recent studies have demonstrated that a Dnmt3a mutant that no longer binds to active chromatin results in progressive DNA hypermethylation across bivalent chromatin domains marked by H3K27me3 and de-repression of developmental regulatory genes in the adult hypothalamus [60]. An isoform-specific knockout of DNMT3A demonstrated that its N-terminal binding to ubiquitinated H2A (H2AK119ub) guides de novo DNA methylation over bivalent genes within the nervous system [61]. Furthermore, a Dnmt3a knockout specifically targeted to excitatory neurons resulted in stunted maturation of synapses, elevated H3K27me3 levels, and fetal-like DNA methylation patterns in the postnatal period [62]. Together, these experiments have shown the mechanistic connections between de novo DNA methylation and placeholder nucleosomes over poised chromatin that are being reflected in the broad epigenomic signatures of human neurodevelopmental disorders.
Examples are shown of two disorders caused by a large chromosomal duplication, 15q11-q13 duplication syndrome (Dup15q, left panel), or aneuploidy, Down syndrome (trisomy 21, right panel). In Dup15q syndrome, duplications are either supernumerary (left) or interstitial (right) and depend on parent of origin, as only maternal (pink chromosome) duplications are associated with ASD. Elevated levels of the imprinted gene UBE3A are predicted to initiate the pathogensis of Dup15q syndrome. UBE3A is an E3 ubiquitin ligase that targets a different E3 ubiquitin ligase called RING1B, part of the PRC1 complex, that monoubiquitinates H2A and H2A.Z, resulting in a maintenance of bivalency (acetylation and ubiquitination). The epigenomic signature of Dup15q brain and neuronal cell line models has revealed hypomethylation at H2A.Z bivalent regions over synaptic genes due to reduced RING1B levels. In contrast, Down syndrome brain is characterized by both hypermethylation and hypomethylation and two chromosome 21-encoded proteins are distinctly implicated in each process. Elevated DNMT3L increases methylation over regions of bivalent chromatin marked by H3K4me3 and H3K27me3, including the chromosome 21 locus RUNX1, encoding a developmental transcription factor. The regions hypomethylated in Down syndrome newborn blood were enriched for RUNX1 binding sites, suggesting that RUNX1 targets these sites for demethylation from its known association with TET2. While the mechanisms behind the epigenomic signatures of Dup15q and Down syndromes are distinct, synaptic gene pathways are apparent in both and the RUNX1 locus shows differential methylation in both syndromes. Created with BioRender.com.
15q11-q13 duplication syndrome (Dup15q) is one of the most common CNVs identified in ASD cases and ASD comorbidity is observed in 85% of Dup15q cases [63]. Due to the same chromosomal breakpoints responsible for large deletions in 15q11-q13, Dup15q syndrome results from duplication that is either extrachromosomal or interstitial [64]. While duplications can occur on either parental chromosome, ASD is only observed in individuals with maternal duplication [63, 64]. The AS gene UBE3A is maternally expressed exclusively in neurons due to the paternal expression of the UBE3A antisense [63]. Because the 15q11-q13 locus is parentally imprinted, the strongest methylation signature observed in Dup15q brain is over a ~7 Mb region that is strikingly hypomethylated on the maternal allele by 20 kb window WGBS analysis, resulting in opposite DNA methylation directions in the 15q11-q13 deletion syndromes AS (hypermethylation) versus PWS (hypomethylation) and a more modest hypomethylation in Dup15q syndrome compared to controls [51]. The opposite pattern of maternal hypermethylation and paternal hypomethylation was observed specifically at CpG islands within the 7 Mb imprinted locus [51], reminiscent of what is observed on the inactive X chromosome in females [65]. The enrichment of both hypo- and hypermethylated probes over the imprinted 15q11-q13 locus in Dup15q syndrome was replicated in a 450k array analysis of three brain regions [52]. This 450k array study in brain also showed a significant overlap in the epigenomic signature between Dup15q and idiopathic ASD [52], a finding that was also replicated in a subsequent WGBS analysis of idiopathic ASD, Dup15q syndrome, and Rett syndrome [66].
Functional follow-up analyses using human neuronal cell line models revealed further insights into the potential mechanism responsible for the epigenomic signature of Dup15q syndrome and how multiple “hits” may impact the strength of the epigenomic signature. A human neuronal cell line model of Dup15q syndrome [67] was cultured in the presence or absence of the environmental pollutant polychlorinated biphenyl (PCB 95) [51], which had previously observed to be elevated in Dup15q brain samples compared to controls or idiopathic ASD [68]. Long-term clonal cultures of this Dup15q model acquired a second duplication on 22q12.3-q13.33, resulting in a multi-hit model of two chromosomal duplications plus the environmental exposure. Each additional hit increased the number of differentially methylated genes, mostly hypomethylated, with functions at the synaptic membrane [51]. Because most hypomethylated genes showed decreased expression in long-term cultures, an epigenetic change to chromatin modifications was investigated. Specifically, a known nuclear target of the ubiquitin ligase activity of UBE3A is RING1 [69], a component of the polycomb regulatory complex 1 (PRC1) repressor that is a ubiquitin ligase for the histone components H2A and H2A.Z [59]. Ubiquitinated H2A.Z is a poised developmental mark of large chromatin domains with lower levels of DNA methylation [59]. As shown in Fig. 1, bivalent H2A.Z marked the Dup15q hypomethylated genes and elevated levels of UBE3A correlated with reduced levels of RING1B. PCB 95 further reduced the levels of H2A.Z [51]. Together, these results suggested a multi-hit intersecting pathway between genetic susceptibility and an environmental exposure observed through the shared epigenomic signature.
Down syndrome (DS) is another neurodevelopmental disorder caused by a chromosomal copy number change, trisomy 21. Estimates of ASD incidence is DS have ranged from 5–39% dependent on the study [70]. Multiple studies have demonstrated that gene expression and epigenetic dysregulation in DS tissues occur genome-wide and are not necessarily enriched on chromosome 21 [71,72,73,74,75,76]. Gene loci hypermethylated in DS were most consistent across tissues, while hypomethylated loci were more tissue-specific. A WGBS study in newborn blood from DS versus other developmental delay or typically developing controls demonstrated a 28 kb domain on chromosome 21, spanning the RUNX1 locus [73], which has been consistently hypermethylated across DS studies and tissues [71,72,73,74,75,76]. RUNX1 encodes a developmental transcription factor important in both hematopoiesis and neurodevelopment [77, 78]. Interestingly, the regions hypomethylated in DS newborn blood were enriched for binding sites of RUNX1 [73]. Mechanistically, RUNX1 binding has been shown to demethylate its binding sites through recruitment of DNA demethylation enzymes (TET2, TET3, TDG, and GADD45) [79]. Therefore, these results are consistent with the mechanism of early life DS transcriptional and epigenetic dysregulation being in part due to both the overexpression and epigenetic modification of RUNX1.
