Abstract
Histologically, drug-induced liver injury could be classified into acute hepatitis, chronic hepatitis, acute cholestasis, chronic cholestasis, and cholestatic hepatitis. The correlation between these histologic patterns and long-term clinical outcomes has not been well established. Therefore, we conducted a retrospective cohort study to investigate the association of histologic patterns and long-term clinical outcomes defined as biochemical normalization, persistent abnormal liver biochemistry or death at designated time points. In this study, biochemical classification was determined by R-values; histologic injury pattern was determined by morphological features. Predictive ability of clinical outcomes by these two classifications was assessed using Receiver Operating Characteristic Curves. Logistic regression was performed to identify histologic factors associated with outcomes. Totally, 88 patients with drug-induced liver injury were included for final analysis. Biochemical and histologic classification were consistent in 50 (57%) cases. 53 (60%) cases showed biochemical normalization within 6 months, and a further 11 (13%), 16 (18%), and 6 (7%) cases within 1, 2, and 3 years, respectively. Compared with biochemical classification, histologic injury pattern had better predictive ability for abnormal biochemistry at 6 months (Areas under Receiver Operating Characteristic Curves 0.92 versus 0.60, P < 0.001) and 1 year (Areas under Receiver Operating Characteristic Curves 0.94 versus 0.69, P < 0.001). Interlobular bile duct loss in >25% portal areas was independently associated with abnormal biochemistry at 6 months, 1 year, and 2 years. In conclusion, histologic injury pattern is better correlated with clinical outcome at 6 months and 1 year than biochemical classification. Moderate bile duct loss is an important histologic feature associated with persistent biochemical abnormality at 6 months, 1 year, and 2 years.
Similar content being viewed by others
Introduction
The annual incidence of drug-induced liver injury was estimated at 14–24 per 100,000 person-years according to the epidemiological studies reported from different countries [1,2,3]. Many drugs can cause unpredictable liver injury, and drug intake is one of the most common reasons for clinically significant liver injury and adoption of postmarket regulatory actions [4,5,6]. While timely withdrawal of the offending drug(s) is usually followed by resolution of liver damage [7], a significant minority of patients can have persistent abnormal liver biochemistry or progress to severe liver damage and even end-stage liver disease [8, 9].
According to the R-value determined by the ratio of alanine aminotransferase and alkaline phosphatase with their upper limits of normal at disease onset, drug-induced liver injury is clinically classified into hepatocellular (R ≥ 5), mixed (2 < R < 5), and cholestatic (R ≤ 2) injury patterns [4]. Patients with cholestatic and mixed phenotypes have been thought to require longer periods to achieve biochemical normalization [10]. However, data from the Spanish drug-induced liver injury registry seem to challenge this view, as the median days to restoration were reported as 83, 76, and 115 days for hepatocellular, mixed, and cholestatic cases, respectively, with no significant differences among the groups [11]. Hence, prediction of clinical outcome based on R-value-determined clinical pattern may not always be reliable.
Liver biopsy examination can aid in the timely diagnosis of drug-induced liver injury, by excluding other potential etiologies, determining the major injury pattern as well as the severity of liver injury. Although drug-induced liver injury can present with a wide range of histologic findings [12, 13] as a result of complex interactions between a specific drug and host factors [14], Kleiner et al. [15] found that five out of 18 proposed histologic injury patterns (namely, acute hepatitis, chronic hepatitis, acute cholestasis, chronic cholestasis, and cholestatic hepatitis) account for up to 83% of cases. Specific histologic features like fibrosis, necrosis, microvesicular steatosis, bile duct loss, and cholangiolar cholestasis have been associated with more severe liver injury index, and have provided the foundations for further association studies [15]. However, the correlation between histologic patterns and postbiopsy long-term clinical outcomes of drug-induced liver injury has not been well established.
In light of the imperfect correlations between histologic findings and prebiopsy biochemical findings, i.e., only 50% of drug-induced liver injury cases have matched biochemical and histologic injury patterns [16], we hypothesized that the histologic injury patterns of drug-induced liver injury might be better correlated with long-term clinical outcomes rather than biochemical classification. Therefore, we aimed to explore and compare the associations between biochemical classification, histologic patterns, and clinical outcomes in drug-induced liver injury patients.
