Abstract
Extramural venous invasion (EMVI) is an established independent prognostic factor in colorectal carcinoma where it is linked to hematogenous spread (i.e., liver metastases), influencing the decision for adjuvant chemotherapy. However, its prognostic significance in small intestinal neuroendocrine tumors (NETs) has not been studied, nor is it routinely assessed or reported. We reviewed primary small bowel NETs (14 jejunum, 82 ileum, 8 not specified) from 104 patients (52 women; median age 60.5, range: 24–84). EMVI was identified in 58 cases (55.8%), including in 13 of 21 equivocal cases using an elastin stain. In univariate analysis, EMVI was associated with lymphovascular and perineural invasion, tumor stage, and lymph node and distant metastases, whereas in multivariate analysis, only distant metastases remained significant (p < 0.001). Liver metastases were present in 55 cases (52.9%) and were significantly associated in univariate analysis with lymphovascular and perineural invasion, tumor stage, lymph node metastases, and EMVI, whereas in multivariate analysis, only EMVI remained significant (p < 0.001; odds ratio (OR) = 59.42). Eight patients developed metachronous liver metastases during follow-up (mean 22.9 ± 22.0 months, range: 4.7–73.2) and all (100%) were positive for EMVI. In contrast, of 49 patients who never developed liver metastases over significantly longer follow-up (mean 71.0 ± 32.4 months, range: 6.6–150.4; p < 0.001), only 7 (14.3%) had EMVI (p < 0.001). In Kaplan–Meier analysis, 8 of 15 patients with EMVI (53.3%) developed metachronous liver metastases, compared with 0 of 42 patients without EMVI (p < 0.001). In contrast, nonhepatic distant metastases, seen in 26 (25.0%) patients, were not associated with EMVI in multivariate or Kaplan–Meier analyses. Our data demonstrate that EMVI is common in small bowel NETs and strongly correlates with development of liver metastases. Therefore, its evaluation is critical and should be assessed in combination with adjuvant techniques such as elastin staining, if necessary. Moreover, inclusion of EMVI in pathology reporting guidelines should be considered.
Similar content being viewed by others
Introduction
Well-differentiated neuroendocrine neoplasms (NENs), also termed neuroendocrine tumors (NETs) and referred to as “carcinoids” when occurring in the gastrointestinal (GI) tract, are relatively rare epithelial neoplasms with morphologic and/or immunohistochemical features supporting neuroendocrine differentiation, such as expression of synaptophysin and/or chromogranin [1,2,3,4,5]. Most small intestinal NETs are thought to arise from enterochromaffin cells and typically exhibit organoid architecture (trabecular, nested, cord- or ribbon-like), uniform small nuclei, and coarsely granular chromatin, similar to NENs in other organs. Well-differentiated NENs of the tubular GI tract are further subclassified according to the anatomical location of the primary tumor wherein staging parameters slightly differ, particularly in regards to T (tumor) stage. The current American Joint Committee on Cancer (AJCC) staging manual (8th edition) takes into account a combination of tumor size and depth of invasion for pT stage, evaluates the number of regional lymph nodes involved (together with size of mesenteric deposits) for pN stage, and assesses distant metastases for pM stage, designated pM1a when confined to the liver [6]. In contrast, grading (low, intermediate, or high) is fairly uniform across all gastroenteropancreatic NENs regardless of primary site, and involves established cutoffs in the mitotic count (determined on hematoxylin & eosin (H&E)-stained sections) and/or Ki-67 proliferation index (evaluated on MIB-1 immunohistochemical stains) [7].
The incidence of NENs has increased substantially in recent decades and the small intestine is the most common primary site within the GI tract (~27–52%, depending on the study population), with 50–67% of these found in the ileum, particularly the distal part, and 8–11% in the jejunum [8, 9]. Small intestinal NETs are slightly more common in women (53%) and as many as 30–50% are multicentric [10]. While about a third remain localized, small bowel NETs pose a therapeutic challenge as more than half (and even up to 90%, in some studies) are already metastatic at the time of diagnosis [11,12,13,14,15]. Common sites of metastases include locoregional lymph nodes (involved in an average of 60–70% of cases) and the liver (20–30%, on average), the latter sometimes (<10%) associated with the occurrence in patients of the so called “carcinoid syndrome,” a constellation of debilitating, hormone-induced symptoms [9, 16]. However, confounding these data is the fact that many small bowel NETs are completely asymptomatic and are identified incidentally or discovered in autopsy series [17]. Long-term recurrence is very common (50%) [18,19,20]. Nevertheless, prognosis is generally favorable with 5-year survival close to 80–100% for localized disease and around 50% for patients with metastases. Beyond grade, stage, and associated variables (nodal metastases, mesenteric involvement, etc.), other prognostic features and biomarkers for NETs have not been well defined or validated [13, 21, 22].
Extramural venous invasion (EMVI) had been recognized early on as an important and independent factor in the prognosis of colorectal carcinoma (CRC), particularly as it pertains to the development of distant, visceral metastases [23,24,25,26,27,28]. It is thought to confer different prognostic information from intramural “small vessel” or lymphovascular invasion (LVI) and, as such, it should be distinguished and separately designated in pathology reports [29,30,31,32,33,34,35]. In particular, LVI is associated with lymph node metastases and is of particular importance in lower stage tumors that might not otherwise be candidates for adjuvant treatment or targets of continued surveillance. Similarly, perineural space invasion, defined as the presence of neoplastic cells within nerve structures with tumor spread along nerve sheaths, has a reported prevalence in CRC anywhere from 2 to more than 50% and may provide a route to local spread and tumor recurrence, but its contribution to distant metastatic disease is less clear [32, 36,37,38,39,40]. Conversely, EMVI has a stronger association with poor prognosis and visceral hematogenous disease spread, thought to be mediated by direct access to the portal circulation in tumors drained by the inferior mesenteric vein [29, 32, 37, 41].