Of the potential known epigenetic regulators encoded on chromosome 21, the DNA methyltransferase gene, DNMT3L has the most accumulated evidence supporting its role in the hypermethylated loci observed across tissue types in DS [80, 81]. DNMT3L is a catalytically inert homolog of DNMT3A and DNMT3B that serves as their protein partner and regulator of de novo DNA methylation [82, 83]. Overexpression of DNMT3L in in human neurons at three stages of differentiation demonstrated a hypermethylated signature shared with that of DS brain and other DS tissues [81]. In this study, DNMT3L-induced hypermethylation occurred predominantly at regions of bivalent chromatin that lose H3K4me3 during neuronal differentiation. In DNMT3L overexpressing cells, hypermethylation of RUNX1 was observed in the neuroblast and differentiated neurons, but not those in process of differentiation [81]. Together, these findings (summarized in Fig. 1) demonstrate that early neuronal DNMT3L overexpression recreates a facet of the genome-wide DS DNA methylation signature, specifically the gene loci that consistently display hyper-methylation in DS such as RUNX1. These results also suggest that DNMT3L may be a major contributor to the hypermethylated bivalent chromatin signature in DS, while RUNX1 binding and demethylation may be a contributor to the blood-specific hypomethylated signature of DS. However, there are also consequences to cellular viability, DNA repair, and metabolism associated with aneuploidy independent of chromosome 21 dosage effects that have been demonstrated in fibroblasts from DS in common with other trisomies [84] that are also consistent with gene ontologies enriched in DS epigenomic signatures [72, 73, 81].
Evidence for epigenetic signatures in human idiopathic ASD brain and surrogate tissues
While the discovery of distinct epigenomic signatures distinguishing syndromic forms of ASD is possible with a relatively low sample size and different tissue types besides brain, this same approach has been more challenging in finding a robust DNA methylation signature for idiopathic ASD. Postmortem brain samples from individuals with idiopathic ASD have been the tissue type used for initial EWAS, because of the predicted involvement in ASD symptomology. Several studies have used the Illumina Infinium 450k platform to investigate different brain regions (prefrontal and temporal cortex, cerebellum) from 6–12 ASD samples compared to matched controls, but no individual differentially methylated probe (DMP) reached genome-wide significance [52, 85,86,87]. However, using an approach of identifying differentially methylated regions (DMR), Ladd-Acosta et al identified three DMRs in temporal cortex (PRRT1, ZNF57, C11orf21) and one in cerebellum (SDHAP3). Furthermore, Wong et al. 2019 used a systems approach of grouping comethylated gene loci into modules, identifying a number of significant gene ontologies associated with idiopathic ASD, including homophylic adhesion, synapse part, and calcium ion binding [52] that were convergent with other idiopathic ASD and Dup15q syndrome analyses [51, 85, 86].
The use of a sequencing-based WGBS approach allowed the discovery of 483 DMRs in idiopathic ASD prefrontal cortex, although this number of regions was 5–10x lower that those identified in two syndromic forms of ASD, Dup15q and Rett syndromes by the same analyses [66]. In addition to enrichment for genes with functions in nervous system development, ASD DMRs were also enriched for regions of open chromatin in microglia, the major immune cell type in brain [66]. In this study, ASD DMRs were also enriched for binding sites of known methyl-sensitive transcription factors, including IRF3, NRF1, YY1, and RFX5. Furthermore, a group of 65 genes overlapped for enrichment in DMRs identified in all three disorders (idiopathic ASD, Dup15q, Rett) that included many of those with known genetic risk for ASD (OTX1, GRIK4, and ADCY5), as well as also differentially expressed in ASD (MYT1L, ZFHX3, RBFOX1) [66]. Furthermore, the genes associated with ASD DMRs in this study showed significant overlaps with those identified from other transcriptome and epigenomic studies in ASD cerebral cortex [66]. Together, these studies support an epigenomic signature associated with idiopathic ASD in the prefrontal cortex that shares some similarities to those of syndromic ASD.
While these biological insights gained from brain EWAS studies have been important, there are many limitations to these studies, including the limited number of samples and the inability to control for confounding variables, such as medications and time and cause of death. Furthermore, the inaccessibility of sampling brain DNA from living individuals makes it not useful as a predictive biomarker. Therefore, multiple studies have attempted to use EWAS in accessible tissues such as blood, with limited success. Hannon et al performed a 450k EWAS on DNA samples isolated from whole blood matched to 4 brain regions (prefrontal cortex, entorhinal cortex, superior temporal gyrus, and cerebellum) from 122 typically developing individuals, concluding that interindividual variation in whole blood was not a strong predictor of interindividual variation in the brain [88]. This finding is consistent with two relatively large studies that did not identify a distinguishable epigenomic signature of idiopathic ASD from blood samples obtained in children after diagnosis [89, 90]. Though these studies failed to identify any individual DMPs at genome-wide significance, there were correlations observed between top DMPs and quantitative autistic trait scores in one study [90] or with some brain DMPs in another [89]. A 450k EWAS study of 95 buccal epithelial samples from ASD versus TD individuals born to mothers ≥35 years identified 9 genes and synaptic pathways associated with ASD from alternative methods of DMRs and comethylated modules [91]. A large 450k EWAS using newborn blood spots was successful in associating polygenic risk score for ASD with methylation variation at some specific chromosomal loci, but did not identify any DMP associated with ASD alone [92].
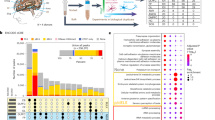
Potentially more promising for identifying predictive epigenetic biomarkers for ASD are those studies that have performed EWAS on newborn samples obtained at delivery in prospective studies of enriched ASD risk. Two prospective cohorts in the US have recruited mothers of a prior child with ASD who are pregnant, thus enriching ASD diagnosis ten-fold compared to population risk: Markers of Autism Risk in Babies – Learning Early Signs (MARBLES) and Early Autism Risk Longitudinal Investigation (EARLI). Both studies collect and store maternal biospecimens during pregnancy, cord blood and placenta at delivery, and follow up children through 36 months with biospecimen collections and quantitative evaluations of social and cognitive outcomes [93, 94]. Both array-based and EWAS studies have been used to investigated ASD epigenomic signatures of idiopathic ASD using samples from these cohorts [95,96,97,98,99,100,101,102,103]. Because the use of WGBS allowed the more extensive coverage of regions of the genome that are the most variable between individuals (discussed above), the focus of this summary is on the two most recent and largest studies using WGBS-based EWAS on placenta [96] versus cord blood39 that have led to new mechanistic insights into ASD (Fig. 2).
At birth, the fetal derived byproducts of placenta and cord blood are usually discarded, but were collected as part of the prospective MARBLES and EARLI cohorts of enriched idiopathic ASD risk. Since they are derived from different lineages of the early embryo, placenta and cord blood from the same individuals have each revealed distinct mechanisms from their epigenomic signatures. Analyses of placenta (left panel) resulted in the discovery of the neuronal hypoxia inducible, placenta associated (NHIP) locus on 22q13.33. NHIP methylation levels were associated with both genetics (upstream insertion) and folic acid (prenatal vitamin use in first pregnancy month). In differentiated human neurons, hypoxia and resulting oxidative stress increase NHIP levels. ASD placenta and brain samples show significantly lower NHIP levels, suggesting a protective effect. NHIP is primate-specific and encodes a conserved micropeptide that associates with nuclear chromatin. NHIP elevated transcript levels alter many downstream gene targets enriched for regulators of chromatin and ASD genetic risk. In contrast, the idiopathic ASD epigenomic signature from cord blood samples revealed an enrichment for X-linked differentially methylated genes, as well as those involved in early neurodevelopment. Differential methylation was observed at the XACT locus, a primate-specific noncoding RNA expressed exclusively from the active X chromosome that represses XIST during the establishment of X chromosome inactivation. In human pluripotent stem cell culture, XACT is implicated in the phenomenon of X chromosome erosion, characterized by the partial loss of epigenetic silencing of X-linked genes and regions of bivalent chromatin on the X chromosome in females. While many X linked genes implicated in genetic risk for ASD were observed to be differentially methylated in ASD cord blood DNA, three specific examples (MECP2, CDKL5, and HDAC8) involved in syndromic forms of ASD are shown. Created with BioRender.com.