Patients and methods
Study design
This was a single-center retrospective observational study. Clinically suspected drug-induced liver injury cases diagnosed at our institution from January 2009 to December 2013 were retrospectively reviewed and their archived liver biopsies retrieved. The medical records for all patients with a diagnosis of “likely”, “possible/probable,” “favoring”, or “consistent with” drug-induced liver injury were reviewed. Causality for each case was ascertained by specialized hepatologists according to: (1) compatible temporal relationship between drug exposure and appearance of liver injury; (2) serological, biochemical, imaging, and histologic data to exclude alternative etiologies; and (3) kinetics of liver biochemistry after de-challenge. Roussel Uclaf Causality Assessment Method scores were calculated according to the clinical information. Biochemical criteria for the identification of drug-induced liver injury are used as reaching any of the following items: alanine aminotransferase ≥5 upper limits of normal; alkaline phosphatase ≥2 upper limits of normal, without bone-diseases-related alkaline phosphatase elevation; alanine aminotransferase ≥3 upper limits of normal and total bilirubin ≥2 upper limits of normal [17].
The biochemical classifications of liver injury were categorized according to the R-value, defined as the ratio of alanine aminotransferase and alkaline phosphatase with their upper limits of normal at disease onset. Specifically, R-value ≥ 5 indicated hepatocellular injury, R-value ≤ 2 indicated cholestatic injury, and R-value 2–5 indicated mixed-type injury according to the criteria of the international consensus meeting for drug-induced liver injury [4].
The liver biopsies of the patients were reviewed and rescored by an experienced hepatologist with pathology experience (XZ) who was blinded to the patients’ clinical and laboratory data using a standard structured form proposed by Kleiner and colleagues (Supplementary Material 1). The final pattern of the histological changes was also categorized according to the classification and criteria of Kleiner et al. [15]. Each liver biopsy was required to be longer than 1.2 cm and included at least ten portal tracts.
Follow-up information was obtained through the (electronic) medical health record system or by telephone calls to determine the clinical course and clinical outcome. Patients whose liver biochemistry returned to the normal range in <1 year were followed up at minimum to biochemical normalization. Patients requiring >1 year to achieve biochemical normalization were followed up at least every 3 months after onset until biochemical normalization or death/liver transplantation.
Although it is generally believed that drug-induced liver injury persisting beyond 6 months should be considered chronic [9, 18], longer cut-off points like 1 year have also been considered when defining chronicity [11]. In the present study, we analyzed the clinical outcomes of patients at 6 months, 1 year, 2 years, and 3 years.
The clinical outcomes were as follows: (1) recovery—biochemical normalization (alanine aminotransferase and aspartate aminotransferase for hepatocellular, alkaline phosphatase for mixed and cholestatic cases, and total bilirubin <1.5 upper limits of normal) without radiological evidence of persistent liver injury; (2) chronicity—persistently elevated alanine aminotransferase or aspartate aminotransferase for hepatocellular, alkaline phosphatase for mixed and cholestatic cases, and total bilirubin >1.5 upper limits of normal, or radiological evidence of persistent liver injury at the designated time point; and (3) death or liver transplantation.
Finally, the associations between biochemical phenotypes, histologic classification, and clinical outcomes were conducted.
Study patients
Between January 2009 and December 2013, patients hospitalized in Beijing Friendship Hospital with clinical and histologic suspicion of drug-induced liver injury were considered as potential cases for the study.
The inclusion criteria were: (1) existing causality between suspected drug exposure and symptoms/abnormal liver biochemistry; (2) Roussel Uclaf Causality Assessment Method score ≥ 3; (3) liver biopsy suggesting “likely”, “possible/probable,” “favoring” or “consistent with” diagnosis of drug-induced liver injury; and (4) availability of adequate follow-up information.
The exclusion criteria were: (1) underlying liver diseases (viral, alcoholic, nonalcoholic, autoimmune, metabolic, congenital hepatitis, biliary obstruction, or altered baseline liver biochemistry of unknown etiology); (2) systemic diseases affecting the liver (thyroid, heart, or kidney disease, HIV infection); (3) history of liver or bone marrow transplantation; (4) liver biopsy suboptimal for scoring and classification; (5) interval from drug-induced liver injury onset to liver biopsy exceeding 6 months; and (6) inadequate clinical information during onset, loss of follow-up, or incomplete follow-up.