Nevertheless, extreme variability is seen in the reported incidence of EMVI in CRC (10–90%), owing to differences in the patient population and sample composition (especially as it relates to tumor stage), the pathologist’s experience and expertise, the use of special stains and techniques, as well as the understanding and application of diagnostic criteria [35, 42,43,44,45,46]. For example, the use of tangential sectioning along the radial edge of the tumor, in order to maximize the number of veins sampled in cross-section, has been shown to almost double the detection rate of EMVI [47,48,49]. The most widely examined and recommended technique in the evaluation of EMVI in CRC has been the use of elastin stains, which has been shown to lead to an approximately threefold increase in detection [50,51,52,53]. These advances have led academic societies and pathology experts to recommend that EMVI should be routinely diagnosed in at least 20–30% of CRC specimens and that efforts to reach that goal should be undertaken and documented [32, 44, 54]. Indeed, most systematic reviews and meta-analyses have concluded that the overall incidence of EMVI in CRC is or should be around 25% [28, 35].
In contrast, there are no published studies on the presence of EMVI in small intestinal NETs and its presence or absence is not routinely assessed or documented in pathology diagnostic reports, nor is it included in synoptic protocol guidelines and recommendations for these tumors. To this end, the aim of our study was to determine the incidence, clinicopathological associations, and prognostic significance of EMVI in small bowel NETs.
Materials and methods
Study cases
The study was approved by the Mount Sinai Institutional Review Board. Cases of small intestinal NETs were retrieved from surgical pathology records over an 8-year period (June 2009–May 2017). Inclusion criteria consisted of surgical resection specimens of neoplasms diagnosed as primary, well-differentiated NENs of the small intestine (including jejunum, ileum, and small bowel, not otherwise specified [NOS]). Specimens with NENs of the stomach, pancreas, duodenum, or large intestine (including cecum, appendix, colon, and rectum) were excluded. Mixed neuroendocrine-non-neuroendocrine neoplasms (MiNENs), adenocarcinomas, and poorly differentiated (small cell or large cell) neuroendocrine carcinomas were excluded. Consultation cases submitted to our department from referring institutions were included only when all microscopic slides and/or tissue blocks were available for review. Specimens from endoscopic procedures (including biopsies, endoscopic mucosal resections, and endoscopic submucosal dissections) were excluded. Cases with lacking or insufficient clinical data in patient medical records were excluded.
Clinicopathological data
Data on demographic and clinicopathological features, including patient age and sex, tumor grade, primary site, multifocality, and stage (pT, pN, pM, and overall stage group), the presence of small vessel LVI, and perineural space invasion, as well as resection margin status were obtained from surgical pathology reports and patient medical records. Cases wherein the anatomical site of the primary tumor in the small intestine was not listed in the medical record (including endoscopy and radiology reports and intraoperative notes), could not be further determined or inferred from the gross pathology specimen, and was listed in the pathology requisition by the clinician or surgeon as “small bowel” with no additional information were designated as small bowel, NOS. Tumor grade was established according to consensus criteria [55,56,57] with grade 1 (G1) NETs defined as those with <2 mitotic figures per ten high power fields (HPF; ×400 magnification, area equivalent to 2 mm2) and Ki-67 proliferation index < 3% (based on MIB-1 immunohistochemical stain); G2 defined as 2–20 mitotic figures per ten HPFs and/or Ki-67 index 3–20%; and G3 defined as >20 mitotic figures per ten HPFs and/or Ki-67 index > 20%. Tumor areas with the most mitotic figures or highest Ki-67 index (“hot spots”) were used and the highest tumor grade regardless of methodology (mitotic count vs. MIB-1 stain) was recorded. When primary tumor grade was discordant with that of metastatic deposits (lymph nodes, liver, etc.), if any, the higher grade was recorded. Stage parameters (pT, pN, pM1, and overall stage group) were scored according to the current AJCC Cancer Staging Manual [6]. To evaluate patient outcomes, electronic medical records were extensively reviewed and events (distant, hepatic, and nonhepatic metastases) were recorded. The presence of distant metastases was established by imaging studies (including CT, MRI, and gallium scanning modalities, as appropriate) and/or histopathologic examination of tissue. Information on the use of adjuvant treatment (such as with somatostatin analogs [SSAs]), if any, was obtained from medical records. Follow-up time, defined as the length of time from small bowel NET diagnosis to distant metastases or data censoring, was determined in months.
