Abstract
Classification of the putative flat preneoplastic and neoplastic lesions of the urothelium with features subthreshold for urothelial carcinoma in situ remains a challenging, indeed, vexing problem in diagnostic surgical pathology. This area, subtending lesions including flat urothelial hyperplasia, urothelial dysplasia, and atypia of unknown significance, has struggled under evolving classifications, changing criteria, and limited clinical actionability, all confounded by the recognized lack of diagnostic reproducibility. Herein, we review the state of the literature around these lesions, reviewing contemporary criteria and definitions, assessing the arguments in favor and against of retaining hyperplasia, dysplasia, and atypia of unknown significance as diagnostic entities. We clarify the intent of the original definitions for dysplasia as a lesion felt to be clearly neoplastic but with morphologic features that fall short of the threshold of urothelial carcinoma in situ. While several pathologists, including some experts in the field, conflate the term dysplasia with urothelial atypia of unknown significance, the latter is defined as a descriptive diagnosis term to express diagnostic uncertainty of a lesion of whether it is clearly reactive or neoplastic. Both molecular studies and clinical needs are considered, as we outline our approach on diagnosing each of these lesions in clinical practice. Recommendations are made to guide consistency and interoperability in future scholarship, and the place of these lesions in context of evolving trends in the field is considered.
Similar content being viewed by others
Introduction
From the pathobiological, diagnostic, and management perspectives, non-invasive urothelial carcinomas are divided into carcinoma in situ (CIS) and papillary urothelial carcinoma (PUC)1. Bladder carcinogenesis has been conceptualized to evolve via two pathways originating from these two intraurothelial carcinomas, through putative premalignant lesions in urothelial dysplasia for CIS, and from hyperplasia for PUC2,3,4. Hyperplasia is traditionally divided into flat hyperplasia and the inaccurately labeled “papillary” (pseudopapillary tenting) urothelial hyperplasia (PUH)5. The term hyperplasia generally refers to flat hyperplasia, as will be the use of the term in this review. For this discussion, hyperplasia and dysplasia are grouped with CIS as “flat” intraurothelial lesions, to discriminate them from the categories of papillary urothelial neoplasms and PUH, although these flat lesions may not appear strictly flattened on their cystoscopic and histologic appearances. While having precancerous lesions for urothelial carcinoma is a logical concept (as in other carcinomas), establishing these flat intraurothelial lesions as diagnosable, reproducible, and clinically valid entities is fraught with challenges, and their existence as distinct entities has been heavily debated. This is compounded by the limited amount of data published on these entities. Another previously introduced flat lesion in urothelial atypia of unknown significance (AUS) is also disputed5.
There is recent enhancement in our understanding of the genomics of urothelial mucosa including field effects, clonal expansion in normal-appearing urothelium, and malignant transformation6,7,8. Further, recent developments in the urological management of superficial bladder cancers such as enhanced cystoscopic techniques (e.g., narrow-band imaging or fluorescent cystoscopy), follow-up surveillance protocols, and standardization of repeat “restaging” transurethral resections (TURs) may be leading to increased sampling of bladder mucosa subject to the field effects of urothelial carcinogenic influence9,10,11. For these reasons, our approach to early incipient lesions, subtle residual neoplasms, and related changes, generally, these challenging flat intraurothelial lesions, need to be re-appraised.
Recent experience of ours with a survey in this area raised our awareness of marked variation in diagnostic approach to one of the putative entities in this flat intraurothelial lesion category, that of dysplasia, an archetype of the challenges in reproducibility and supporting literature and data12. For example, though a majority of urologic pathologist survey respondents reported that they had diagnosed dysplasia in the prior 10 years, the circumstances under which the term had been used was widely variable, including only in de novo cases in a minority (14%) versus a small majority (61%) who would only use the term after prior diagnosis of a urothelial neoplasm, or a remaining minority (25%) who diagnose dysplasia in either setting. Significant majorities of respondents (83%) endorsed the concept as relevant to the pathogenesis of urothelial neoplasms, though only a minority could identify an important primary source supporting dysplasia as an entity from recent years. Respondents were split nearly equally in their recommendations for (56%) or against (44%) continued use of the term, dysplasia, in diagnostic practice.
Building from these unresolved survey observations, and with an eye to furthering academic discussions, research, and refinements to inform future tumor classifications, this review summarizes the origin and context of theses flat intraurothelial lesions, including the arguments in favor and against whether these lesions should exist as distinct diagnostic entities. We assess the published evidence, consider clinical needs, and report our summary opinions, approaches, and recommendations for the diagnosis of these lesions in routine practice. In short, for each of these three intraepithelial lesions, hyperplasia, dysplasia, and AUS, in turn, we review concept and definition of each, delineate the “pro” and “con” positions for and against their existence, and describe our recommendations for routine practice. Assessment of each of these concepts follows, as well as final remarks hoping to make useful suggestions on how to standardize future scholarly inquiry into this area.
Historical background
Since the remarkable discovery of urothelial CIS in the bladder by Koss about 70 years ago, there has been frequent modification in the categories of flat intraurothelial lesions (Table 1)13. In 1952, Melicow and Hollowell14 reported the presence of hyperplasia, metaplasia, papillary excrescences, and Bowenoid changes (intraurothelial carcinoma) in grossly normal-appearing bladder mucosa. In 1975, Koss15 proposed a 3-tiered system to classify flat intraurothelial lesions for the 2nd series of the Armed Forces Institute of Pathology (AFIP) fascicle on “Tumors of the Urinary Bladder” that included non-neoplastic (hyperplasia), atypical (atypical hyperplasia) and malignant (CIS) lesions. In 1982, Nagy et al.16 proposed an expanded dysplasia categories of mild, moderate, and severe in addition to CIS. In 1984 however, Mostofi and Sesterhenn17 argued that dysplasia should be considered as CIS since 30% of patients with moderate dysplasia developed papillary or invasive urothelial carcinoma and recommended replacing dysplasia with categories of CIS grades I, II and III. In a workshop for CIS of bladder in 1984, the categories were divided into slight, moderate, and marked dysplasia/CIS and were also referred as intraurothelial neoplasia 1, 2, and 3, respectively18. In 1986, Murphy19 proposed a simplified division in dysplasia (mild dysplasia) and CIS (moderate and severe dysplasia) that was later incorporated in the 1994 3rd AFIP fascicle on “Tumors of the Kidney, Urinary Bladder and Other Related Urinary Structures”20.