Using MARBLES as the discovery cohort and EARLI as the replication cohort, Zhu et al 2022 analyzed a total of 204 placenta samples (83 ASD, 107 controls) using WGBS to identify ASD-associated methylation changes [96]. 134 ASD associated DMRs were identified in the discovery group that were enriched for bivalent chromatin regions in placenta and enhancers in fetal brain. Remarkably, a large block of CpGs in 22q13.33 was significantly hypomethylated in ASD and this finding replicated across both MARBLES and EARLI cohorts. This 22q13.33 block of comethylated regions was previously identified as a CoRSIV and a region of bivalent chromatin in placenta, but did not contain any well characterized genes. There was a transcript within the 22q13.33 block annotated as LOC105373085 which additional functional studies showed to be expressed in brain and induced in response to neuronal differentiation and oxidative stress. This transcript was therefore renamed NHIP for neuronal hypoxia inducible, placenta associated. Using RNAseq on both postmortem ASD and control brain samples as well as NHIP-overexpressing cell lines, NHIP was shown to regulate expression of other known ASD-risk genes involved in chromatin and transcriptional regulation. A common structural variant within the NHIP locus was independently associated with increased ASD risk, reduced expression of NHIP, and reduced methylation. Interestingly, placentas from mothers who took a folic acid vitamin in the first month of pregnancy, a known protective anti-oxidant methyl-donor [104, 105], showed increased NHIP methylation, essentially counteracting the genetic risk at NHIP [96]. Functionally, NHIP encodes a 20 amino acid peptide that localizes to neuronal nuclei in human brain. While this peptide is well conserved amongst primates, NHIP is not detected in other mammals, suggesting a recent evolved function in the response to hypoxia and oxidative stress in primates [96]. Within primates, humans have evolved the highest brain-to-body ratio, with a human fetal brain consuming up to 60% of the body’s oxygen and energy consumption, despite making up ~13% of body mass [106]. Together, these results suggest that transient NHIP expression in response to hypoxia is neuroprotective.
In contrast to placenta which is derived from the trophectoderm layer of the preimplantation embryo in all mammals, cord blood is derived from the hematopoietic cell lineage of the embryo proper [107]. So perhaps not surprisingly, distinct properties of ASD associated DMRs were identified in the analysis of cord blood compared to placenta using similar WGBS and DMR approaches [95, 96, 98]. Unlike the placental analysis for which the variable of offspring sex was adjusted for [96], cord blood WGBS analysis was most informative when samples were stratified by sex because of the strong enrichment for cord blood ASD DMRs on the X chromosome [95]. Subjects in this study were 74% male from both MARBLES and EARLI high-familial risk prospective cohorts. Replication across different cohorts identified 537 ASD DMR genes in males and 1762 ASD DMR genes in females by gene association. ASD DMR genes identified from cord blood were significantly enriched for brain and embryonic expression and identification in prior epigenetic studies of ASD in post-mortem brain. Like what ASD DMRs revealed in postmortem brain, those identified in cord blood were significantly enriched for binding sites of methyl-sensitive transcription factors relevant to fetal brain development. The major finding that ASD DMRs identified brain and early developmental functions rather than immune functions [95] was in distinct contrast to a transcriptome analysis of the same samples which only identified blood and immune functions [108]. Furthermore, autosomal ASD DMRs from cord blood were significantly enriched for promoter and bivalent chromatin states in both sexes, while sex differences were observed for X-linked ASD DMRs [95]. Interestingly, the enrichment of differentially methylated genes on the X chromosome included the primate-specific noncoding transcript XACT [95], which is expressed from the active X chromosome in males and females and has been implicated in the phenomenon of X chromosome erosion in human pluripotent cells [109, 110]. Furthermore, many of the genes associated with ASD DMRs in cord blood were known syndromic ASD risk genes [95], including MECP2 and CDKL5 (Rett syndrome) [111], as well as histone deacetylase HDAC8 (Cornelia de Lange syndrome 5) [112].
Future perspectives
The multiple lines of evidence presented above have established that epigenetic changes in the form of distinct DNA methylation signatures characterize both syndromic and idiopathic neurodevelopmental disorders on the autism spectrum. There is also strong evidence that in general, most inter-individual variation in DNA methylation is best explained by GxE models, meaning that common genetic risk is predicted to dynamically interact with common but variable environmental factors. Since most ASD cases are unlikely to have a single genetic or environmental cause, the use of epigenomic signatures to detect the multivariate intersection is likely to improve understanding of common genetic risk for ASD. Importantly, the DNA methylome signature of ASD polygenic risk is highly supportive of most common risk involving GxE more than other neuropsychiatric disorders [40]. This is insightful considering a recent study showing that polygenic risk score for schizophrenia is only predictive for those with early life complications [112]. Thus, being able to accurately interpret the unique signatures of the DNA methylome at birth within the heterogeneous mixture of ASD offers important insights to improved diagnosis and therapy.
Since ASD is currently diagnosed by behavioral testing that does not detect all cases until 3 years of age [113], there is a current need for early biomarkers of ASD risk, so that at risk toddlers may receive behavioral interventions at an age where they are most effective [114, 115]. In the future, newborn genetic screens may be widened to include the known syndromic forms of ASD [116, 117]. However, since these genetic tests need to be performed one at a time on limited DNA isolated from newborn blood spots, a more efficient screen could be an epigenetic one, perhaps using an algorithmic predictive strategy like EpiSign [117], discussed above. For such a screening to be both sensitive and specific for both syndromic as well as idiopathic forms of ASD, replication with larger sample sizes and careful design of the most appropriate genomic regions to assay is imperative to glean the benefits of both array-based and sequencing-based platforms (Box 2). The latest genomic advancements, including the entire telomere to telomere (T2T) human genome [118, 119] and DNA methylome [120] maps should be used when choosing the regions with the most inter-individual variation and differences between ASD and control from the analyses of early life tissues. Cell-specific methylome maps in brain [121,122,123] are another emerging resource also of critical importance for designing improved assays for clinical use. Single cell technologies of brain cell transcriptomes and epigenomes have been reviewed elsewhere [123, 124]. More research is clearly needed in prospective human cohorts in which samples and data collected during pregnancy and childbirth are stored and widely distributed. This is a major goal of the Environmental effects of Child Health Outcomes (ECHO) US-wide birth cohort study [125] that has the potential to be used for future EWAS and transgenerational investigations [126] into intersections of genetic and environmental risk factors for ASD and other adverse health outcomes.