Extramural venous invasion
All H&E-stained sections were retrieved and independently and blindly scored by two GI pathologists (QL and ADP) for the presence of EMVI, defined as the unequivocal presence of NET deposits within the lumen of large veins located in the subserosal soft tissue. Cases with disagreement were resolved by consensus on a multiheaded microscope. Veins were identified by the lack of internal elastic lamina, the presence of a relatively thin smooth muscle layer (tunica media), a low wall-thickness-to-vessel-diameter ratio, and their close proximity to large muscular arteries or their smooth-bordered projection into subserosal adipose tissue (“orphan arteriole” and “protruding tongue” signs, respectively) [35]. In accordance with AJCC Cancer Staging guidelines, the designation of EMVI (as with tumor deposits) is applicable to all pT categories and its presence does not influence or upstage the pT stage of the tumor [6]. Equivocal cases on H&E-stained sections (i.e., where extramural tumor deposits were not clearly surrounded by the wall of a large vein) were selectively stained with a modified Verhoeff’s-van Gieson (VVG) stain. Briefly, tissue sections (5 μm thick, fixed in 10% neutral buffered formalin) were deparaffinized, hydrated, and stained in fresh Verhoeff’s solution (five parts 5% alcoholic hematoxylin, two parts 10% aqueous ferric chloride, and two parts Gram–Weigert’s iodine solution). Subsequently, sections were differentiated in 2% aqueous ferric chloride, treated with 5% aqueous sodium thiosulfate, and counterstained in van Gieson’s solution (1 part 1% aqueous acid fuchsin and 20 parts saturated aqueous picric acid solution). Cases were defined as positive for EMVI on VVG stain, when clearly identifiable NET deposits were completely or almost completely surrounded by a rim of irregular, black-staining elastin fibers, indicating the presence of venous wall remnants.
Statistical analysis
Continuous variables (patient age and follow-up time) were compared using Student’s t test. Categorical variables (patient sex, anatomical site of the tumor, tumor multicentricity, surgical resection margin status, tumor grade, presence of lymphovascular and perineural space invasion, pT, pN, pM, use of SSA, presence of liver and/or other distant metastases, and presence of EMVI) were compared using Fisher’s exact or likelihood ratio chi-squared tests. The following group comparisons were made: low grade (G1) vs. intermediate and high (G2/G3) grade; intramural (pT1/T2) vs. extramural (pT3/T4) tumor spread; negative (pN0) vs. positive (pN1/2) lymph nodes; and absence (pM0) vs. presence (pM1) of distant metastases. NETs from the small bowel, NOS, were not incorporated into the analysis on anatomical site. Multivariate logistic regression analysis was performed using all characteristics as independent variables with calculated OR and 95% confidence intervals (CI). Survival analysis with Kaplan–Meier curves was used to test for differences in the development of hepatic and nonhepatic distant metastases as a function of the presence or absence of EMVI at the time of surgical resection of the small intestinal NET. Log-rank test was employed for statistical significance. All statistical analyses were carried out using Statistical Package for the Social Sciences software (build 1.0.0.1327; copyright 2019, IBM) with p < 0.05 considered significant throughout.
Results
Clinicopathological characteristics
Resection specimens of primary small bowel NETs from 104 patients fulfilled inclusion criteria (Table 1). The patients, 52 (50.0%) of whom were female, had a mean age of 60.4 ± 12.3 years and a median age of 60.5 years (range: 24–84). The anatomical site of the primary tumor was most often the ileum (82 cases, 78.8%) and 46 tumors (44.2%) were multifocal, while 15 cases (14.4%) had positive resection margin(s). Most tumors (72 cases, 69.2%) were low grade (G1) and the most common primary tumor stage (62 cases, 59.6%) was invasion through muscularis propria into subserosal soft tissue (pT3). Most tumors (92 cases, 88.5%) were positive for intramural, small vessel LVI, and half (52 cases, 50.0%) showed perineural space invasion. Most tumors (82 cases, 78.8%) were positive for regional lymph node metastases, including 26 cases (25.0%) with 12 or more positive lymph nodes or mesenteric tumor deposits larger than 2 cm (pN2). More than half of the cases (62, 59.6%) had or later developed distant metastases (pM1, overall stage group IV), including 36 (34.6%) to the liver (pM1a), 7 (6.7%) to other sites (e.g., lungs, bones, breast; pM1b), and 19 (18.3%) to both (pM1c). The overall TNM stage was low in 13 cases (12.5%), including 4 (3.8%) stage I and 9 (8.7%) stage II tumors.
Extramural venous invasion
EMVI, defined as the presence of NET deposits within the lumen of large veins in the perienteric adipose tissue, was evaluated in all cases. In 83 (79.8%), the presence or absence of EMVI was sufficiently determined by reviewing H&E-stained sections and, of these, 45 (54.2%) were positive for EMVI. For the remaining 21 (20.2%) cases that were initially considered equivocal for EMVI, serial sections were stained for elastin using a modified VVG stain (Fig. 1). Of these, 13 cases (61.9%) were deemed positive for EMVI on VVG stain. Overall, 58 (55.8%) cases were positive for EMVI and the use of an elastin stain improved the rate of EMVI detection from 45 to 58 cases, a 28.9% increase. The number of blocks with extramural tissue examined from cases that were positive for EMVI (mean 5.8 ± 3.1, median 5, range: 2–15) was comparable with the number from cases negative for EMVI (mean 6.3 ± 2.6, median 6, range: 2–12), the difference not being significant (p = 0.38). In univariate analysis, the presence of LVI, perineural space invasion, higher tumor pT stage, positive lymph nodes, and distant metastases were significantly associated with EMVI, whereas patient sex and age, anatomical site of primary tumor and tumor multicentricity, margin status, and grade were not (Table 1). Multivariate logistic regression showed that only distant metastases (pM1) remained significantly associated with EMVI (p < 0.001), and this association was investigated further.
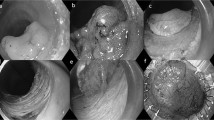
a Representative hematoxylin & eosin (H&E)-stained section showing tumor deposits (arrow) in subserosal soft tissue, near a large muscular artery (top). b Verhoeff’s-van Gieson (VVG)-stained serial section from the same tissue block (as in a) highlights tumor cells surrounded by black wisps of elastin, indicating invasion within the lumen of a large vein (arrow). Note the artery (top) with both internal and external elastic laminae. Based on the VVG stain, this tumor was scored as being positive for EMVI. c Representative H&E section from a different NET with extramural tumor deposits (arrow) amid fibroadipose tissue, again in the vicinity of a large muscular artery (right). d VVG-stained serial section from the same tissue block as in c shows no black-staining elastin fibers around tumor cells (arrow). Note again the muscular artery (right). On the basis of this VVG stain, this tumor was recorded as being negative for EMVI (H&E and VVG stains; original magnification: ×200).