Statistical analysis
Categorical variables were presented as count/percentage, continuous variables as means ± standard deviations or medians and interquartile ranges. Comparisons of different groups were performed using the chi-square test for categorical variables and analysis of variance or the Kruskal–Wallis test for continuous variables, as appropriate.
The difference of diagnostic potency between biochemical versus histologic injury pattern and clinical outcomes was assessed using Receiver Operating Characteristic Curves after calculating predictive P values at designated time points. Area under Receiver Operating Characteristic Curves values were compared with the De Long method.
Logistic regression model was used to identify the potential clinical and histologic risk factors for clinical outcomes. First, we build univariate regression model to reveal relationship between potential clinical/histologic risk factors and clinical outcomes. These relationships were illustrated as odds ratio and 95% confidence interval. Then, multiple regression model was performed. Variables with a P value < 0.1 in univariate models would be put into multiple regression model.
For all analyses, P < 0.05 was considered to be significant. All analyses were performed using SPSS V.24.0 software (SPSS Inc., Chicago, IL) and Medcalc Software version 12.2.1.0 (Medcalc, Mariakerke, Belgium).
Results
Patient inclusion and clinical characteristics
From January 2009 to December 2013, a total of 214 hospitalized patients with clinically suspected drug-induced liver injury underwent a liver biopsy in Beijing Friendship Hospital. Of these, 133 patients were identified as drug-induced liver injury without other underlying liver diseases, after excluding three cases with intervals from drug-induced liver injury onset to biopsy exceeding 6 months and 42 cases lacking necessary clinical information (e.g., for calculation of R-value at onset) and/or robust follow-up information for further analysis. Finally, 88 drug-induced liver injury patients were included for analysis (Fig. 1). Comparison of the clinical and histologic characteristics between the 88 included and 42 excluded cases showed no statistically significant differences (Supplementary Material 2, Tables 2.1 and 2.2).
Of the 88 included patients, the mean age was 49 years (range, 10–79 years), 69 (78%) were female, and hepatocellular injury was the predominant biochemical classification determined by the R-value (52 patients, 59%). The baseline characteristics were similar among the different clinical classifications (hepatocellular, mixed, and cholestatic) determined by R-value, except for alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, cholesterol, prothrombin time activity, and hemoglobin (Table 1).
Of the 88 cases, Roussel Uclaf Causality Assessment Method causality assessment defined 18 cases (20%) as “possible” drug-induced liver injury, 63 cases (72%) as “probable” drug-induced liver injury, and 7 (8%) cases as “highly probable” drug-induced liver injury.
Biochemical phenotypes, histologic patterns, and clinical outcomes of the 88 patients
According to the biochemical classification determined by the R-value, 52, 18, and 18 cases were clinically classified as hepatocellular, mixed, and cholestatic phenotypes, respectively. The histologic injury patterns were characterized as hepatitis in 52 cases, cholestatic hepatitis in 18 cases, and cholestasis in 18 cases. When correlating the histologic injury patterns with the biochemical classification, biochemical and histologic classification were consistent in 50 (57%) cases. The distributions of biochemical phenotypes and histologic patterns of the 88 cases are shown in Fig. 2. Table 2 shows the distribution of selected histologic findings according to clinical phenotypes.
The most common insulting agents were herbal products, accounting for 44 (50%) of the 88 drug-induced liver injury cases. The causative drugs and their corresponding histologic patterns are shown in Supplementary Material 3.
The median biochemical recovery time of the 88 patients was 122 days (range, 16–1460 days). For the different biochemical phenotypes, the median biochemical recovery time was 99 days (range, 16–981 days) for hepatocellular injury type, 141 days (range, 30–1460 days) for mixed injury type, and 333 days (range, 21–928 days) for cholestatic injury type, with no significant difference (P = 0.104). However, the biochemical recovery time differed significantly amongst the different histologic patterns (P < 0.001), with median values for acute hepatitis, chronic hepatitis, cholestatic-hepatitis, acute cholestasis, and chronic cholestasis of 69 days (range, 12–182 days), 298 days (range, 54–981 days), 117 days (range, 27–635 days), 139 days (range, 21–205 days), and 600 days (range, 366–1460 days), respectively (Fig. 3).