In 1996, Amin et al.21 laid down the categories that would become the foundation for future classification of flat intraurothelial lesions, which included reactive urothelial atypia, AUS, dysplasia and CIS. These four categories were recommended by the 1998 World Health Organization (WHO)/International Society of Urological Pathology (ISUP) consensus conference, with dysplasia being synonymous with low-grade intraurothelial neoplasia and CIS synonymous with high-grade intraurothelial neoplasia5. Further, hyperplasia was subdivided into flat and papillary urothelial hyperplasia (PUH). In the 2004 WHO blue book, references were made to these different categories with chapters devoted for hyperplasia and dysplasia, although these were not formally included in the tumor classification; interestingly, hyperplasia used as an overarching term was recognized to have flat and/or papillary architecture22.
In 2011, for practicality in diagnosis and to enhance their reproducibility, experts participating in the International Consultation on Urological Disease (ICUD)-European Association of Urology (EAU) Consultation on Bladder Cancer, proposed a simplified morphologic analogy of flat and papillary lesions (Table 2), with the cytomorphologic and pattern alterations in hyperplasia, dysplasia and CIS analogous to that in papillary urothelial neoplasm of low malignant potential (PUNLMP), low-grade PUC, and high-grade PUC, respectively23,24. Subsequently, in the 2016 WHO blue book, dysplasia was officially listed as a category, while hyperplasia was changed to urothelial proliferation of undetermined malignant potential (UPUMP) and added in the tumor classification1. In part, the rationale for a UPUMP category, including flat hyperplasia and PUH, was preferred for the 2016 WHO blue book because such lesions are not purely a reactive/reversible process (i.e., hyperplasia) and because subsets are believed to encompass preneoplastic changes. Most recently, in the 2021 white paper by the Genitourinary Pathology Society (GUPS) on Classification and Grading of Flat and Papillary Urothelial Neoplasia, flat hyperplasia was recommended to be superseded by the new term atypical urothelial proliferation (AUP)-flat while PUH should be replaced by AUP-tented, essentially forgoing the term UPUMP25.
With this historic perspective as guide, further evolution of these terms and concepts is anticipated from future WHO classifications and key diagnostic references, not least due to the ongoing limited data and conflicting priorities. For these lesions, it remains challenging to: (1) provide terms for histologic patterns seen by pathologists, while (2) still promulgating categories with at least some meaningful clinical/management implications, and yet desiring to (3) maintain categories with bona fide relation to scientific concepts believed to underlie the pathways of tumorigenesis in urothelial neoplasms.
Flat urothelial hyperplasia
Contemporary definitions and criteria
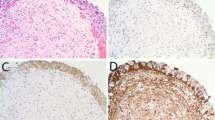
Hyperplasia is defined as marked thickening of urothelial cell layers that entirely lack cytological atypia (Fig. 1)5,22. Although in the past, specific cut-offs (>7 cell layers) were provided, generally, counting the number of cell layers is not recommended as thickening is typically marked; there is usually a component of increased cellularity per unit area within the thickened urothelium. As detailed above, the hyperplasia concept became included in the 2016 WHO blue book as UPUMP1, and the 2021 GUPS white paper recommended separating flat from papillary hyperplasia, with the former to be designated as AUP-flat25.
Assessment of value as a diagnostic entity
Arguments in favor of retaining hyperplasia
Simplicity in diagnosis
Identification of hyperplasia relies on the presence of markedly increased non-atypical urothelial cell layers5,22. Thus, diagnosis of hyperplasia is not expected to suffer as high intra- and interobserver variations observed in the other controversial flat intraurothelial lesions. The simple morphologic criteria and relative ease in identification should encourage documentation of hyperplasia in routine practice.
Presence of molecular alterations seen in carcinoma
Deletion of chromosome 9 is present in 37–70% of hyperplasia26,27,28. Other chromosomal losses, gains and amplification were also detected in hyperplasia27,28,29. FGFR3 mutation, common in low-grade PUC, is detected in 23% of hyperplasia, including those with no prior or concomitant urothelial neoplasm (de novo hyperplasia)28. Polysomy of at least one chromosome is detected in 17% of hyperplasia cases, compared to 68% in CIS30. A recent whole organ mapping of bladders with cancer detected TERT mutations in 33% of hyperplasia compared to 46% in CIS and 100% of invasive cancer31. Presence of these alterations suggests that at least a significant subset of hyperplasia is preneoplastic or neoplasia-associated.
Correspondence to clinical lesions and symptoms
Hyperplasia may present as a discernable bladder mucosal bump that may draw the attention of the examining urologist on cystoscopy with concern for a small papillary tumor. This is likely not an uncommon scenario, since thickening in hyperplasia is often pronounced; obvious enough to be sampled on cystoscopy. Furthermore, hyperplasias are frequently detected as “false positive” lesion in photodynamic diagnosis27,32. A recent study on hyperplasia reported as UPUMP that included de novo lesions (38%) and those with flat architecture (69% of de novo), showed that most de novo hyperplasias had associated symptoms including 61% of patients presenting with hematuria33. Reporting hyperplasia thus provides a pathologic diagnosis, corresponding to and explaining a cystoscopic abnormality and possible clinical symptoms.
Adjacency to prior endoscopic resection sites
Repeat TUR is now highly encouraged after diagnosis of high-grade Ta and T1 PUC, and standard in the absence of muscularis propria in the specimen9,10,11. Repeat TUR targets (at least) the prior TUR site and presence of hyperplasia at the edge of the ulcer likely represents either the continuity or “shoulder” of the initially resected PUC or associated precursor; alternatively, it may also represent a reactive process in response to the prior procedure. Reporting hyperplasia in follow-up TUR thus informs that the surrounding periphery of the prior tumor is sampled, harbors abnormality (i.e., hyperplasia), and if purely the change, no longer contains residual PUC.