Liver metastases
Among study patients, 55 (52.9%) had liver metastases (Table 2). In univariate analysis, lymphovascular and perineural invasion, higher tumor pT stage, positive lymph nodes, and EMVI were significantly associated with the presence of liver metastases. Patient sex and age, tumor site, tumor multicentricity, resection margin status, and tumor grade were not associated with liver metastases. Of 55 patients who originally had or eventually developed liver metastases, 51 (92.7%) had EMVI diagnosed at the time of resection of the small bowel NET, including identified through the use of an elastin stain, whereas out of 49 patients that did not develop liver metastases, only 7 (14.3%) had EMVI (p < 0.001). In multivariate logistic regression analysis, EMVI was the only characteristic that was significantly associated with the presence of liver metastases in our patient cohort (p < 0.001) with an OR of 59.4 (95% CI: 13.4–263.5).
Patient outcomes
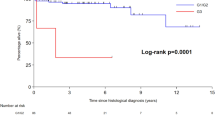
In terms of overall survival, there were only five deaths during the study period: two patients with liver metastases died of disease and three patients without liver metastases died with no evidence of disease. The low mortality rate precluded any meaningful analysis of overall survival. Instead, we evaluated hepatic and nonhepatic distant metastases as primary outcome endpoints in order to identify variables influencing tumor progression and disease-free survival. Of 55 patients with liver metastases, 27 (49.1%) were diagnosed at the time of small bowel NET resection and another 20 (36.4%) were diagnosed before the actual tumor diagnosis, i.e., patients had presented with liver metastases. The remaining eight patients (14.5%) developed liver metastases subsequently (i.e., metachronous), during a mean clinical follow-up of 22.9 ± 22.0 months (median 13.8, range: 4.7–73.2) and all eight (100%) were positive for EMVI (Supplementary Table 1). In contrast, 49 patients never developed liver metastases over a significantly longer mean clinical follow-up of 71.0 ± 32.4 months (median 61.4, range: 6.6–150.4; p < 0.001) and only seven (14.3%) of these had EMVI (p < 0.001). Besides EMVI, in univariate analysis, female sex, perineural space invasion, and positive lymph nodes also correlated with the development of metachronous liver metastases. However, in multivariate analysis excluding EMVI, none of these variables retained significance. To examine for the possible effect of medical therapy on the endpoint of liver metastases, we also recorded the use of adjuvant SSAs, after surgery and NET diagnosis (Supplementary Table 1). There was no significant difference in the number of patients on SSA, with five of eight (62.5%) patients with liver metastases receiving treatment, compared with 19 of 49 (38.8%) without liver metastases (p = 0.10). No patients received adjuvant chemotherapy (other than SSA) or radiation prior to developing liver metastases. Kaplan–Meier curves were also used to analyze differences in the development of metachronous liver metastases (i.e., disease-free survival) after surgical resection of small bowel NETs (Fig. 2a). Of 15 patients with EMVI at the time of NET diagnosis, 8 (53.3%) developed metachronous liver metastases during follow-up, compared with none out of 42 patients without EMVI (p < 0.001; log-rank test).
a Kaplan–Meier curves of cumulative disease-free survival (DFS), defined as the absence of liver metastases, for NETs with and without EMVI at the time of surgical resection. b Kaplan–Meier curves of DFS defined as the absence of distant metastases to sites other than the liver (i.e., lungs, bones, breast, etc.) for NETs with and without EMVI at the time of diagnosis. Cross hatches (+) indicate censoring of patients (death or end of follow-up) and vertical step lines indicate events (i.e., diagnosis of liver or nonliver metastases). p values given for log-rank test.
Nonliver distant metastases
In order to determine whether EMVI was associated with all types of distant metastases or specifically correlated with disease spread to the liver, we identified cases that developed metastases to other organs, such as the lungs, bones, breast, etc. Such nonliver distant metastases were seen in 26 (25.0%) cases overall (Table 2). In univariate analysis, tumor multifocality, positive lymph nodes, and EMVI were significantly associated with the presence of nonliver distant metastases, whereas patient sex and age, anatomical site of the primary tumor, resection margin status, tumor grade, lymphovascular or perineural space invasion, and tumor pT stage were not. After multivariate logistic regression analysis, tumor multifocality remained closely associated with the presence of nonliver distant metastases, albeit without retaining significance (p = 0.061), whereas EMVI did not. Finally, 11 (42.3%) of the 26 patients developed nonliver metastases subsequently to NET diagnosis and, of those, 6 (54.5%) were positive for EMVI, a similar proportion to the 38 of 78 (48.7%) patients without any nonhepatic distant metastases being positive for EMVI (p = 0.72). A Kaplan–Meier curve (Fig. 2b) showed that while there was a trend toward patients with EMVI at the time of NET diagnosis to develop metachronous, nonliver distant metastases compared with patients without EMVI, the result was not statistically significant (p = 0.09; log-rank test).