Median biochemical recovery time of the 88 drug-induced liver injury cases according to biochemical (a) and histologic (b) injury patterns. Median biochemical recovery time was not statistically different among different biochemical classifications, but significantly different among different histologic injury pattern
Of the 88 cases, 53 (60%) resolved within 6 months, with a further 11 (13%) within 1 year, 16 (18%) within 2 years, and 6 (7%) within 3 years. Of the two remaining patients, one died from acute myocardial infarction on day 85 after drug-induced liver injury onset, and the other did not achieve biochemical normalization after the third year and died of decompensated biliary cirrhosis at the fourth year.
Comparison of biochemical versus histologic injury patterns correlating with outcomes at 6 months, 1 year, and 2 years
Notably, from the viewpoint of histologic patterns, the majority of drug-induced liver injury patients with histologic acute hepatocellular (97%) and acute cholestatic (83%) patterns had normalization of liver biochemistry within 6 months. For those labeled with histologic chronic hepatitis and chronic cholestasis patterns, most cases (74 and 92%, respectively) had persistent (>6 months) liver biochemistry abnormalities. Compared with the recovery rates of 67% for hepatocellular, 61% for mixed, and 39% for cholestatic injury types according to the R-value based clinical biochemical phenotypes, classifications based on pathological injury patterns showed obviously better correlations with clinical outcomes at 6 months. The same trend was seen at 1 year and 2 years (Fig. 4).
Outcomes at designated time points according to biochemical and histologic injury patterns. In figure a–c, H stands for hepatocellular biochemical pattern, M mixed biochemical pattern, C cholestatic biochemical pattern; In figure d–f, AH stands for acute hepatitic histologic pattern, CH chronic hepatitic histologic pattern, M cholestatic hepatitic histologic pattern, AC acute cholestatic histologic pattern, CC chronic cholestatic histologic pattern
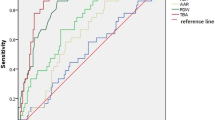
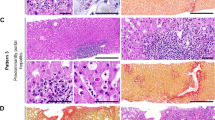
Comparison between the biochemical and histologic injury patterns with outcomes at designated time point was further assessed using Areas under Receiver Operating Characteristic Curves after calculating predictive P values. Histologic injury pattern was significantly better correlated with outcomes (abnormal biochemistry) at 6 months (Areas under Receiver Operating Characteristic Curves being 0.92 and 0.60, P < 0.001) and 1 year (Areas under Receiver Operating Characteristic Curves being 0.94 and 0.69, P < 0.001), but at 2 years, the difference is not significant (Areas under Receiver Operating Characteristic Curves being 0.95 and 0.79, P = 0.14, Fig. 5). Figure 6 illustrated examples of histologic images from five histologic injury patterns.
Examples of drug-induced liver injury cases included. a Acute hepatic liver injury pattern with bridging necrosis (H&E, 200×), with biochemical normalization within 182 days. b Chronic hepatic liver injury pattern with mild fibrosis (Ishak stage 2), with biochemical normalization within 321 days (Sweet, 100×). c Cholestatic hepatic liver injury pattern (H&E, 200×), with biochemical normalization within 240 days. d Acute cholestatic liver injury (H&E, 100×), with biochemical normalization within 50 days. e Cholestatic hepatic liver injury with bile duct injury and bile duct loss <25% (H&E, 400×), with biochemical normalization within 240 days; f Chronic cholestatic liver injury with bile duct loss >50%, with biochemical normalization within 480 days (H&E, 400×)
Clinical outcomes at different time points based on liver histologic features, which are determinant components of histologic patterns
We examined two clinical features (age and sex) and histological features that may be associated with clinical outcomes (focusing on achievement of biochemical normalization) at 6 months, 1 year, and 2 years after drug-induced liver injury onset using logistic regression.
At 6 months, the univariate-unadjusted logistic analysis showed significantly different abnormal biochemistry rates according to severity of ductal paucity (>25% versus ≤25%: odds ratio, 8.130; 95% confidence interval, 2.070–31.940; P = 0.003), degree of chronic portal inflammation (less than moderate in some or all portal areas versus more than moderate or marked in all portal areas: odds ratio, 4.952; 95% confidence interval, 1.657–14.802; P = 0.004), and fibrosis stage (by Ishak) [19] (insignificant fibrosis (< Ishak stage 3) versus significant fibrosis ( ≥ Ishak stage 3): odds ratio, 7.738; 95% confidence interval, 2.259–26.503; P = 0.001), but not for age, sex, degree of necrosis, degree of cholestasis, ductular reaction, or interface hepatitis. In the multivariate analysis, degree of ductal paucity (> 25% versus ≤ 25%: odds ratio, 6.212; 95% confidence interval, 1.369–28.185; P = 0.018) and fibrosis stage (insignificant fibrosis versus significant fibrosis: odds ratio, 6.972; 95% confidence interval, 1.881–25.841; P = 0.004) were the two histologic features independently associated with outcomes at 6 months.