Potential to progress to neoplasia
Both flat hyperplasia and PUH are suggested to have association with or may represent precursors to early non-invasive low-grade neoplasia, with PUH having a closer connection33,34. The study by Lowenthal et al.33 showed subsequent development of urothelial neoplasia in 40% of bladders with UPUMP (hyperplasia and PUH), including in 16% with flat architecture (flat hyperplasia), suggesting that hyperplasia can progress to neoplasia33. However, one can argue that apparent “progression” was perhaps driven by the antecedent (to the UPUMP) neoplasia that was present in many of these cases (i.e., recurrence) rather than as progression of UPUMP per se. Nonetheless, progression to neoplasia was also reported in 17% of the group of de novo UPUMPs studied, including 5% UPUMP with flat architecture33. Thus, limited data suggest a low but real risk for progression of hyperplasia to neoplasia including those in the de novo setting.
Arguments against retaining hyperplasia
Nonspecificity of molecular alterations
Studies showed that normal-appearing urothelium may contain clones harboring mutations identified also in cancer consistent with mucosal field effects6,7,8, such that the specificity of such mutations to hyperplasia may be questioned. Interestingly, mutations are also detected in normal-appearing urothelium in bladders removed for transplant donation with no concurrent neoplasia6. The recent study by Weyerer et al.31 on bladder cancer specimens, detected TERT mutation in 17% of normal-appearing urothelium compared to 33% of hyperplasia. Another study showed polysomy of one or more chromosomes in 14% of normal urothelium compared to 17% in hyperplasia30. Thus, one may argue that the molecular changes detected are contingent of the mucosal field change and not necessarily attributable to the hyperplastic lesion. Nonetheless, the reproducibly increasing proportion of mutations observed across the histologic spectrum ranging from normal to hyperplasia to carcinoma is a counterargument to suggest progression to neoplasia31.
Diagnostic conflation of reactive and preneoplastic processes
Only a subset of hyperplasia exhibits detectable, carcinoma-associated molecular alterations26,27,29,30,31. Further, most studies that identified genomic and chromosomal alterations in lesions meeting diagnostic criteria for hyperplasia employed bladder samples from patients with prior or concurrent neoplasia such that such findings might represent an epiphenomenon of cancer field effects rather than autonomous drivers of hyperplastic changes26,27,29,30,31. Hyperplasia as a reversible, reactive, or adaptive process is known in many organs, and in theory should also exist in the bladder as response to irritative stimuli. This line of thinking is also consistent with the prior observations that de novo hyperplasia is more commonly flat (73%) than papillary (PUH; 26%), with the former more weakly associated with neoplasia33. On the other hand, mutations may be detected in de novo flat hyperplasia with no prior or concomitant neoplasia, suggesting hyperplasia may also be truly preneoplastic28. In summary, it is likely that lesions meeting diagnostic criteria for flat hyperplasia encompass subsets with reactive changes and subsets with preneoplastic etiologies. Such a conflation of lesions arising by different processes (reactive and preneoplastic), despite the shared histopathology, questions the construct validity of hyperplasia as a distinct biologic entity.
Uncertain relationship to PUH
There have been significant reclassifications of flat hyperplasia and PUH since the time of the 1998 WHO/ISUP consensus, through intervening WHO classifications, up to the 2021 GUPS White Paper, reflective of their uncertain relationship1,5,25. These two lesions were initially considered separate in the 1998 WHO/ISUP consensus, considered under one spectrum in the 2004 WHO blue book, merged in the 2016 WHO blue book as UPUMP, and lately, recommended again to be separated by GUPS1,5,22,25. Studies distinguishing flat hyperplasia and PUH are very limited. One study showed that UPUMP with papillations (PUH) had higher progression rates to neoplasia than UPUMP with flat architecture in (flat hyperplasia) (52% versus 18%) and the difference was significant in de novo cases; however, the analysis was limited by the small number of cases33. While limited evidence suggests differences, additional studies are needed to show that flat hyperplasia is truly distinct from PUH.
Lack of clinical actionability
Recurrences of low-grade and high-grade PUCs are frequent, occurring in 50% and 60% of cases, respectively1. It is now standard for patients with non-invasive high-grade PUC to undergo a second or restaging TUR and standardized surveillance cystoscopy protocol for at least 5 years9,10,11. Many hyperplasia lesions occur during follow-up of these patients with PUC, either as a recurrence or as a residual or adjacent shoulder lesion on follow-up TUR performed several weeks after the initial TUR. Thus, in many cases, management and surveillance plans would be primarily dictated by the more significant prior PUC diagnosis rather than by the subsequent hyperplasia, the diagnosis of which may be ignored by clinicians. However, one can argue that de novo hyperplasia may still warrant surveillance because of the small risk of progression to neoplasia.
Our recommended approach in clinical practice
The traditional simplistic criteria for the diagnosis of flat hyperplasia—markedly thickened urothelium, often with increased cellular density and lacking atypia—have remained consistent since the 1998 WHO/ISUP consensus5. Tangentially sectioned and/or distorted urothelium may mimic hyperplasia. However, the effect of tangential sectioning is typically focal, and not seen in other fragments. Further, the increase in cell layers is not that striking and the increase in cellularity is not that dense; both of features may recede on deeper levels. The term AUP-flat may also be used as a synonym; e.g., “Urinary bladder, biopsy, right lateral wall: Urothelial hyperplasia (Atypical urothelial proliferation—flat)”25. Presence of cellular disorganization, nuclear rounding, pleomorphism, nuclear hyperchromasia, nucleolomegaly and mitosis, depending on the degree, should warrant the diagnosis of dysplasia (see discussion for criteria below) or (most commonly) CIS1,35,36,37,38. Although some degree of undulations, papillations (or accordion pleated appearance) without true papillary cores may be seen with hyperplasia, when prominent, the term AUP-tended may be used. Identification on repeat TUR as a pure lesion (without residual PUC) should prompt a comment that the hyperplasia may represent as the lateral extension of the prior PUC, and document specifically that no residual PUC is seen. Diagnosis of the uncommon de novo flat hyperplasia should also prompt a comment for reasonable clinical follow-up. These recommendations, therefore, emphasize the utmost importance of accessing patient diagnostic and clinical history, requisite correlation with cystoscopic reports describing the lesion sampled, and judicious use of recuts and deeper level sections to document or exclude more worrisome findings (cytologic atypia or true papillary cores) deeper in tissue blocks. We do not employ immunohistochemistry or any other adjunctive assay in the diagnosis of hyperplasia.