Advances in machine learning are already beginning to be used to determine the most sensitive and specific predictors of ASD diagnosis from large-scale EWAS datasets [127,128,129]. The major challenge to the field at this stage is that the training on small sample sizes become problematic in overfitting data based on too little and likely highly biased data inputs. Major efforts towards recruitment of larger numbers of research subjects from diverse and medically underserved populations is therefore important if the resulting predicted are going to be beneficial for screening of all newborns. Ideally, multiple layers of information about each subject, including genetic variants, DNA methylation signatures, measured metabolite levels, brain imaging measurements, as well as a variety of surveyed information concerning social determinants of health would be integrated into machine learning predictive algorithms. In addition to simple yes/no diagnoses, these could potentially become sophisticated enough to predict some of the clinical variability within syndromic disorders, such as epilepsy within Dup15q, or comorbidities within idiopathies ASD.
Moving to an even earlier stage of potential preventative intervention that the newborn stage, prenatal screening from cell free fetal DNA within maternal blood holds potential for early identification and interventions to improve brain development during pregnancy for at risk fetuses. Currently, non-invasive prenatal screening is performed in the first trimester as an alternative to chorionic villus sampling for prenatal screening of Down syndrome. This procedure involves sampling and sequencing the cell-free fetal DNA (cffDNA) fraction from blood of pregnant mothers [130, 131]. cffDNA originates from the trophoblasts of the placenta, based on genetic evidence from cases of anembryonic pregnancies or confined placental mosaicism [132,133,134]. DNA methylation evidence also has demonstrated that cffDNA contains partially methylated domains [135, 136] which are uniquely characteristic of placenta [137, 138]. Currently, cancer epigenomic signatures from cell free DNA derived from tumor cells is showing promise for early detection and tissue origin of multiple cancer types [139,140,141]. Therefore, profiling epigenomic signatures or specific placental gene loci such as NHIP from cffDNA within maternal blood could provide an early marker of ASD risk in the child. Early identification of risk could prompt existing interventions during pregnancy that reduce medical complications and environmental exposures. Some preventative epigenomic screenings could even be performed pre-conception, as there has be some predictive success within small studies of sperm methyome differences in fathers of ASD offspring [103, 142]. There are clearly ethical concerns and limitations to pre-conception and prenatal epigenomic screening for ASD risk to avoid the negative impacts associated with false positives. Clearly, more research is needed on epigenomic screening and preventative intervention of ASD risk.
Following up on specific mechanistic insights provided by epigenomic signatures from both syndromic and idiopathic ASD early life samples is expected to inform future therapies and early interventions. Future investigations should seek to understand the overlap between ASD-associated DNA methylation signatures with additional epigenetic layers, including histone acetylation and long noncoding RNAs identified as differential in ASD brain [143, 144]. Comparisons to brain organoid cultures derived from ASD patient-derived or genetically engineered stem cells (reviewed in [145, 146]) are also expected to inform mechanism of developmental epigenetic changes in ASD. Since the epigenomic signatures provided from bulk tissues are often complex, use of emerging single cell approaches to define ASD signatures in perinatal tissues such as placenta and cord blood is expected to be informative. For instance, single cell methylomes have been instrumental in dissecting our specific neuronal cell types in human brain [147, 148]. These approaches have recently enabled multi-omic analyses of the methylome, transcriptome, chromatin accessibility, and chromatin loops for human cortical cell types [121]. When these multi-omic cellular maps were compared to GWAS loci for neuropsychiatric traits, they demonstrated a significant enrichment for closed chromatin within layer 6 excitatory neurons, for example. The future use of multi-omic single cell methods in comparisons of ASD to control samples is expected to shed additional light on convergent mechanisms in the pathogenesis of ASD, including the dynamic changes to DNA methylation and bivalent chromatin highlighted in this review.
But in addition to the cell type specific mechanisms, there may also be some tissue independent and universal defining mechanisms across different etiologies of ASD that are revealed from epigenomic signatures of syndromic and/or idiopathic ASD. An example discussed in this review is the NHIP regulatory gene locus that was discovered in placenta, but highly expressed in brain and responsive to hypoxia in differentiated human neurons [96]. Hypoxia and oxidative stress are pathways in common to multiple environmental insults as well as placental complications during pregnancy. Since elevated NHIP expression in response to oxidative stress appears to be protective for the development of ASD, the NHIP gene or encoded micropeptide could be promising as a therapeutic approach during pregnancy or early infancy in high-risk populations. In summary, the discoveries made from epigenomic investigations using the latest genomic approaches are likely to yield clinically relevant advancements in the future that would not have been possible through traditional genetic approaches such as GWAS.
References
Autism, Developmental Disabilities Monitoring Network Surveillance Year Principal I, Centers for Disease C, Prevention. Prevalence of autism spectrum disorders--autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveill Summ. 2007;56:12–28.
CDC. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR. 2012;61:1–19.
Maenner MJ, Shaw KA, Baio J, EdS, Washington A, Patrick M, et al. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill Summ. 2020;69:1–12.
MJ M, KA S, AV B, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ. 2021;70:1–16.
Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168:904–12.
Dawson G. Dramatic increase in autism prevalence parallels explosion of research into its biology and causes. JAMA Psychiatry. 2013;70:9–10.
Maenner MJ, Rice CE, Arneson CL, Cunniff C, Schieve LA, Carpenter LA, et al. Potential impact of DSM-5 criteria on autism spectrum disorder prevalence estimates. JAMA Psychiatry. 2014;71:292–300.
Delobel-Ayoub M, Ehlinger V, Klapouszczak D, Maffre T, Raynaud JP, Delpierre C, et al. Socioeconomic disparities and prevalence of autism spectrum disorders and intellectual disability. PLoS One. 2015;10:e0141964.
Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology. 2009;20:84–90.
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–102.
Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E, et al. Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry. 2015;72:415–23.
Tick B, Bolton P, Happe F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57:585–95.
Deng W, Zou X, Deng H, Li J, Tang C, Wang X, et al. The Relationship Among Genetic Heritability, Environmental Effects, and Autism Spectrum Disorders: 37 Pairs of Ascertained Twin Study. J Child Neurol. 2015;30:1794–9.
Yu TW, Chahrour MH, Coulter ME, Jiralerspong S, Okamura-Ikeda K, Ataman B, et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013;77:259–73.
De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–15.
Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–21.
Zhou X, Feliciano P, Shu C, Wang T, Astrovskaya I, Hall JB, et al. Integrating de novo and inherited variants in 42,607 autism cases identifies mutations in new moderate-risk genes. Nat Genet. 2022;54:1305–19.
Fu JM, Satterstrom FK, Peng M, Brand H, Collins RL, Dong S, et al. Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nat Genet. 2022;54:1320–31.
Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51:431–44.
Heyn H. A symbiotic liaison between the genetic and epigenetic code. Front Genet. 2014;5:113.
Davis CA, Hitz BC, Sloan CA, Chan ET, Davidson JM, Gabdank I, et al. The Encyclopedia of DNA elements (ENCODE): data portal update. Nucleic Acids Res. 2018;46:D794–801.
Higgins GA, Allyn-Feuer A, Handelman S, Sadee W, Athey BD. The epigenome, 4D nucleome and next-generation neuropsychiatric pharmacogenomics. Pharmacogenomics. 2015;16:1649–69.
Amin V, Harris RA, Onuchic V, Jackson AR, Charnecki T, Paithankar S, et al. Epigenomic footprints across 111 reference epigenomes reveal tissue-specific epigenetic regulation of lincRNAs. Nat Commun. 2015;6:6370.