Discussion
This retrospective study of 104 small intestinal NETs identified EMVI as a significant prognostic factor for the development of liver metastases. The demographic characteristics of our patient cohort (50% female, mean age 60.4 years) and basic clinicopathological features of the tumors in this study (78.8% ileal, 44.2% multifocal) are in keeping with the published attributes of small bowel NETs [8, 13, 14, 16, 58]. However, the proportion of patients with advanced disease in this group (78.8% lymph node-positive, 52.9% with liver metastases) is slightly higher than the average reported in the literature. Possibly accounting for this, our institution functions as a referral center for the treatment of NENs, particularly of the small and large intestines, which may have led to an enrichment in our study of more difficult to treat, higher-stage cases as a result. Nevertheless, these parameters were incorporated in multivariate analysis and their possible contribution in our cohort was appropriately assessed.
EMVI was present in 55.8% of cases. While there are no published reports on the incidence of EMVI in intestinal NETs to compare it with, this rate is higher than the average incidence of EMVI in CRC, which is generally reported to be between 20 and 40%, on average [32, 37]. This difference is not completely explained by any disparity in the propensity for metastases between the two tumor types, since the average rate of liver metastases among small intestinal NENs is only slightly higher than that seen with CRC (20–30% vs. 15–25%, respectively) [59, 60]. Interestingly, liver metastases are more common with left-sided as opposed to right-sided CRC, so a more anatomically proximal location to the liver (as would be seen with small intestinal tumors as opposed to colorectal ones) is also not likely to account for this discrepancy [59, 60].
A recent study of mesenteric tumor deposits in small intestinal NETs found that they were more common (60%) than corresponding deposits in CRC and were significantly associated with LVI and liver metastases [61]. To the extent that at least some of these lesions represent mesenteric venous invasion, as the authors suggested, our results are in line with these findings. Importantly, 51.3% of the patients in our study had liver metastases, slightly higher than the average rate in both CRC and small bowel NENs [11, 14,15,16]. A higher overall tumor stage in our cases could account for the higher observed rate of EMVI, particularly since we found that liver metastases were significantly associated with EMVI. Alternatively, one could hypothesize that it may be easier to recognize EMVI in histological sections from NETs compared with sections from adenocarcinomas, even though NET cells are phenotypically more likely to be mistaken for lymphocytes, often incite retraction artifact, and generally lack tumor necrosis and the micropapillary architecture that is often characteristic of vascular tumor emboli in CRC.
The use of an elastin stain has been shown to increase detection of EMVI in CRC by two- to threefold [46,47,48,49]. In our study, adjuvant VVG staining increased the rate of EMVI positivity by 13 cases, from 45 (43.3%) to 58 (55.8%), a 28.9% (or ~1.3-fold) increase. A possible reason for this lower rate of increase may be that we started out from a higher incidence to begin with, prior to the evaluation of adjuvant elastin stains. Studies of EMVI incidence in CRC had an initial rate of detection on H&E-stained sections between 19 and 24% before using elastin stains and therefore ample opportunity for a more impressive fold increase [47,48,49]. It is possible that, in combination with an elastin stain for vessels, the use of a counterstain to help identify tumor cells (e.g., a silver stain for neuroendocrine cells) would increase the rate of EMVI detection even further. However, technical issues might preclude the combination of silver and elastin stains, not to mention the difficulty in identifying both tumor cells and vessel walls through their black color. Perhaps more feasible would be the use of multiplex immunohistochemistry employing markers for both the vein wall and invading NET cells to document EMVI (e.g., smooth muscle actin and synaptophysin), something that has been occasionally utilized in CRC [33, 62, 63].
A number of CRC studies have tried to differentiate intramural from extramural vascular invasion, the former incorporating invasion into small lymphatic vascular channels as well as intramural veins, without necessarily distinguishing between the two [35]. Nevertheless, they are both associated with poor prognosis and adverse outcomes even though results have been diverse and contradicting [29,30,31, 64,65,66]. Small vessel LVI is mostly associated with lymph node metastases whereas large EMVI is largely a risk factor for liver metastases. Thus, current recommendations and synoptic protocols for CRC call for the distinction between small and large vessel tumor invasion and further separate intramural from EMVI [67]. However, the significance of intramural venous invasion (i.e., within the submucosa or muscularis propria) is less clear. Since published protocols for the examination of small intestinal specimens with NETs currently only identify “LVI” as a data element without additional modifiers [68], it is our hope that results such as those presented herein will encourage the use of distinct subcategories for vascular invasion in NETs as well.
The concept of multistep carcinogenesis encompasses the metachronous, step-wise progression of disease beyond the primary tumor [69]. Thus, CRC cells travel to regional lymph nodes first before disseminating to the liver or peritoneal surfaces, and lung metastases develop only after considerable disease spread has occurred. This is consistent with higher rates of lymph node involvement compared with liver metastases seen with most intestinal tumors, including CRCs and NETs, and with the relatively low incidence of metastases to other organs. EMVI is a particularly strong prognostic factor in CRC, especially as it pertains to the development of distant metastases. This has led to the concept of an “anatomical vascular highway” accessed by tumor cells via deep, extramural growth and venous invasion and allowing for extensive and rapid tumor dissemination [32]. Accordingly, small intramural LVI is associated with regional lymph node metastases and lymphatic drainage through the thoracic duct to the systemic circulation, whereas EMVI is associated with hematogenous liver metastases via the inferior mesenteric vein drainage to the portal system. These disparate modes of tumor spread would lend support to the argument for separating LVI from EMVI during histopathological examination of these tumors.