Urothelial dysplasia
Contemporary definitions and criteria
Dysplasia shows appreciable cytological and architectural features felt to be neoplastic but insufficient for the diagnostic threshold of CIS (Fig. 2)1. Dysplasia is no longer stratified into two- or three-tiered categories by its degree of changes; CIS must be used instead to diagnose lesions with features previously designated as severe or marked dysplasia. The term low-grade intraurothelial neoplasia, while originally synonymous with dysplasia is now regarded as obsolete. Some authors may use the term “urothelial atypia, cannot exclude dysplasia” or merge dysplasia with AUS (discussed below) as “dysplasia/AUS” due to their similar management and diagnostic discrimination issues1,39. Recently, the 2021 GUPS white paper suggested using the term “early low-grade PUC” for the challenging scenario presented by lesions demonstrating overall flat dysplasia with foci of early papillary formation25. Although this preferred term is less ambiguous than “dysplasia with early papillary features”, it must also be acknowledged that this is a descriptive designation and it is not yet entirely clear if such lesions truly can evolve into a well-developed PUC. As defined by the WHO and by the ICUD, dysplasia represents a putative flat precursor lesion and morphologically analogous to low-grade PUC, and due to it being a single category (compared to the previous categories of mild, moderate and severe dysplasia or CIS I and II) the threshold for the diagnosis should be high for lesions being considered as clearly preneoplastic but falling short of CIS (high-grade disease).
Assessment of value as a diagnostic entity
Arguments in favor of retaining dysplasia
Increasing endoscopic saliency?
It is believed to be unlikely for dysplasia to cause symptoms and be detected clinically in the de novo setting. However, the recent use of enhanced cystoscopy, active surveillance protocols and standardization of repeat TUR may lead to sampling of early flat lesions during follow-up of patients with prior neoplasia. Studies suggest that photodynamic diagnosis (blue light) and narrow-band imaging has higher sensitivity than the traditional white light cystoscopy in detecting CIS and dysplasia40,41,42. For flat lesions, previously CIS was often sampled as a “red lesion” visible due to associated angioplasia, whereas formerly invisible lower grade lesions representing dysplasia, may now be encountered increasingly.
Recently improved diagnostic criteria
It is acknowledged by experts that dysplasia has traditionally suffered from poor interobserver variations39. The aforementioned survey conducted by the authors documented this challenge, including comments by participants reflecting variable use of the term describing a bona fide low-grade neoplasm by some, while others employing dysplasia to convey diagnostic uncertainty (whether in degree of atypia vis a vis CIS or reactive versus neoplastic nature). However, recent attempts through ICUD at refining diagnostic criteria for dysplasia by promulgation of the concept of a morphologic analogy between dysplasia and low-grade PUC as architecturally flat versus papillary patterns of the same degree of cytologic changes, provides some degree of simplicity and practicality in the diagnostic assessment23,24. While admittedly there are no studies yet to document any improvement in interobserver agreement after the introduction of this approach, the broad understanding of the cytologic features of low-grade PUC and its reasonable reproducibility as a diagnostic entity provides a criteria and context that can be more easily extrapolated to its flat morphologic counterpart in dysplasia.
Presence of molecular alterations seen in carcinoma
Similar to aforementioned hyperplasia, whole-bladder genomic characterization showed presence of mutations in dysplasia that overlap in cancer, with both showing significantly different mutational landscapes and with lesser copy number change8. Significant allelic losses on multiple chromosomes are detected in dysplasia43. Dyplasia harbors deletions in chromosome 9 and p53 at lower frequencies than in CIS44. Dysplasia also shows TERT promoter mutation at a lesser frequency than CIS31. The type of TERT mutations present can be similar to that in concomitant CIS, indicating a related clonal process. The presence of these cancer-related alterations supports dysplasia as a precancerous flat process related to CIS.
Potential to progress to carcinoma
Several older studies have shown that 14–19% of dysplasia develops into biopsy-proven carcinoma45,46,47,48. One study on de novo dysplasia (no prior or concomitant neoplasia) showed progression to carcinoma in 19%, including to CIS and invasive cancer, at a mean progression interval of 2.5 years46. Overall, studies support that diagnosis of dysplasia is a risk factor for development of or perhaps even a precursor for CIS and invasive cancer. More contemporary data with longitudinal follow-up information is an important scholarship gap in this area of bladder cancer.
Adjacency to prior endoscopic resection sites
Just as in hyperplasia, identification of dysplasia at a prior TUR site, often at the edge of the ulcer or scar, likely represents the shoulder of the initially resected PUC or CIS. Anecdotally, dysplasia, as a residual lesion adjacent to the prior PUC, usually shows thickened cell layers (hyperplasia), though by definition cytologic atypia (analogous to low-grade carcinoma) is also present. Thus, reporting dysplasia in repeat TURs can inform that the surrounding periphery of the prior tumor, while not harboring residual or recurrent carcinoma, nonetheless harbors residual neoplastic abnormalities.
Analogy to other organ systems and mucosal types
Precancerous lesions are well-recognized in other non-urothelial carcinomas including lower anogenital tract squamous cell carcinomas (SCCs)49. For example, HPV-associated lesions of the lower anogenital tract encompass low-grade squamous intraepithelial lesion and high-grade squamous intraepithelial lesion49. In the bladder, besides squamous dysplasia, keratinizing squamous metaplasia, particularly if extensive, is also a known risk factor for SCC that warrants clinical follow-up50,51. These recognized precancerous lesions provide precedence for analogous lesions such as dysplasia (and, for that matter, hyperplasia) in the urothelial tract.
Designation of uncertain/borderline lesions
Although reproducibility is an issue, use of diagnosis of dysplasia allows the greater chance for diagnostic capture of challenging (e.g., nonpleomorphic, Pagetoid spread, or other subtle patterns) CIS lesions. One recent study resulted in the reclassification of 18.5% of lesions originally diagnosed as dysplasia or AUS by generalist surgical pathologists into CIS after review by two genitourinary pathologists52. The lack of a third “buffer” category between normal and CIS may risk the inappropriate classification of challenging CIS patterns into the clinically inappropriate category engendered by a normal urothelium diagnosis. In contrast, diagnosis of dysplasia might allow referral for histologic review, rebiopsy, or follow-up cytology, as examples. Dysplasia and CIS represent a continuum of flat lesions presenting features that may appear conflicting, including examples that are hyperplastic (as above), those that are low grade “atypical,” and those that are high-grade “atypical”. Certainly, the threshold for CIS is different for different observers, despite the increased inclusivity of “less atypical” (i.e., less severe cytologic atypia) lesions in CIS under contemporary criteria. Nonetheless, it is emphasized that current criteria are intended to increase the number of lesions diagnosed as CIS and to triage a higher proportion of such cases into surveillance and CIS management protocols.