Chadwick LH. The NIH Roadmap Epigenomics Program data resource. Epigenomics. 2012;4:317–24.
Vlahos A, Mansell T, Saffery R, Novakovic B. Human placental methylome in the interplay of adverse placental health, environmental exposure, and pregnancy outcome. PLoS Genet. 2019;15:e1008236.
Provencal N, Binder EB. The effects of early life stress on the epigenome: From the womb to adulthood and even before. Exp Neurol. 2015;268:10–20.
Schanen NC. Epigenetics of autism spectrum disorders. Hum Mol Genet. 2006;15:R138–50.
Hogart A, Wu D, Lasalle JM, Schanen NC. The comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13. Neurobiol Dis. 2008;38:181–91.
Samaco R, Hogart A, Lasalle J. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005;14:483–92.
Hogart A, Patzel KA, LaSalle JM. Gender influences monoallelic expression of ATP10A in human brain. Hum Genet. 2008;124:235–42.
Jiang YH, Sahoo T, Michaelis RC, Bercovich D, Bressler J, Kashork CD, et al. A mixed epigenetic/genetic model for oligogenic inheritance of autism with a limited role for UBE3A. Am J Med Genet. 2004;131:1–10.
Delahanty RJ, Kang JQ, Brune CW, Kistner EO, Courchesne E, Cox NJ, et al. Maternal transmission of a rare GABRB3 signal peptide variant is associated with autism. Mol Psychiatry. 2011;16:86–96.
Nagarajan RP, Hogart AR, Gwye Y, Martin MR, Lasalle JM. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1:172–82.
Nagarajan RP, Patzel KA, Martin M, Yasui DH, Swanberg SE, Hertz-Picciotto I, et al. Mecp2 promoter methylation and X chromosome inactivation in autism. Autism Res. 2008;1:169–78.
Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62.
Furukawa R, Hachiya T, Ohmomo H, Shiwa Y, Ono K, Suzuki S, et al. Intraindividual dynamics of transcriptome and genome-wide stability of DNA methylation. Sci Rep. 2016;6:26424.
Liang G, Weisenberger DJ. DNA methylation aberrancies as a guide for surveillance and treatment of human cancers. Epigenetics. 2017;12:416–32.
Teh AL, Pan H, Chen L, Ong ML, Dogra S, Wong J, et al. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Res. 2014;24:1064–74.
Cheung WA, Shao X, Morin A, Siroux V, Kwan T, Ge B, et al. Functional variation in allelic methylomes underscores a strong genetic contribution and reveals novel epigenetic alterations in the human epigenome. Genome Biol. 2017;18:50.
Czamara D, Eraslan G, Page CM, Lahti J, Lahti-Pulkkinen M, Hamalainen E, et al. Integrated analysis of environmental and genetic influences on cord blood DNA methylation in new-borns. Nat Commun. 2019;10:2548.
Garg P, Joshi RS, Watson C, Sharp AJ. A survey of inter-individual variation in DNA methylation identifies environmentally responsive co-regulated networks of epigenetic variation in the human genome. PLoS Genet. 2018;14:e1007707.
Gu J, Stevens M, Xing X, Li D, Zhang B, Payton JE, et al. Mapping of variable DNA methylation across multiple cell types defines a dynamic regulatory landscape of the human genome. G3 (Bethesda). 2016;6:973–86.
Gunasekara CJ, Scott CA, Laritsky E, Baker MS, MacKay H, Duryea JD, et al. A genomic atlas of systemic interindividual epigenetic variation in humans. Genome Biol. 2019;20:105.
Li S, Ye Z, Mather KA, Nguyen TL, Dite GS, Armstrong NJ, et al. Early life affects late-life health through determining DNA methylation across the lifespan: A twin study. EBioMedicine. 2022;77:103927.
LaSalle J. Autism genes keep turning up chromatin. OA Autism. 2013;1:14.
Aref-Eshghi E, Schenkel LC, Lin H, Skinner C, Ainsworth P, Pare G, et al. Clinical validation of a genome-wide DNA methylation assay for molecular diagnosis of imprinting disorders. J Mol Diagn. 2017;19:848–56.
Aref-Eshghi E, Rodenhiser DI, Schenkel LC, Lin H, Skinner C, Ainsworth P, et al. Genomic DNA methylation signatures enable concurrent diagnosis and clinical genetic variant classification in neurodevelopmental syndromes. Am J Hum Genet. 2018;102:156–74.
Aref-Eshghi E, Kerkhof J, Pedro VP, Groupe DIF, Barat-Houari M, Ruiz-Pallares N, et al. Evaluation of DNA methylation episignatures for diagnosis and phenotype correlations in 42 mendelian neurodevelopmental disorders. Am J Hum Genet. 2020;106:356–70.
Kerkhof J, Squeo GM, McConkey H, Levy MA, Piemontese MR, Castori M, et al. DNA methylation episignature testing improves molecular diagnosis of Mendelian chromatinopathies. Genet Med. 2022;24:51–60.
Foroutan A, Haghshenas S, Bhai P, Levy MA, Kerkhof J, McConkey H, et al. Clinical utility of a unique genome-wide DNA methylation signature for KMT2A-related syndrome. Int J Mol Sci. 2022;23:3.
Dunaway KW, Islam MS, Coulson RL, Lopez SJ, Vogel Ciernia A, Chu RG, et al. Cumulative impact of polychlorinated biphenyl and large chromosomal duplications on DNA methylation, chromatin, and expression of autism candidate genes. Cell Rep. 2016;17:3035–48.
Wong CCY, Smith RG, Hannon E, Ramaswami G, Parikshak NN, Assary E, et al. Genome-wide DNA methylation profiling identifies convergent molecular signatures associated with idiopathic and syndromic autism in post-mortem human brain tissue. Hum Mol Genet. 2019;28:2201–11.
Siu MT, Butcher DT, Turinsky AL, Cytrynbaum C, Stavropoulos DJ, Walker S, et al. Functional DNA methylation signatures for autism spectrum disorder genomic risk loci: 16p11.2 deletions and CHD8 variants. Clin Epigenetics. 2019;11:103.
Lomvardas S, Maniatis T. Histone and DNA modifications as regulators of neuronal development and function. Cold Spring Harb Perspect Biol. 2016;8:7.
Gallegos DA, Chan U, Chen LF, West AE. Chromatin regulation of neuronal maturation and plasticity. Trends Neurosci. 2018;41:311–24.
Williams LA, LaSalle JM. Future prospects for epigenetics in autism spectrum disorder. Mol Diagn Ther. 2022;26:569–79.
Murphy PJ, Wu SF, James CR, Wike CL, Cairns BR. Placeholder nucleosomes underlie germline-to-embryo DNA methylation reprogramming. Cell. 2018;172:993–1006 e13.
Kuroda MI, Kang H, De S, Kassis JA. Dynamic competition of polycomb and trithorax in transcriptional programming. Annu Rev Biochem. 2020;89:235–53.
Ku M, Jaffe JD, Koche RP, Rheinbay E, Endoh M, Koseki H, et al. H2A.Z landscapes and dual modifications in pluripotent and multipotent stem cells underlie complex genome regulatory functions. Genome Biol. 2012;13:R85.