The univariate-unadjusted odds ratios of clinical and histologic features for the outcomes and the multivariate-adjusted odds ratios at 1 year and 2 years are shown in Supplementary Material 4 (Table 4.1–4.3) and Table 3. In the multivariate analysis, degree of duct paucity was the only histologic feature independently associated with outcomes at both 1 year (odds ratio, 13.727; 95% confidence interval, 3.058–61.627; P = 0.001) and 2 years (odds ratio, 8.414; 95% confidence interval, 1.111–63.710; P = 0.039).
Analysis of patients with different degrees of ductal paucity
The clinical features of patients with different degrees of ductal paucity are presented in Table 4. Patients with ductal paucity >25% had significantly lower initial alanine aminotransferase levels (151 IU/L for ductal paucity >50%; 154 IU/L for bile duct loss 25–50%), higher alkaline phosphatase levels (257 IU/L for ductal paucity > 50%; 227 IU/L for bile duct loss 25–50%), and lower R-values (2.4 for ductal paucity >50%; 1.3 for bile duct loss 25–50%) than patients with <25% ductal paucity (initial alanine aminotransferase level, 423 IU/L, P = 0.049; initial alkaline phosphatase level, 161 IU/L, P = 0.012; initial R-value, 1.1, P = 0.002). The interval from diagnosis of drug-induced liver injury to biopsy in patients with ductal paucity >50% (109 days) was clearly longer than that in patients with <50% ductal paucity (19 days for bile duct loss 25–50%; 25 days for bile duct loss <25%), although this was not statistically significant, perhaps due to the small number of patients (seven cases) with >50% ductal paucity. Moreover, the speed of biochemical normalization was lowest in patients with ductal paucity >50%.
Discussion
In this study, we analyzed the biochemical phenotypes and histologic injury patterns associated with the clinical outcomes (focusing on biochemical normalization) of drug-induced liver injury patients in a single medical institution. We found that histologic pattern was better correlated with clinical outcome at 6 months and 1 year than biochemical classifications determined by the R-value. These findings are not surprising, since in the Drug-induced Liver Injury Network prospective study [20], by analyzing paired liver biopsy slides in 12 patients, chronic cholestasis and chronic hepatitis pattern were the most common pattern of liver injury in those with a second biopsy, which is in line with our findings that patients with chronic cholestasis and chronic hepatitis type need longer time to achieve biochemical normalization.
Specific histologic features of drug-induced liver injury patients were associated with clinical outcomes at different time points. In particular, ductal paucity (interlobular bile duct loss in >25% portal areas) and fibrosis (≥Ishak stage 3) indicated a higher likelihood of sustained biochemical abnormalities at 6 months, with the former also associated with biochemical abnormalities at 1 and 2 year/s. Bile duct loss and fibrosis progression have been observed in the second biopsy, indicating that patients with histologic features of duct loss and fibrosis are associated with persistent abnormal biochemistry [20]. Thus, to assess the prognosis of drug-induced liver injury patients, more attention should be paid to histologic injury patterns and histologic features such as the degree of ductal paucity and fibrosis. Although it was not possible to compare the effects of vanishing bile duct syndrome on clinical outcomes by logistic regression owing to the small number of patients (seven cases) with >50% ductal paucity, it was observed that patients with ductal paucity >25% required a significantly longer period to achieve biochemical normalization, at least at the time points of 6 months, 1 year, and 2 years.
Our study validated observations from a previous cross-sectional study that clinical injury patterns determined by the R-value are dominated by hepatocellular injury type (59% in the present study; 52% in the previous study [16]). In the present study, biochemical and histologic classification were consistent in 50 (57%) cases. Concerning injury patterns, patients with biochemical hepatocellular injury were more likely to have acute or chronic hepatitis changes on biopsy and less likely to have acute or chronic cholestasis, and vice versa in patients with clinically cholestatic injury type, consistent with the study by Kleiner et al. [15]. in 2014. Also in Kleiner’s study, the severity of hepatic injury was associated with specific histologic features such as higher degrees of necrosis, fibrosis stage, which has been a foundation for our studies correlating drug-induced liver injury pathology with outcomes.