Arguments against retaining dysplasia
Semantic issues with the word, dysplasia
One challenge with the word, dysplasia, is that it has different meanings in different contexts in pathology. For example, one of the authors (M.B.A.) participating in the 1998 WHO/ISUP consensus conference, recalls that one reason for the introduction of the alternative terminology of low-grade intraurothelial neoplasia concerned desire to avoid connoting developmental dysplasia in the sense it conveys in pediatric and developmental pathology5. To some, dysplasia implies squamous or glandular mucosal changes; to those wishing to use dysplasia as a term implying diagnostic uncertainty (falling short of urothelial CIS) rather than a specific low-grade neoplastic lesion, it implies too specific an entity.
Poor diagnostic reproducibility
Perhaps the strongest counterargument for the existence of dysplasia is its poor diagnostic reproducibility, which is widely acknowledged by experts36,39,53. Diagnostic preference for the term dysplasia significantly varies among pathologists, even with the aid of ancillary FISH and immunohistochemistry54. Even through the conduct of this review, some published images of dysplasia we encountered in the previously published papers are deemed by some of us as either CIS or normal urothelium. The study by Milord et al.55 provided an objective nuclear size for dysplasia (2.4X lymphocyte size) versus CIS (3.6X lymphocyte size); however, the size of nuclei in dysplasia also overlaps with that in non-neoplastic (reactive, etc.) urothelium. It is for this poor reproducibility that previous data on dysplasia is not considered strong.
Lack of supportive ancillary testing
The commonly-used immunostains for supporting the diagnosis of CIS, including CK20, CD44 and p53, are not useful in the diagnosis of dysplasia54,56. A recent study showed that in biopsies with equivocal urothelial atypia, the presence or lack of a prior diagnosis of bladder cancer is a more reliable predictor of recurrence than is CK20 and p53 staining56. Increased Ki-67 staining is also less reliable to aid in diagnosis of dysplasia57, (and its utility for flat neoplasia including CIS versus reactive processes is increasingly being questioned36. The overlap in chromosomal alterations in CIS, dysplasia, and normal urothelium certainly also questions the utility of FISH44. Likewise, overlap in TERT promoter mutation status makes it not helpful when identifying dysplasia in patients with prior neoplasia31. One recent study showed that TERT promoter mutation is reportedly absent in normal urothelium of deceased transplant organ donors6, and use in uncommon de novo setting remains to be investigated. Thus, diagnosis of dysplasia relies solely on morphological features already fraught with reproducibility concerns, and current technologies do not offer reliable ancillary studies for diagnostic support.
Nonspecificity of molecular alterations
Studies have shown that mutations in bladder cancer may also be present in normal-appearing urothelium representing field effects6,7,8. Some of these mutations are shared with concomitant carcinoma8. Thus, if molecular alterations are used as supporting evidence for neoplastic change, such an approach will run the risk of reactive atypia being overinterpreted as dysplasia or CIS.
Infrequent sampling in the de novo setting
Since dysplasia may not cause symptoms, it is unlikely to be sampled in the de novo setting. The most common indication for cystoscopy is hematuria, and, unlike other cancers such as colorectal cancer, endoscopic screening is not recommended for asymptomatic bladder patients58. Patients who are diagnosed with non-invasive PUC undergo follow-up surveillance, and it is in this scenario where most lesions meeting diagnostic criteria for dysplasia are encountered9. A central tenet of oncology is that early detection of precancerous lesion is an important step towards cancer prevention; in this context, the minimal chance of encountering and making a diagnosis of primary dysplasia diminishes its impact, as a useful entity, in cancer prevention.
Limited clinical significance of secondary dysplasia
There is no evidence that dysplasia will require or benefit from active intervention, including repeat TUR or intravesical instillations. Recommending follow-up for dysplasia is also likely of lesser significance, as most examples are detected in the secondary setting, where patients are already under the surveillance protocols dictated by the higher-risk prior urothelial neoplasia diagnosis. Under such protocols, a diagnostic entity that, neoplastic or not, engenders no different management that a non-neoplastic diagnosis, may be ignored by clinicians.
Our recommended approach in clinical practice
We recommend that diagnosis of dysplasia, when rarely made, should be based on documentation of morphologic features meeting criteria for dysplasia rather than use of dysplasia as a term to convey diagnostic uncertainty1,5,35,36,38,39. We convey diagnostic uncertainty between reactive urothelial atypia and flat neoplasia using the term AUS, as described below. Distinction of dysplasia from CIS relies on the degree of the morphologic changes. Dysplasia shows variable, often slight loss of nuclear polarity relative to basement membrane and crowding with mild nuclear changes in nucleomegaly, nuclear outline variation and hyperchromasia. Prominent pleomorphism with large nuclei (>5X lymphocyte size), dense coarse chromatin, brisk mitotic activity (particularly atypical mitoses, or mitotic activity towards the surface) and florid nucleolomegaly (including multiple nucleoli, on occasion) are strongly associated with CIS.
Caution is advised in making the rare diagnosis of de novo dysplasia. The authors of this manuscript make such a diagnosis only very exceptionally. When providing such an exceptional diagnosis, a comment describing the lesion and noting cytologic atypia lower in degree than CIS is given, with inclusion of a suggestion of close clinical follow-up and possibly rebiopsy if any symptoms persist. Dysplasia is more likely to be seen in patients with low-grade PUC. Occasionally, in repeat TURs, dysplasia at a prior tumor site may represent the residual shoulder of a prior PUC or continuity of a prior CIS, and a comment should be made of this likely scenario. In any case, because of the interobserver variations in dysplasia diagnosis, sharing the case with colleagues or in a consensus conference is strongly advised.