Sendzikaite G, Hanna CW, Stewart-Morgan KR, Ivanova E, Kelsey G. A DNMT3A PWWP mutation leads to methylation of bivalent chromatin and growth retardation in mice. Nat Commun. 2019;10:1884.
Gu T, Hao D, Woo J, Huang TW, Guo L, Lin X, et al. The disordered N-terminal domain of DNMT3A recognizes H2AK119ub and is required for postnatal development. Nat Genet. 2022;54:625–36.
Li J, Pinto-Duarte A, Zander M, Cuoco MS, Lai CY, Osteen J, et al. Dnmt3a knockout in excitatory neurons impairs postnatal synapse maturation and increases the repressive histone modification H3K27me3. Elife (Cambridge). 2022;11:e66909.
LaSalle JM, Reiter LT, Chamberlain SJ. Epigenetic regulation of UBE3A and roles in human neurodevelopmental disorders. Epigenomics. 2015.
Hogart A, Wu D, LaSalle JM, Schanen NC. The comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13. Neurobiol Dis. 2010;38:181–91.
Cotton AM, Lam L, Affleck JG, Wilson IM, Penaherrera MS, McFadden DE, et al. Chromosome-wide DNA methylation analysis predicts human tissue-specific X inactivation. Hum Genet. 2011;130:187–201.
Vogel Ciernia A, Laufer BI, Hwang H, Dunaway KW, Mordaunt CE, Coulson RL, et al. Epigenomic convergence of neural-immune risk factors in neurodevelopmental disorder cortex. Cereb Cortex. 2020;30:640–55.
Meguro-Horike M, Yasui DH, Powell W, Schroeder DI, Oshimura M, Lasalle JM, et al. Neuron-specific impairment of inter-chromosomal pairing and transcription in a novel model of human 15q-duplication syndrome. Hum Mol Genet. 2011;20:3798–810.
Mitchell MM, Woods R, Chi LH, Schmidt RJ, Pessah IN, Kostyniak PJ, et al. Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ Mol Mutagenesis. 2012;53:589–98.
Zaaroor-Regev D, de Bie P, Scheffner M, Noy T, Shemer R, Heled M, et al. Regulation of the polycomb protein Ring1B by self-ubiquitination or by E6-AP may have implications to the pathogenesis of Angelman syndrome. Proc Natl Acad Sci USA. 2010;107:6788–93.
Hahn LJ, Hamrick LM, Kelleher BL, Roberts JE. Autism spectrum disorder-associated behaviour in infants with down syndrome. J Health Sci Educ. 2020;4:2.
Letourneau A, Santoni FA, Bonilla X, Sailani MR, Gonzalez D, Kind J, et al. Domains of genome-wide gene expression dysregulation in Down’s syndrome. Nature. 2014;508:345–50.
Laufer BI, Hwang H, Vogel Ciernia A, Mordaunt CE, LaSalle JM. Whole genome bisulfite sequencing of Down syndrome brain reveals regional DNA hypermethylation and novel disorder insights. Epigenetics. 2019;14:672–84.
Laufer BI, Hwang H, Jianu JM, Mordaunt CE, Korf IF, Hertz-Picciotto I, et al. Low-pass whole genome bisulfite sequencing of neonatal dried blood spots identifies a role for RUNX1 in Down syndrome DNA methylation profiles. Hum Mol Genet. 2021;29:3465–76.
Mao R, Zielke CL, Zielke HR, Pevsner J. Global up-regulation of chromosome 21 gene expression in the developing Down syndrome brain. Genomics. 2003;81:457–67.
Bacalini MG, Gentilini D, Boattini A, Giampieri E, Pirazzini C, Giuliani C, et al. Identification of a DNA methylation signature in blood cells from persons with Down Syndrome. Aging (Albany NY). 2015;7:82–96.
Jones MJ, Farre P, McEwen LM, Macisaac JL, Watt K, Neumann SM, et al. Distinct DNA methylation patterns of cognitive impairment and trisomy 21 in Down syndrome. BMC Med Genomics. 2013;6:58.
Mevel R, Draper JE, Lie ALM, Kouskoff V, Lacaud G. RUNX transcription factors: orchestrators of development. Development. 2019;146:17.
Gao L, Tober J, Gao P, Chen C, Tan K, Speck NA. RUNX1 and the endothelial origin of blood. Exp Hematol. 2018;68:2–9.
Suzuki T, Shimizu Y, Furuhata E, Maeda S, Kishima M, Nishimura H, et al. RUNX1 regulates site specificity of DNA demethylation by recruitment of DNA demethylation machineries in hematopoietic cells. Blood Adv. 2017;1:1699–711.
Lu J, McCarter M, Lian G, Esposito G, Capoccia E, Delli-Bovi LC, et al. Global hypermethylation in fetal cortex of Down syndrome due to DNMT3L overexpression. Hum Mol Genet. 2016;25:1714–27.
Laufer BI, Gomez JA, Jianu JM, LaSalle JM. Stable DNMT3L overexpression in SH-SY5Y neurons recreates a facet of the genome-wide Down syndrome DNA methylation signature. Epigenetics Chromatin. 2021;14:13.
Chedin F, Lieber MR, Hsieh CL. The DNA methyltransferase-like protein DNMT3L stimulates de novo methylation by Dnmt3a. Proc Natl Acad Sci USA. 2002;99:16916–21.
Chen ZX, Mann JR, Hsieh CL, Riggs AD, Chedin F. Physical and functional interactions between the human DNMT3L protein and members of the de novo methyltransferase family. J Cell Biochem. 2005;95:902–17.
Hwang S, Cavaliere P, Li R, Zhu LJ, Dephoure N, Torres EM. Consequences of aneuploidy in human fibroblasts with trisomy 21. Proc Natl Acad Sci USA. 2021;118:6.
Nardone S, Sams DS, Zito A, Reuveni E, Elliott E. Dysregulation of cortical neuron DNA methylation profile in autism spectrum disorder. Cereb Cortex. 2017;27:5739–54.
Nardone S, Sams DS, Reuveni E, Getselter D, Oron O, Karpuj M, et al. DNA methylation analysis of the autistic brain reveals multiple dysregulated biological pathways. Transl Psychiatry. 2014;4:e433.
Ladd-Acosta C, Hansen KD, Briem E, Fallin MD, Kaufmann WE, Feinberg AP. Common DNA methylation alterations in multiple brain regions in autism. Mol Psychiatry. 2014;19:862–71.
Hannon E, Lunnon K, Schalkwyk L, Mill J. Interindividual methylomic variation across blood, cortex, and cerebellum: implications for epigenetic studies of neurological and neuropsychiatric phenotypes. Epigenetics. 2015;10:1024–32.
Andrews SV, Sheppard B, Windham GC, Schieve LA, Schendel DE, Croen LA, et al. Case-control meta-analysis of blood DNA methylation and autism spectrum disorder. Mol Autism. 2018;9:40.
Wong CC, Meaburn EL, Ronald A, Price TS, Jeffries AR, Schalkwyk LC, et al. Methylomic analysis of monozygotic twins discordant for autism spectrum disorder and related behavioural traits. Mol Psychiatry. 2014;19:495–503.