It is generally believed that patients with clinically cholestatic and mixed injury type require longer times to achieve normalization [7, 10, 21]. Although our study initially appeared to support this view, with the median duration to biochemical normalization of 99, 141, and 333 days for hepatocellular, mixed, and cholestatic cases, respectively, these findings were not significantly different among the groups (P = 0.104), similar to the results obtained by the Spanish drug-induced liver injury registry [11]. Hence, the prediction of different recovery times based on biochemical classification may not always be reliable.
The histologic findings of drug-induced liver injury cover a broad spectrum of injury patterns even within the same category of insulting drugs [13, 22,23,24,25,26], and specific features have been associated with clinical severity determined by clinical and laboratory findings at onset [15]. Our findings further emphasized the importance and contribution of the pathological classifications proposed by Kleiner et al. [15] based on the liver biopsy findings in providing prognostic information.
Destruction and loss of small bile ducts are frequently observed in chronic cholestasis of different etiologies, of which drug-induced liver injury is an important differential diagnosis [27]. Extensive bile duct loss (>50% ductal paucity) is referred to as vanishing bile duct syndrome [28]. Although vanishing bile duct syndrome is rare, studies have reported that the prognosis of drug-induced vanishing bile duct syndrome is not very favorable [29,30,31]. In the Drug-induced Liver Injury Network study, 7% of drug-induced liver injury patients had bile duct loss [32], of whom more than half had severe bile duct loss (>50% portal areas without bile ducts) and was associated with poor outcomes. In our study, bile duct paucity in >25% portal areas was already predictive of persistent abnormal biochemistry at 6 months, 1 year, and 2 years. More attention should therefore be focused on drug-induced liver injury patients with this histologic feature in liver biopsies to better assess their prognosis. Identifying this subgroup of patients to avoid further liver injury from other etiologies, or if possible, to prevent bile duct destruction, or relieve cholestasis may be essential. Unfortunately, the pathogenesis behind drug-induced vanishing bile duct syndrome is largely unknown, and there is no proven therapy that can reverse this condition to date.
The main strengths of the present study were the thorough clinical information and adequate follow-up information of the patients, the availability of a liver biopsy for review in every case; having experienced hepatologist with pathology experience re-evaluating the liver biopsy features while blinded to patient information, and the use of the scoring system proposed by Kleiner et al. [15], which is widely accepted for drug-induced liver injury. These aspects have facilitated the association analyses among the clinical classifications, histologic patterns, and clinical outcomes.
Our study had some limitations. Firstly, this was a single-center retrospective study, which limited the patient population to those undergoing liver biopsy within 6 months after onset and those with thorough clinical and follow-up information. Inevitably, patients with chronic liver injury were followed more closely or more likely to undergo biopsy, and patients with severe bile duct loss were more likely to have a biopsy later after injury onset, thereby introducing a possibility for selection bias and lead time bias. However, when we compared the 42 cases excluded due to incomplete clinical information or follow-up data against the 88 drug-induced liver injury patients included for analysis, we found that the clinical and pathological features were mostly similar. Secondly, we used biochemical findings as surrogate markers for patient outcomes such as recovery and chronicity at different time points, as the occurrence of end-point events like liver cirrhosis was too infrequent for statistical analysis. Thirdly, the sample size of the included patients may allow limited subgroup analysis of associations between less frequent histologic features (e.g., degree of ductal paucity) and clinical outcomes, hence these findings should be further validated by prospective studies with larger sample size.
In conclusion, compared with biochemical classification, histologic injury pattern is better associated with clinical outcome at 6 months and 1 year after drug-induced liver injury onset. Interlobular duct loss >25% is a critical histologic feature associated with persistent biochemical abnormalities at 6 months, 1 year, and 2 years. The present findings are relevant for counseling, monitoring, and management of drug-induced liver injury patients.
References
Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–5.
Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25, e19-e20.
Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156:2230–41.
Kullak-Ublick GA, Andrade RJ, Merz M, End P, Benesic A, Gerbes AL, et al. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut. 2017;66:1154–64.