Due to the low mortality rate among patients in our study, we were unable to investigate the effect of EMVI on overall survival as a primary outcome endpoint. A much larger population-based study would be required in order to examine this possible and crucial association. Nevertheless, the strong correlation we identified between the presence of EMVI and liver metastases, particularly metachronous ones, makes it a critical parameter to evaluate during pathologic examination. From a diagnostic perspective, the identification of EMVI on surgical resection specimens from primary small bowel NETs would further identify the liver as a major focus for additional clinical investigation. Moreover, it may contribute information in terms of the biological pathways used by tumor cells during the development of distant metastases, which may then provide targets for therapeutic intervention in the future.
Nonliver distant metastases were present at a lower rate than liver metastases in this patient cohort and were not significantly associated with the presence of EMVI. We found that 100% of NETs with metachronous liver metastases were positive for EMVI, compared with 54.5% of tumors with metachronous spread to other organs. While perhaps a larger number of cases would have achieved statistical significance, our results nevertheless suggest that nonliver metastases are less significantly related to EMVI compared with hepatic metastases. This raises the possibility that nonhepatic metastases are instead associated with other tumor characteristics, such as overall disease burden and tumor aggressiveness, rather than vascular access [32]. Supporting this idea, tumor multifocality was the variable with the strongest correlation to the presence of nonliver distant metastases in our multivariate analysis (albeit not quite reaching significance), lending some credence to the hypothesis that overall NET burden may be associated with the development of distant metastases beyond the liver.
There are some limitations in this study. First, as a retrospective study, it is inherently subject to selection bias. However, this was minimized by using and following strictly defined and comprehensive inclusion and exclusion criteria, by employing nonconstricting search parameters, and by retrieving and screening all consecutive cases that resulted from our pathology records search. Underscoring this, the clinicopathological characteristics of our cases were very similar to previously published studies. Sampling bias can also be a limitation, but in our study similar numbers of tissue blocks were examined from cases eventually diagnosed as positive or negative for EMVI. Interestingly, this is not something that has been formally addressed in CRC, where there is significant literature on EMVI. A meta-analysis concluded that CRC studies with higher average numbers of tumor blocks tended to have a higher rate of EMVI detection [35]. However, some of these studies reported the total number of tumor blocks taken, not necessarily the number of sections that contained extramural tissue. Another limitation is chronology bias, as diagnostic parameters and staging guidelines change over time and certainly they have done so since 2009 when our first cases were collected [70]. In order to ameliorate this, we reevaluated all original pathology reports and reassigned parameters (including grade and stage information) according to current criteria and recommendations. Critical sections and slides, such as for the determination of EMVI, were reviewed and a significant proportion (20.2%) was subjected to additional VVG staining for the identification of elastin fibers. Finally, transfer bias due to patients lost to follow-up is always a concern when outcome data are measured. Our study had significant follow-up, particularly for patients that did not develop liver or nonliver metastases, giving us confidence that metastases were not underreported. In order to achieve internal and external validity, our study was carefully designed such that investigators were blinded to outcomes and independently assessed the various prognostic parameters (including the main independent variable of EMVI). Confounding variables were accounted for by using multivariate logistic regression and our results are generalizable and appropriate, given similar findings in CRC. Even so, confirmation of our findings in future studies would be essential.
In conclusion, we found that the presence of EMVI strongly correlates with the development of liver metastases in NETs of the small intestine. It is therefore clinically important to identify and report EMVI during the histopathologic evaluation of resection specimens from these tumors, even with the use of adjunct methods (such as elastin stains) in equivocal cases. Moreover, the inclusion of EMVI status as a variable in consensus guidelines for the synoptic reporting of small bowel NETs (e.g., in society-sponsored cancer examination protocols) should be thoughtfully considered.
References
Assarzadegan N, Montgomery E. What is new in 2019 World Health Organization (WHO) classification of tumors of the digestive system: review of selected updates on neuroendocrine neoplasms, appendiceal tumors, and molecular testing. Arch Pathol Lab Med. 2020.
Klimstra DS, Modlin IR, Adsay NV, Chetty R, Deshpande V, Gonen M, et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010;34:300–13.
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–8.
Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770–86.
Perren A, Basturk O, Bellizzi AM, Scoazec JY, Sipos B. Small intestinal and ampullary neuroendocrine neoplasms. In: Lokuhetty D, White VA, Watanabe R, Cree IA, editors. WHO classification of tumours—digestive system tumors. 5th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2017. p. 131–4.
Woltering EA, Bergsland EK, Beyer DT, O’Dorisio TM, Rindi G, Klimstra DS, et al. Neuroendocrine tumors of the jejunum and ileum. In: Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. editors. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017. p. 375–87.
van Velthuysen ML, Groen EJ, van der Noort V, van de Pol A, Tesselaar ME, Korse CM. Grading of neuroendocrine neoplasms: mitoses and Ki-67 are both essential. Neuroendocrinology. 2014;100:221–7.
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42.
Modlin IM, Champaneria MC, Chan AK, Kidd M. A three-decade analysis of 3,911 small intestinal neuroendocrine tumors: the rapid pace of no progress. Am J Gastroenterol. 2007;102:1464–73.
Choi AB, Maxwell JE, Keck KJ, Bellizzi AJ, Dillon JS, O’Dorisio TM, et al. Is multifocality an indicator of aggressive behavior in small bowel neuroendocrine tumors? Pancreas. 2017;46:1115–20.
Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8–21.
Moris D, Ntanasis-Stathopoulos I, Tsilimigras DI, Vagios S, Karamitros A, Karaolanis G, et al. Update on surgical management of small bowel neuroendocrine tumors. Anticancer Res. 2018;38:1267–78.
Norlen O, Stalberg P, Oberg K, Eriksson J, Hedberg J, Hessman O, et al. Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J Surg. 2012;36:1419–31.
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72.
Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72.
Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–59.