Berko ER, Suzuki M, Beren F, Lemetre C, Alaimo CM, Calder RB, et al. Mosaic epigenetic dysregulation of ectodermal cells in autism spectrum disorder. PLoS Genet. 2014;10:e1004402.
Hannon E, Schendel D, Ladd-Acosta C, Grove J, I P-BASDG, Hansen CS, et al. Elevated polygenic burden for autism is associated with differential DNA methylation at birth. Genome Med. 2018;10:19.
Hertz-Picciotto I, Schmidt RJ, Walker CK, Bennett DH, Oliver M, Shedd-Wise KM, et al. A prospective study of environmental exposures and early biomarkers in autism spectrum disorder: design, protocols, and preliminary data from the MARBLES study. Environ Health Perspect. 2018;126:117004.
Newschaffer CJ, Croen LA, Fallin MD, Hertz-Picciotto I, Nguyen DV, Lee NL, et al. Infant siblings and the investigation of autism risk factors. J Neurodev Disord. 2012;4:7.
Mordaunt CE, Jianu JM, Laufer BI, Zhu Y, Hwang H, Dunaway KW, et al. Cord blood DNA methylome in newborns later diagnosed with autism spectrum disorder reflects early dysregulation of neurodevelopmental and X-linked genes. Genome Med. 2020;12:88.
Zhu Y, Gomez JA, Laufer BI, Mordaunt CE, Mouat JS, Soto DC, et al. Placental methylome reveals a 22q13.33 brain regulatory gene locus associated with autism. Genome Biol. 2022;23:46.
Dou JF, Middleton LYM, Zhu Y, Benke KS, Feinberg JI, Croen LA, et al. Prenatal vitamin intake in first month of pregnancy and DNA methylation in cord blood and placenta in two prospective cohorts. Epigenetics Chromatin. 2022;15:28.
Zhu Y, Mordaunt CE, Yasui DH, Marathe R, Coulson RL, Dunaway KW, et al. Placental DNA methylation levels at CYP2E1 and IRS2 are associated with child outcome in a prospective autism study. Hum Mol Genet. 2019;28:2659–74.
Crary-Dooley FK, Tam ME, Dunaway KW, Hertz-Picciotto I, Schmidt RJ, LaSalle JM. A comparison of existing global DNA methylation assays to low-coverage whole-genome bisulfite sequencing for epidemiological studies. Epigenetics. 2017;12:206–14.
Schroeder DI, Schmidt RJ, Crary-Dooley FK, Walker CK, Ozonoff S, Tancredi DJ, et al. Placental methylome analysis from a prospective autism study. Mol Autism. 2016;7:51.
Bakulski KM, Dou JF, Feinberg JI, Aung MT, Ladd-Acosta C, Volk HE, et al. Autism-associated DNA methylation at birth from multiple tissues is enriched for autism genes in the early autism risk longitudinal investigation. Front Mol Neurosci. 2021;14:775390.
Bakulski KM, Dou JF, Feinberg JI, Brieger KK, Croen LA, Hertz-Picciotto I, et al. Prenatal multivitamin use and MTHFR genotype are associated with newborn cord blood DNA methylation. Int J Environ Res Public Health. 2020;17:24.
Feinberg JI, Bakulski KM, Jaffe AE, Tryggvadottir R, Brown SC, Goldman LR, et al. Paternal sperm DNA methylation associated with early signs of autism risk in an autism-enriched cohort. Int J Epidemiol. 2015;44:1199–210.
Schmidt RJ. Maternal folic acid supplements associated with reduced autism risk in the child. Evid Based Med. 2013;18:e53.
Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology. 2011;22:476–85.
Steiner P. Brain fuel utilization in the developing brain. Ann Nutr Metab. 2019;75:8–18.
Vazin T, Freed WJ. Human embryonic stem cells: derivation, culture, and differentiation: a review. Restor Neurol Neurosci. 2010;28:589–603.
Mordaunt CE, Park BY, Bakulski KM, Feinberg JI, Croen LA, Ladd-Acosta C, et al. A meta-analysis of two high-risk prospective cohort studies reveals autism-specific transcriptional changes to chromatin, autoimmune, and environmental response genes in umbilical cord blood. Mol Autism. 2019;10:36.
Vallot C, Ouimette JF, Makhlouf M, Feraud O, Pontis J, Come J, et al. Erosion of X chromosome inactivation in human pluripotent cells initiates with XACT coating and depends on a specific heterochromatin landscape. Cell Stem Cell. 2015;16:533–46.
Vallot C, Patrat C, Collier AJ, Huret C, Casanova M, Liyakat Ali TM, et al. XACT noncoding RNA competes with XIST in the control of X chromosome activity during human early development. Cell Stem Cell. 2017;20:102–11.
Kadam SD, Sullivan BJ, Goyal A, Blue ME, Smith-Hicks C. Rett syndrome and CDKL5 deficiency disorder: from bench to clinic. Int J Mol Sci. 2019;20:20.
Deardorff MA, Bando M, Nakato R, Watrin E, Itoh T, Minamino M, et al. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature. 2012;489:313–7.
Ozonoff S, Iosif AM. Changing conceptualizations of regression: What prospective studies reveal about the onset of autism spectrum disorder. Neurosci Biobehav Rev. 2019;100:296–304.
Pierce K, Glatt SJ, Liptak GS, McIntyre LL. The power and promise of identifying autism early: insights from the search for clinical and biological markers. Ann Clin Psychiatry. 2009;21:132–47.
Frye RE, Vassall S, Kaur G, Lewis C, Karim M, Rossignol D. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7:792.
Tassone F. Newborn screening for fragile X syndrome. JAMA Neurol. 2014;71:355–9.
Bailey DB Jr, Skinner D, Warren SF. Newborn screening for developmental disabilities: reframing presumptive benefit. Am J Public Health. 2005;95:1889–93.
Hoyt SJ, Storer JM, Hartley GA, Grady PGS, Gershman A, de Lima LG, et al. From telomere to telomere: The transcriptional and epigenetic state of human repeat elements. Science. 2022;376:eabk3112.
Nurk S, Koren S, Rhie A, Rautiainen M, Bzikadze AV, Mikheenko A, et al. The complete sequence of a human genome. Science. 2022;376:44–53.
Gershman A, Sauria MEG, Guitart X, Vollger MR, Hook PW, Hoyt SJ, et al. Epigenetic patterns in a complete human genome. Science. 2022;376:eabj5089.
Luo C, Liu H, Xie F, Armand EJ, Siletti K, Bakken TE, et al. Single nucleus multi-omics identifies human cortical cell regulatory genome diversity. Cell Genom. 2022;2:3.
Liu H, Zhou J, Tian W, Luo C, Bartlett A, Aldridge A, et al. DNA methylation atlas of the mouse brain at single-cell resolution. Nature. 2021;598:120–8.
Armand EJ, Li J, Xie F, Luo C, Mukamel EA. Single-cell sequencing of brain cell transcriptomes and epigenomes. Neuron. 2021;109:11–26.
Chen Y, Song J, Ruan Q, Zeng X, Wu L, Cai L, et al. Single-cell sequencing methodologies: from transcriptome to multi-dimensional measurement. Small Methods. 2021;5:e2100111.