Zhu X, Kruhlak NL. Construction and analysis of a human hepatotoxicity database suitable for QSAR modeling using post-market safety data. Toxicology. 2014;321:62–72.
Downing NS, Shah ND, Aminawung JA, Pease AM, Zeitoun JD, Krumholz HM, et al. Postmarket safety events among novel therapeutics approved by the US Food and Drug Administration between 2001 and 2010. JAMA. 2017;317:1854–63.
Bjornsson E, Kalaitzakis E, Av KV, Alem N, Olsson R. Long-term follow-up of patients with mild to moderate drug-induced liver injury. Aliment Pharm Ther. 2007;26:79–85.
Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21.
Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950–66, 967.
Andrade RJ, Lucena MI, Kaplowitz N, Garcia-Munoz B, Borraz Y, Pachkoria K, et al. Outcome of acute idiosyncratic drug-induced liver injury: long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581–8.
Medina-Caliz I, Robles-Diaz M, Garcia-Munoz B, Stephens C, Ortega-Alonso A, Garcia-Cortes M, et al. Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J Hepatol. 2016;65:532–42.
Barge S, Ziol M, Nault JC. Autoimmune-like chronic hepatitis induced by olmesartan. Hepatology. 2017;66:2086–8.
Kleiner DE. Histopathological challenges in suspected drug-induced liver injury. Liver Int. 2018;38:198–209.
Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena MI. Drug-induced liver injury: interactions between drug properties and host factors. J Hepatol. 2015;63:503–14.
Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, et al. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014;59:661–70.
Ettel M, Gonzalez GA, Gera S, Eze O, Sigal S, Park JS, et al. Frequency and pathological characteristics of drug-induced liver injury in a tertiary medical center. Hum Pathol. 2017;68:92–98.
Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharm Ther. 2011;89:806–15.
Yu YC, Mao YM, Chen CW, Chen JJ, Chen J, Cong WM, et al. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int. 2017;11:221–41.
Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9.
Fontana RJ, Hayashi PH, Barnhart H, Kleiner DE, Reddy KR, Chalasani N, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110:1450–9.
Fontana RJ, Hayashi PH, Gu J, Reddy KR, Barnhart H, Watkins PB, et al. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology. 2014;147:96–108.
Kleiner DE. The histopathological evaluation of drug-induced liver injury. Histopathology. 2017;70:81–93.
Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60:679–86.
Bjornsson ES. Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol. 2015;89:327–34.
Bjornsson ES. Drug-induced liver injury due to antibiotics. Scand J Gastroenterol. 2017;52:617–23.
Devarbhavi H, Andrade RJ. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014;34:145–61.
Visentin M, Lenggenhager D, Gai Z, Kullak-Ublick GA. Drug-induced bile duct injury. Biochim Biophys Acta. 2018;1864:1498–506.
Reau NS, Jensen DM. Vanishing bile duct syndrome. Clin Liver Dis. 2008;12:203–17.
Ludwig J. Idiopathic adulthood ductopenia: an update. Mayo Clin Proc. 1998;73:285–91.
Desmet VJ. Vanishing bile duct syndrome in drug-induced liver disease. J Hepatol. 1997;26(Suppl 1):31–35.
Bjornsson ES, Jonasson JG. Idiosyncratic drug-induced liver injury associated with bile duct loss and vanishing bile duct syndrome: rare but has severe consequences. Hepatology. 2017;65:1091–3.
Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, et al. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65:1267–77.
Acknowledgements
This work was funded by grant from the National Natural Science Foundation of China (no. 81670545).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Study approval
This study was approved by the Institutional Ethical Review Board.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tian, Qj., Zhao, Xy., Wang, Y. et al. Histologic pattern is better correlated with clinical outcomes than biochemical classification in patients with drug-induced liver injury. Mod Pathol 32, 1795–1805 (2019). https://doi.org/10.1038/s41379-019-0314-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0314-9
This article is cited by
-
Chinese guideline for the diagnosis and treatment of drug-induced liver injury: an update
Hepatology International (2024)
-
Identification of MRI features associated with injury type, severity, and prognosis in drug-induced liver injury
European Radiology (2022)
-
Leberbiopsiediagnostik im Wandel
Der Pathologe (2020)
-
Drug-Induced Liver Injury – Stellenwert der Pathologie
Der Pathologe (2020)