Atypia of unknown significance
Contemporary definitions and criteria
The term AUS is a descriptive diagnosis, recommended when cytologic atypia is present, but it is difficult to opine if this is inflammatory atypia or neoplastic, and to a degree that dysplasia or for that matter CIS cannot be ruled out with certainty, as occurs in a background of inflammation (Fig. 3)1,5,22. As mentioned above, some experts prefer merging AUS with dysplasia, although for clarity, dysplasia is a term used when the urothelium has appreciable cytologic and architectural changes that are felt to be preneoplastic, yet they fall short of the diagnostic threshold for urothelial CIS.
Assessment of value as a diagnostic entity
Arguments in favor of retaining AUS
Just like in dysplasia, AUS can create a buffer category that allows further review and follow-up of equivocal lesions. For example, a patient may be re-evaluated after the associated inflammation subsides. Such a diagnostic category can help avoid missing dysplasia or potentially CIS that is masked by inflammation. One study showed reclassification of a subset of AUS into CIS after review by genitourinary pathologists with the aid of ancillary CK20, CD44, and p53 panel of immunostains52. We strongly suggest employing and interpreting immunohistochemical findings strictly in the context of morphology and the clinical scenario.
Arguments against retaining AUS
AUS is not truly a biologic entity but a descriptive term that may be used by some pathologists as a waste basket diagnosis. Clinical follow-up of patients diagnosed with AUS showed none developing carcinoma45,59. Cheng et al.45 followed 35 AUS (median 3.5 years) and Ziemba et al.59 followed 12 AUS (mean 35 months) with no risk for carcinoma development. Just as with dysplasia, reproducibility is an issue in diagnosing AUS52,54.
Our recommended approach in clinical practice
In this category, as is the case for any cystoscopic sample from the urinary bladder and especially these challenging flat lesions, correlating clinical information and history (e.g., prior cancer diagnosis, presence of any indwelling catheters, stones, infection, etc.), and cystoscopic impressions of lesions are of the utmost importance when considering AUS. As above, a panel of CK20, AMACR, p53, and CD44 may aid in the distinction of CIS versus AUS. A comment about the potential clinical significance of this diagnosis along with recommendations for follow-up to clinicians, is advisable as the descriptive diagnosis is still actionably vague and does not provide any guidance. In diagnosis on surveillance cystoscopy for prior neoplasia, greater caution in using this term is advised, since the risk for dysplasia or CIS is higher in this setting. In such settings the authors most frequently diagnose “urothelial atypia, see comment” and if appropriate favoring a specific etiology. In fact, the authors favor this approach, and tend to consider the concept of AUS as the most appropriate term to employ for equivocal lesions to convey diagnostic uncertainty.
Concluding remarks and future directions
Clearly, in the above context of convincing arguments for and against each of these flat lesions, the question of need for future studies, including interinstitutional efforts and prospective experience, begs itself. The process of reviewing prior studies and opinion in this area revealed that the evolution in both concepts and nomenclature over the years has hindered comparison of findings and extrapolation between observations made under different classification systems. This was especially apparent in our attempts to compare findings from older studies of flat lesions to recent findings for UPUMP, which may include both truly flat and tented lesions. This experience leads us to recommend that future studies standardize methods and criteria so that findings can be mapped to whatever future categories are to be used. From the top, we recommend close attention to and documentation of the clinical setting of lesions sampled and studied. Perhaps the most convincing findings in recent studies of flat urothelial lesions is that the prognostic value of diagnosis of hyperplasia and/or dysplasia for recurrence and progression to carcinoma is much higher in the secondary setting than the de novo setting33,56. Certainly, the de novo setting is where future efforts can shed real light on the risk of diagnosis of these lesions; but, above all, careful delineation of de novo versus secondary presentation is paramount to understand their biologic potential.
From the standpoint of standardization of criteria, review of prior studies suggests that careful documentation of the criteria used for classification is necessary to compare across studies and nomenclatures operative at different times. We would recommend careful attention to documentation of architecture (flat versus tented/pseudopapillary); assessment of the presence, or absence and degree of epithelial hyperplasia; and the criteria/definition used for diagnosis of the presence or absence and degree of cytologic atypia. The clinical and histologic size and focality of flat lesions, parameters prognostic for practically all of the better characterized categories of urothelial neoplasia, bears documentation as well. Also, we recommend use of digital imaging to provide enduring documentation of lesions studied, as is often done for the source data of molecular studies. Recent innovations in image segmentation and measurement provide the opportunity to objectify histopathologic criteria previously only qualitative, experiential or Gestalt in nature60.
Lastly, we would note the apparent limitations observed, at least regarding diagnostic support, of molecular studies performed on these lesions. The successes of molecular assessment in surgical pathology are not to be underemphasized, but, nonetheless to date, have provided limited clarity for flat urothelial lesions. This is in contrast to the apparent value of ascertainment of clinical context (e.g., de novo, versus secondary). With recent growth in understanding of the genetic predispositions to urothelial carcinoma, we cannot help but speculate whether reproducible features of different precursor lesions may pertain to differing genetic contexts. For example, recent scholarship documents the relationship of urothelial carcinoma, especially of the upper tract, to Lynch syndrome61,62,63, though essentially nothing is known of whether precursor/preneoplastic lesions in the spectrum of the flat lesions discussed herein relate to syndrome-associated urothelial carcinomas in the way that they do for colorectal carcinoma. More comprehensively, closer attention to demographics (e.g., age, gender identify), genetic context (e.g., SNPs), or factors from population medicine (diet, exposures, comorbidities) have any relevance to lesions in this category.