Buckley JP, Barrett ES, Beamer PI, Bennett DH, Bloom MS, Fennell TR, et al. Opportunities for evaluating chemical exposures and child health in the United States: the Environmental influences on Child Health Outcomes (ECHO) Program. J Expo Sci Environ Epidemiol. 2020;30:397–419.
Breton CV, Landon R, Kahn LG, Enlow MB, Peterson AK, Bastain T, et al. Exploring the evidence for epigenetic regulation of environmental influences on child health across generations. Commun Biol. 2021;4:769.
Huang Y, Sun X, Jiang H, Yu S, Robins C, Armstrong MJ, et al. A machine learning approach to brain epigenetic analysis reveals kinases associated with Alzheimer’s disease. Nat Commun. 2021;12:4472.
Rauschert S, Melton PE, Heiskala A, Karhunen V, Burdge G, Craig JM, et al. Machine learning-based DNA methylation score for fetal exposure to maternal smoking: development and validation in samples collected from adolescents and adults. Environ Health Perspect. 2020;128:97003.
Brasil S, Neves CJ, Rijoff T, Falcao M, Valadao G, Videira PA, et al. Artificial intelligence in epigenetic studies: shedding light on rare diseases. Front Mol Biosci. 2021;8:648012.
Lo YM, Patel P, Wainscoat JS, Sampietro M, Gillmer MD, Fleming KA. Prenatal sex determination by DNA amplification from maternal peripheral blood. Lancet. 1989;2:1363–5.
Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–7.
Masuzaki H, Miura K, Yoshiura KI, Yoshimura S, Niikawa N, Ishimaru T. Detection of cell free placental DNA in maternal plasma: direct evidence from three cases of confined placental mosaicism. J Med Genet. 2004;41:289–92.
Alberry M, Maddocks D, Jones M, Abdel Hadi M, Abdel-Fattah S, Avent N, et al. Free fetal DNA in maternal plasma in anembryonic pregnancies: confirmation that the origin is the trophoblast. Prenat Diagn. 2007;27:415–8.
Chen C, Cram DS, Xie F, Wang P, Xu X, Li H, et al. A pregnancy with discordant fetal and placental chromosome 18 aneuploidies revealed by invasive and noninvasive prenatal diagnosis. Reprod Biomed Online. 2014;29:136–9.
Sun K, Jiang P, Chan KC, Wong J, Cheng YK, Liang RH, et al. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc Natl Acad Sci USA. 2015;112:E5503–12.
Jensen TJ, Kim SK, Zhu Z, Chin C, Gebhard C, Lu T, et al. Whole genome bisulfite sequencing of cell-free DNA and its cellular contributors uncovers placenta hypomethylated domains. Genome Biol. 2015;16:78.
Schroeder DI, Blair JD, Lott P, Yu HOK, Hong D, Crary F, et al. The human placenta methylome. Proc Natl Acad Sci USA. 2013;110:6037–42.
Schroeder DI, Jayashankar K, Douglas KC, Thirkill TL, York D, Dickinson PJ, et al. Early developmental and evolutionary origins of gene body DNA methylation patterns in mammalian placentas. PLoS Genet. 2015;11:e1005442.
Barefoot ME, Loyfer N, Kiliti AJ, McDeed APT, Kaplan T, Wellstein A. Detection of cell types contributing to cancer from circulating, cell-free methylated DNA. Front Genet. 2021;12:671057.
Locke WJ, Guanzon D, Ma C, Liew YJ, Duesing KR, Fung KYC, et al. DNA methylation cancer biomarkers: translation to the clinic. Front Genet. 2019;10:1150.
Vrba L, Futscher BW, Oshiro M, Watts GS, Menashi E, Hu C, et al. Liquid biopsy, using a novel DNA methylation signature, distinguishes pancreatic adenocarcinoma from benign pancreatic disease. Clin Epigenetics. 2022;14:28.
Garrido N, Cruz F, Egea RR, Simon C, Sadler-Riggleman I, Beck D, et al. Sperm DNA methylation epimutation biomarker for paternal offspring autism susceptibility. Clin Epigenetics. 2021;13:6.
Sun W, Poschmann J, Cruz-Herrera Del Rosario R, Parikshak NN, Hajan HS, Kumar V, et al. Histone acetylome-wide association study of autism spectrum disorder. Cell. 2016;167:1385–97 e11.
Parikshak NN, Swarup V, Belgard TG, Irimia M, Ramaswami G, Gandal MJ, et al. Genome-wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature. 2016;advance online publication.
Chen HI, Song H, Ming GL. Applications of human brain organoids to clinical problems. Dev Dyn. 2019;248:53–64.
Bhattacharya A, Choi WWY, Muffat J, Li Y. Modeling developmental brain diseases using human pluripotent stem cells-derived brain organoids - progress and perspective. J Mol Biol. 2022;434:167386.
Luo C, Keown CL, Kurihara L, Zhou J, He Y, Li J, et al. Single-cell methylomes identify neuronal subtypes and regulatory elements in mammalian cortex. Science. 2017;357:600–4.
Luo C, Rivkin A, Zhou J, Sandoval JP, Kurihara L, Lucero J, et al. Robust single-cell DNA methylome profiling with snmC-seq2. Nat Commun. 2018;9:3824.
Medvedeva YA, Lennartsson A, Ehsani R, Kulakovskiy IV, Vorontsov IE, Panahandeh P, et al. EpiFactors: a comprehensive database of human epigenetic factors and complexes. Database (Oxf). 2015;2015:bav067.
Abrahams BS, Arking DE, Campbell DB, Mefford HC, Morrow EM, Weiss LA, et al. SFARI Gene 2.0: a community-driven knowledgebase for the autism spectrum disorders (ASDs). Mol Autism. 2013;4:36.
Morrison J, Koeman JM, Johnson BK, Foy KK, Beddows I, Zhou W, et al. Evaluation of whole-genome DNA methylation sequencing library preparation protocols. Epigenetics Chromatin. 2021;14:28.
Mordaunt CE, Mouat JS, Schmidt RJ, LaSalle JM. Comethyl: a network-based methylome approach to investigate the multivariate nature of health and disease. Brief Bioinform. 2022;23:2.
Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–22.
Sun R, Zhu P. Advances in measuring DNA methylation. Blood Sci. 2022;4:8–15.
Senol Cali D, Kim JS, Ghose S, Alkan C, Mutlu O. Nanopore sequencing technology and tools for genome assembly: computational analysis of the current state, bottlenecks and future directions. Brief Bioinform. 2019;20:1542–59.
Funding
This ongoing research has been supported by NIH, R01 ES029213.
Ethics declarations
Competing interest
The author has a pending patent application on “Methods and Compositions for Determining Risk of Autism Spectrum Disorder.”
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
LaSalle, J.M. Epigenomic signatures reveal mechanistic clues and predictive markers for autism spectrum disorder. Mol Psychiatry 28, 1890–1901 (2023). https://doi.org/10.1038/s41380-022-01917-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01917-9
This article is cited by
-
Impact of IDO activation and alterations in the kynurenine pathway on hyperserotonemia, NAD+ production, and AhR activation in autism spectrum disorder
Translational Psychiatry (2023)
-
Psychosis and autism spectrum disorder: a special issue of Molecular Psychiatry
Molecular Psychiatry (2023)
-
Interaction of the pre- and postnatal environment in the maternal immune activation model
Discover Mental Health (2023)