Berge T, Linell F. Carcinoid tumours. Frequency in a defined population during a 12-year period. Acta Pathol Microbiol Scand A. 1976;84:322–30.
Dieckhoff P, Runkel H, Daniel H, Wiese D, Koenig A, Fendrich V, et al. Well-differentiated neuroendocrine neoplasia: relapse-free survival and predictors of recurrence after curative intended resections. Digestion. 2014;90:89–97.
Glazer ES, Tseng JF, Al-Refaie W, Solorzano CC, Liu P, Willborn KA, et al. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB. 2010;12:427–33.
Le Roux C, Lombard-Bohas C, Delmas C, Dominguez-Tinajero S, Ruszniewski P, Samalin E, et al. Relapse factors for ileal neuroendocrine tumours after curative surgery: a retrospective French multicentre study. Dig Liver Dis. 2011;43:828–33.
Manguso N, Johnson J, Harit A, Nissen N, Mirocha J, Hendifar A, et al. Prognostic factors associated with outcomes in small bowel neuroendocrine tumors. Am Surg. 2017;83:1174–8.
Manguso N, Nissen N, Hendifar A, Harit A, Mirocha J, Friedman M, et al. Prognostic factors influencing survival in small bowel neuroendocrine tumor with liver metastases. J Surg Oncol. 2019;120:926–31.
Talbot IC, Ritchie S, Leighton M, Hughes AO, Bussey HJ, Morson BC. Invasion of veins by carcinoma of rectum: method of detection, histological features and significance. Histopathology. 1981;5:141–63.
Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980;67:439–42.
Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. Spread of rectal cancer within veins. Histologic features and clinical significance. Am J Surg. 1981;141:15–17.
Mori D, Shibaki M, Masuda M, Yamasaki F. Quantitative measurement of venous invasion of colorectal cancer with metachronous liver metastases. Histopathology. 2009;55:654–9.
Petersen VC, Baxter KJ, Love SB, Shepherd NA. Identification of objective pathological prognostic determinants and models of prognosis in Dukes’ B colon cancer. Gut. 2002;51:65–69.
Chand M, Siddiqui MR, Swift I, Brown G. Systematic review of prognostic importance of extramural venous invasion in rectal cancer. World J Gastroenterol. 2016;22:1721–6.
Betge J, Pollheimer MJ, Lindtner RA, Kornprat P, Schlemmer A, Rehak P, et al. Intramural and extramural vascular invasion in colorectal cancer: prognostic significance and quality of pathology reporting. Cancer. 2012;118:628–38.
Gibson KM, Chan C, Chapuis PH, Dent OF, Bokey L. Mural and extramural venous invasion and prognosis in colorectal cancer. Dis Colon Rectum. 2014;57:916–26.
Knijn N, van Exsel UEM, de Noo ME, Nagtegaal ID. The value of intramural vascular invasion in colorectal cancer—a systematic review and meta-analysis. Histopathology. 2018;72:721–8.
Lord AC, Knijn N, Brown G, Nagtegaal ID. Pathways of spread in rectal cancer: a reappraisal of the true routes to distant metastatic disease. Eur J Cancer. 2020;128:1–6.
Liang P, Nakada I, Hong JW, Tabuchi T, Motohashi G, Takemura A, et al. Prognostic significance of immunohistochemically detected blood and lymphatic vessel invasion in colorectal carcinoma: its impact on prognosis. Ann Surg Oncol. 2007;14:470–7.
McClelland D, Murray GI. A comprehensive study of extramural venous invasion in colorectal cancer. PLoS ONE. 2015;10:e0144987.
Messenger DE, Driman DK, Kirsch R. Developments in the assessment of venous invasion in colorectal cancer: implications for future practice and patient outcome. Hum Pathol. 2012;43:965–73.
Knijn N, Mogk SC, Teerenstra S, Simmer F, Nagtegaal ID. Perineural invasion is a strong prognostic factor in colorectal cancer: a systematic review. Am J Surg Pathol. 2016;40:103–12.
Mayo E, Llanos AA, Yi X, Duan SZ, Zhang L. Prognostic value of tumour deposit and perineural invasion status in colorectal cancer patients: a SEER-based population study. Histopathology. 2016;69:230–8.
Yang Y, Huang X, Sun J, Gao P, Song Y, Chen X, et al. Prognostic value of perineural invasion in colorectal cancer: a meta-analysis. J Gastrointest Surg. 2015;19:1113–22.
Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27:5131–7.
Ueno H, Shirouzu K, Eishi Y, Yamada K, Kusumi T, Kushima R, et al. Characterization of perineural invasion as a component of colorectal cancer staging. Am J Surg Pathol. 2013;37:1542–9.
Sato T, Ueno H, Mochizuki H, Shinto E, Hashiguchi Y, Kajiwara Y, et al. Objective criteria for the grading of venous invasion in colorectal cancer. Am J Surg Pathol. 2010;34:454–62.
Dirschmid K, Lang A, Mathis G, Haid A, Hansen M. Incidence of extramural venous invasion in colorectal carcinoma: findings with a new technique. Hum Pathol. 1996;27:1227–30.
Littleford SE, Baird A, Rotimi O, Verbeke CS, Scott N. Interobserver variation in the reporting of local peritoneal involvement and extramural venous invasion in colonic cancer. Histopathology. 2009;55:407–13.
Messenger DE, Driman DK, McLeod RS, Riddell RH, Kirsch R. Current practice patterns among pathologists in the assessment of venous invasion in colorectal cancer. J Clin Pathol. 2011;64:983–9.