References
WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th edn. (WHO Press, 2016)
Czerniak B, Dinney C and McConkey D. Origins of bladder cancer. Annu Rev Pathol 11, 149-174 (2016)
Guo CC and Czerniak B. Bladder cancer in the genomic era. Arch Pathol Lab Med 143, 695-704 (2019)
Amin MB and Young RH. Intraepithelial lesions of the urinary bladder with a discussion of the histogenesis of urothelial neoplasia. Semin Diagn Pathol 14, 84-97 (1997)
Epstein JI, Amin MB, Reuter VE and Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 22, 1435-1448 (1998)
Lawson ARJ, Abascal F, Coorens THH, Hooks Y, O’Neill LO, Latimer C, et al. Extensive heterogeneity in somatic mutation and selection in the human bladder. Science 370, 75-82 (2020)
Li R, Du Y, Chen Z, Xu D, Lin T, Jin S, et al. Macroscopic somatic clonal expansion in morphologically normal human urothelium. Science 370, 82-89 (2020)
Majewski T, Yao H, Bondaruk J, Chung W, Lee S, Lee JG, et al. Whole-organ genomic characterization of mucosal field effects initiating bladder carcinogenesis. Cell Rep 26, 2241-2256.e4 (2019)
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Bladder Cancer. Version 6.21 - December 6, 2021 (2021)
Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol 196, 1021-1029 (2016)
Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European Association of Urology Guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol 81, 75-94 (2022)
Smith S, McKenney J, Paner G, Al-Ahmadie H, Aron M, Berney D, et al. Urothelial dysplasia: diagnostic value in clinical practice 20 years since the 1998 WHO/ISUP consensus. Mod Pathol 33, 977-978 (2020)
Koss LG. Bladder cancer from a perspective of 40 years. J Cell Biochem Suppl 16I, 23-29 (1992)
Melicow MM and Hollowell JW. Intra-urothelial cancer: carcinoma in situ, Bowen’s disease of the urinary system: discussion of thirty cases. J Urol 68, 763-772 (1952)
Koss LG. Tumors of the Urinary Bladder: Atlas of Tumor Pathology. Armed Forces Institute of Pathology 2nd Series, Fascicle 11, (1975)
Nagy GK, Frable WJ and Murphy WM. Classification of premalignant urothelial abnormalities. A Delphi study of the National Bladder Cancer Collaborative Group A. Pathology Annual 17, 219-233 (1982)
Mostofi FK and Sesterhenn IA. Pathology of epithelial tumors & carcinoma in situ of bladder. Prog Clin Biol Res 162A, 55-74 (1984)
Friedell GH, Soloway MS, Hilgar AG and Farrow GM. Summary of workshop on carcinoma in situ of the bladder. J Urol 136, 1047-1048 (1986)
Murphy WM. Atlas of Bladder Carcinoma. American Society of Clinical Pathologists Press (1986)
Murphy MW, Beckwith JB and Farrow GM. Tumors of the kidney, bladder and related urinary structures. Armed Forces Institute of Pathology 3rd Series, Fascicle 11, 219-230 1994
Amin MB, Grignon DJ and Eble JN. Intraepithelial lesions of the urothelium: an interobserver reproducibility study with proposed terminology and histologic criteria. Mod Pathol 10, 69 (1997)
World Health Organization Classification of Tumours. Pathology & Genetics. Tumours of the Urinary System and Male Genital Organs. World Health Organization (2004)
Amin MB, McKenney JK, Paner GP, Hansel DE, Grignon DJ, Montironi R, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Pathology. Eur Urol 63, 16-35 (2013)
Amin MB, Smith SC, Reuter VE, Epstein JI, Grignon DJ, Hansel DE, et al. Update for the practicing pathologist: The International Consultation On Urologic Disease-European Association of Urology Consultation on Bladder Cancer. Mod Pathol 28, 612-630 (2015)
Amin MB, Comperat E, Epstein JI, True LD, Hansel D, Paner GP, et al. The Genitourinary Pathology Society update on classification and grading of flat and papillary urothelial neoplasia with new reporting recommendations and approach to lesions with mixed and early patterns of neoplasia. Adv Anat Pathol 28, 179-195 (2021)
Hartmann A, Moser K, Kriegmair M, Hofstetter A, Hofstaedter F and Knuechel R. Frequent genetic alterations in simple urothelial hyperplasias of the bladder in patients with papillary urothelial carcinoma. Am J Pathol 154, 721-727 (1999)
Obermann EC, Junker K, Stoehr R, Dietmaier W, Zaak D, Schubert J, et al. Frequent genetic alterations in flat urothelial hyperplasias and concomitant papillary bladder cancer as detected by CGH, LOH, and FISH analyses. J Pathol 199, 50-57 (2003)
van Oers JMM, Adam C, Denzinger S, Stoehr R, Bertz S, Zaak D, et al. Chromosome 9 deletions are more frequent than FGFR3 mutations in flat urothelial hyperplasias of the bladder. Int J Cancer 119, 1212-1215 (2006)
Mazzucchelli R, Barbisan F, Stramazzotti D, Montironi R, Lopez-Beltran A and Scarpelli M. Chromosomal abnormalities in macroscopically normal urothelium in patients with bladder pT1 and pT2a urothelial carcinoma: a fluorescence in situ hybridization study and correlation with histologic features. Anal Quant Cytol Histol 27, 143-151 (2005)
Schwarz S, Rechenmacher M, Filbeck T, Knuechel R, Blaszyk H, Hartmann A, et al. Value of multicolour fluorescence in situ hybridisation (UroVysion) in the differential diagnosis of flat urothelial lesions. J Clin Pathol 61, 272-277 (2008)
Weyerer V, Eckstein M, Strissel PL, Wullweber A, Lange F, Tögel L, et al. TERT promoter mutation analysis of whole-organ mapping bladder cancers. Genes (Basel) 12, 230 (2021)
Veeratterapillay R, Gravestock P, Nambiar A, Gupta A, Aboumarzouk O, Rai B, et al. Time to turn on the blue lights: A systematic review and meta-analysis of photodynamic diagnosis for bladder cancer. Eur Urol Open Sci 31, 17-27 (2021)
Lowenthal BM, Sahoo D, Amin MB and Hansel DE. Urothelial proliferation of unknown malignant potential involving the bladder: histopathologic features and risk of progression in de novo cases and cases with prior neoplasia. Arch Pathol Lab Med 144, 853-862 (2020)
Readal N and Epstein JI. Papillary urothelial hyperplasia: relationship to urothelial neoplasms. Pathology 42, 360-363 (2010)