Messenger DE, McLeod RS, Kirsch R. What impact has the introduction of a synoptic report for rectal cancer had on reporting outcomes for specialist gastrointestinal and nongastrointestinal pathologists? Arch Pathol Lab Med. 2011;135:1471–5.
Stewart CJ, Morris M, de Boer B, Iacopetta B. Identification of serosal invasion and extramural venous invasion on review of Dukes’ stage B colonic carcinomas and correlation with survival. Histopathology. 2007;51:372–8.
Dirschmid K, Sterlacci W, Oellig F, Edlinger M, Jasarevic Z, Rhomberg M, et al. Absence of extramural venous invasion is an excellent predictor of metastases-free survival in colorectal carcinoma stage II-a study using tangential tissue sectioning. J Clin Pathol. 2012;65:619–23.
Dirschmid K, Sterlacci W, Woll E, Tschann P, Rhomberg M, Offner F. Incidence of extramural venous invasion in colorectal carcinoma as determined at the invasive tumor front and its prognostic impact. Hum Pathol. 2019;86:102–7.
Sternberg A, Mizrahi A, Amar M, Groisman G. Detection of venous invasion in surgical specimens of colorectal carcinoma: the efficacy of various types of tissue blocks. J Clin Pathol. 2006;59:207–10.
Baumhoer D, Thiesler T, Maurer CA, Huber A, Cathomas G. Impact of using elastic stains for detection of venous invasion in the prognosis of patients with lymph node negative colorectal cancer. Int J Colorectal Dis. 2010;25:741–6.
Howlett CJ, Tweedie EJ, Driman DK. Use of an elastic stain to show venous invasion in colorectal carcinoma: a simple technique for detection of an important prognostic factor. J Clin Pathol. 2009;62:1021–5.
Kirsch R, Messenger DE, Riddell RH, Pollett A, Cook M, Al-Haddad S, et al. Venous invasion in colorectal cancer: impact of an elastin stain on detection and interobserver agreement among gastrointestinal and nongastrointestinal pathologists. Am J Surg Pathol. 2013;37:200–10.
Vass DG, Ainsworth R, Anderson JH, Murray D, Foulis AK. The value of an elastic tissue stain in detecting venous invasion in colorectal cancer. J Clin Pathol. 2004;57:769–72.
Quirke P, Morris E. Reporting colorectal cancer. Histopathology 2007;50:103–12.
Kloppel G, La Rosa S. Ki67 labeling index: assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch. 2018;472:341–9.
Rindi G, Kloppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401.
Rindi G, Kloppel G, Couvelard A, Komminoth P, Korner M, Lopes JM, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757–62.
Hatzaras I, Palesty JA, Abir F, Sullivan P, Kozol RA, Dudrick SJ, et al. Small-bowel tumors: epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch Surg. 2007;142:229–35.
Engstrand J, Nilsson H, Stromberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78.
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–9.
Gonzalez RS, Liu EH, Alvarez JR, Ayers GD, Washington MK, Shi C. Should mesenteric tumor deposits be included in staging of well-differentiated small intestine neuroendocrine tumors? Mod Pathol. 2014;27:1288–95.
Ervine AJ, McBride HA, Kelly PJ, Loughrey MB. Double immunohistochemistry enhances detection of lymphatic and venous invasion in early-stage colorectal cancer. Virchows Arch. 2015;467:265–71.
Lapertosa G, Baracchini P, Fulcheri E, Tanzi R. Prognostic value of the immunocytochemical detection of extramural venous invasion in Dukes’ C colorectal adenocarcinomas. A preliminary study. Am J Pathol. 1989;135:939–45.
Leijssen LGJ, Dinaux AM, Amri R, Taylor MS, Deshpande V, Bordeianou LG, et al. Impact of intramural and extramural vascular invasion on stage II-III colon cancer outcomes. J Surg Oncol. 2019;119:749–57.
Lim SB, Yu CS, Jang SJ, Kim TW, Kim JH, Kim JC. Prognostic significance of lymphovascular invasion in sporadic colorectal cancer. Dis Colon Rectum. 2010;53:377–84.
Santos C, Lopez-Doriga A, Navarro M, Mateo J, Biondo S, Martinez Villacampa M, et al. Clinicopathological risk factors of stage II colon cancer: results of a prospective study. Colorectal Dis. 2013;15:414–22.
College of American Pathologists. Protocol for the examination of resection specimens from patients with primary carcinoma of the colon and rectum, version 4.1.0.0. Northfield, IL: College of American Pathologists; 2020. https://documents.cap.org/protocols/cp-gilower-colonrectum-resection-20-4100.pdf.
College of American Pathologists. Protocol for the examination of specimens from patients with neuroendocrine tumors (carcinoid tumors) of the jejunum and ileum, version 1.0.0.2. Northfield, IL: College of American Pathologists; 2020. https://documents.cap.org/protocols/cp-endocrine-jejunal-Ileal-net-20-1002.pdf.
Sugarbaker PH. Metastatic inefficiency: the scientific basis for resection of liver metastases from colorectal cancer. J Surg Oncol Suppl. 1993;3:158–60.
Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg. 2010;126:619–25.
Acknowledgements
The authors would like to thank Dr. H. Mabel Ko and Dr. John D. Paulsen for critical comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, Q., Polydorides, A.D. Diagnosis and prognostic significance of extramural venous invasion in neuroendocrine tumors of the small intestine. Mod Pathol 33, 2318–2329 (2020). https://doi.org/10.1038/s41379-020-0585-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0585-1
This article is cited by
-
Prognostic differences in grading and metastatic lymph node pattern in patients with small bowel neuroendocrine tumors
Langenbeck's Archives of Surgery (2023)