Amin MB and McKenney JK. An approach to the diagnosis of flat intraepithelial lesions of the urinary bladder using the World Health Organization/ International Society of Urological Pathology consensus classification system. Adv Anat Pathol 9, 222-232 (2002)
McKenney JK. Urothelial carcinoma in situ: diagnostic update. Pathology 53, 86-95 (2021)
Gallan AJ, Choy B and Paner GP. Contemporary grading and staging of urothelial neoplasms of the urinary bladder: new concepts and approaches to challenging scenarios. Surg Pathol Clin 11, 775-795 (2018)
McKenney JK, Gomez JA, Desai S, Lee MW and Amin MB. Morphologic expressions of urothelial carcinoma in situ: a detailed evaluation of its histologic patterns with emphasis on carcinoma in situ with microinvasion. Am J Surg Pathol 25, 356-362 (2001)
McKenney JK. Precursor lesions of the urinary bladder. Histopathology 74, 68-76 (2019)
Drejer D, Beji S, Oezeke R, Nielsen AM, Høyer S, Johansen TEB, et al. Comparison of white light, photodynamic diagnosis, and narrow-band imaging in detection of carcinoma in situ or flat dysplasia at transurethral resection of the bladder: the DaBlaCa-8 study. Urology 102, 138-142 (2017)
Naya Y, Oishi M, Yamada Y, Ueda T, Fujihara A, Nakanishi H, et al. Initial experience of combined use of photodynamic diagnosis and narrow band imaging for detection of flat urothelial lesion. Int J Clin Oncol 20, 593-597 (2015)
Blanco S, Raber M, Leone BE, Nespoli L and Grasso M. Early detection of urothelial premalignant lesions using hexaminolevulinate fluorescence cystoscopy in high risk patients. J Transl Med 8, 122 (2010)
Czerniak B, Li L, Chaturvedi V, Ro JY, Johnston DA, Hodges S, et al. Genetic modeling of human urinary bladder carcinogenesis. Genes Chromosomes Cancer 27, 392-402 (2000)
Hartmann A, Schlake G, Zaak D, Hungerhuber E, Hofstetter A, Hofstaedter F, et al. Occurrence of chromosome 9 and p53 alterations in multifocal dysplasia and carcinoma in situ of human urinary bladder. Cancer Res 62, 809-818 (2002)
Cheng L, Cheville JC, Neumann RM and Bostwick DG. Flat intraepithelial lesions of the urinary bladder. Cancer 88, 625-631 (2000)
Cheng L, Cheville JC, Neumann RM and Bostwick DG. Natural history of urothelial dysplasia of the bladder. Am J Surg Pathol 23, 443-447 (1999)
Zuk RJ, Rogers HS, Martin JE and Baithun SI. Clinicopathological importance of primary dysplasia of bladder. J Clin Pathol 41, 1277-1280 (1988)
Baithun SI, Rogers HS, Martin JE, Zuk RJ and Blandy JP. Primary dysplasia of bladder. Lancet 1, 483 (1988)
Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med 136, 1266-1297 (2012)
Warrick JI, Kaag M, Raman JD, Chan W, Tran T, Kunchala S, et al. Squamous dysplasia of the urinary bladder: a consecutive cystectomy series. Int J Surg Pathol 24, 306-314 (2016)
Guo CC, Fine SW and Epstein JI. Noninvasive squamous lesions in the urinary bladder: a clinicopathologic analysis of 29 cases. Am J Surg Pathol 30, 883-891 (2006)
Lawless ME, Tretiakova MS, True LD and Vakar-Lopez F. Flat urothelial lesions with atypia: interobserver concordance and added value of immunohistochemical profiling. Appl Immunohistochem Mol Morphol 26, 180-185 (2018)
Humphrey PA, Moch H, Cubilla AL, Ulbright TM and Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol 70, 106-119 (2016)
Murata S, Iseki M, Kinjo M, Matsuzaki O, Moriuchi A, Ohtani H, et al. Molecular and immunohistologic analyses cannot reliably solve diagnostic variation of flat intraepithelial lesions of the urinary bladder. Am J Clin Pathol 134, 862-872 (2010)
Milord RA, Lecksell K and Epstein JI. An objective morphologic parameter to aid in the diagnosis of flat urothelial carcinoma in situ. Hum Pathol 32, 997-1002 (2001)
Arias-Stella JA 3rd, Shah AB, Gupta NS and Williamson SR. CK20 and p53 immunohistochemical staining patterns in urinary bladder specimens with equivocal atypia. Arch Pathol Lab Med 142, 64-69 (2018)
Kunju LP, Lee CT, Montie J and Shah RB. Utility of cytokeratin 20 and Ki-67 as markers of urothelial dysplasia. Pathol Int 55, 248-254 (2005)
Provenzale D, Gupta S, Ahnen DJ, Markowitz AJ, Chung DC, Mayer RJ, et al. NCCN Guidelines Insights: Colorectal Cancer Screening, Version 1.2018. J Natl Compr Canc Netw 16, 939-949 (2018)
Ziemba JB, Golan R, Skokan A, Patel D, Feldman MD, Bing Z, et al. The clinical presentation and outcome of urothelial atypia on biopsy of the bladder. Urol Oncol 32, 645-647 (2014)
Vrabie CD and Gangal M. Digital re-classification of equivocal dysplastic urothelial lesions using morphologic and immunohistologic analysis. medRxiv, https://doi.org/10.1101/2020.1110.1104.20206524 (2022)
Harper HL, McKenney JK, Heald B, Stephenson A, Campbell SC, Plesec T, et al. Upper tract urothelial carcinomas: frequency of association with mismatch repair protein loss and lynch syndrome. Mod Pathol 30, 146-156 (2017)
Ju JY, Mills AM, Mahadevan MS, Fan J, Culp SH, Thomas MH, et al. Universal Lynch syndrome screening should be performed in all upper tract urothelial carcinomas. Am J Surg Pathol 42, 1549-1555 (2018)
Gayhart MG, Johnson N, Paul A, Quillin JM, Hampton LJ, Idowu MO, et al. Universal mismatch repair protein screening in upper tract urothelial carcinoma. Am J Clin Pathol 154, 792-801 (2020)
Author information
Authors and Affiliations
Contributions
G.P.P., S.C.S., and M.B.A. performed study concept and design, provided acquisition, analysis and interpretation of published literature, and performed writing; G.P.P., S.C.S., A.H., P.K.A., E.C., and M.B.A. performed review and revision of the paper. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paner, G.P., Smith, S.C., Hartmann, A. et al. Flat intraurothelial lesions of the urinary bladder—do hyperplasia, dysplasia, and atypia of unknown significance need to exist as diagnostic entities? and how to handle in routine clinical practice. Mod Pathol 35, 1296–1305 (2022). https://doi.org/10.1038/s41379-022-01087-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01087-7