Abstract
The cell division cycle 73 gene is mutated in familial and sporadic forms of primary hyperparathyroidism, and the corresponding protein product parafibromin has been proposed as an adjunct immunohistochemical marker for the identification of cell division cycle 73 mutations and parathyroid carcinoma. Here, we present data from our experiences using parafibromin immunohistochemistry in parathyroid tumors since the marker was implemented in clinical routine in 2010. A total of 2019 parathyroid adenomas, atypical adenomas, and carcinomas were diagnosed in our department, and parafibromin staining was ordered for 297 cases with an initial suspicion of malignant potential to avoid excessive numbers of false positives. The most common inclusion criteria for immunohistochemistry were marked tumor weight (146 cases) and/or fibrosis (77 cases) and/or marked pleomorphism (58 cases). In total, 238 cases were informatively stained, and partial or complete loss of nuclear parafibromin immunoreactivity was noted in 40 cases; 10 out of 182 adenomas (5%), 27 out of 46 atypical adenomas (59%), and 7 out of 10 carcinomas (70%), with positive and negative predictive values of 85 and 90%, respectively for the detection of atypical adenomas/carcinomas versus adenomas, and 18 and 98%, respectively for carcinomas versus atypical adenomas/adenomas. Male patients with high-proliferative tumors were overrepresented among cases with aberrant parafibromin immunohistochemistry, and carcinomas more frequently harbored parafibromin aberrancies than atypical adenomas and adenomas (p < 0.001). We conclude that parafibromin immunohistochemistry is a useful marker in the clinical routine when applied on a pre-selected material of cases, with positive immunoreactivity as a confident rule out marker of malignancy.
Similar content being viewed by others
Introduction
Parathyroid carcinoma is a rare malignancy that provides an indisputable diagnostic challenge for practicing endocrine pathologists and clinicians alike [1]. The unequivocal histological criteria includes lymphovascular and perineural invasion, as well as local invasion of surrounding tissues and the development of distant metastases [2, 3]. Many parathyroid tumors, however, do not exhibit these clear-cut microscopic features, but instead show various features that are overrepresented in parathyroid carcinoma compared with adenomas—such as the occurrence of increased glandular weight, aberrant growth patterns, fibrosis, marked nuclear pleomorphism, macronucleoli, mitoses, necrosis, and capsular engagement. If several of these parameters are in a single lesion, the diagnosis atypical adenoma should be considered [2, 3].
In 2002, the cell division cycle 73 tumor suppressor gene (originally entitled hyperparathyroidism 2) was coupled to the autosomal dominant hyperparathyroidism-jaw tumor syndrome, in which the affected kindred develop primary hyperparathyroidism and ossifying fibromas of the mandible [4]. In addition, germline cell division cycle 73 mutations were also found for subset of families with familial isolated hyperparathyroidism, a condition in which primary hyperparathyroidism is the sole manifestation. In following studies, cell division cycle 73 was identified as recurrently mutated in the majority of sporadic parathyroid carcinomas [5], and the corresponding main protein product parafibromin was subsequently shown to be downregulated, as indicated by protein analyses [6,7,8,9,10]. In contrast, only few sporadic adenomas were shown to exhibit cell division cycle 73 gene mutations and loss of parafibromin immunoreactivity. These results from expressional studies in clinical material indicated that parafibromin immunohistochemistry could be used as a clinical marker for the detection of underlying cell division cycle 73 gene mutations, which in turn would imply a malignant parathyroid tumor, not seldom associated to an undetected hyperparathyroidism-jaw tumor syndrome [11,12,13,14,15]. This conception was somewhat complicated by the occurrence of cell division cycle 73 mutations and loss of parafibromin immunoreactivity in subsets of atypical adenomas, possibly indicating that parathyroid tumors acquire cell division cycle 73 mutations and a malignant potential prior to the onset of full-blown histological evidence of malignancy [6, 14, 15]. Indeed, parafibromin has been acknowledged as a multifaceted protein with various molecular functions in both the nuclear and cytoplasmic compartments, including chromatin remodeling and regulation of apoptosis and the cell cycle machinery [16,17,18,19,20]. As most studies agree that the main roles of this protein are tumor-suppressive, this fits the association between loss of protein expression and the development of a malignant parathyroid tumor.
In addition to parafibromin, a number of protein products have been implied as possible immunohistochemical screening markers of parathyroid malignancy [21,22,23,24,25]. Of these, the adenomatous polyposis coli tumor suppressor protein is one of the few reproduced markers with a coupling to Wnt, the main parafibromin-associated pathway, that also exhibits a possible correlation to underlying genetics, namely aberrant methylation of the adenomatous polyposis coli gene promoter in parathyroid tumors [19, 26, 27].
In 2010, our institution introduced the parafibromin and adenomatous polyposis coli markers as immunohistochemical screening markers for parathyroid tumors with uncertain malignant potential and parathyroid carcinomas. The two responsible endocrine pathologists (CCJ and AH) decided to only allow staining of cases with suspicion of malignant potential. This approach was undertaken since a general screening without coupling to clinical or histological suspicion would yield a large number of false-positive results, as indicated by the non-perfect specificity of the method described in previous publications [6,7,8,9]. Therefore, the inclusion criteria for ordering of the stainings were to be specifically stated in the pathology report.
We gathered information regarding the inclusion criteria used for each parathyroid tumor undergoing parafibromin and adenomatous polyposis coli immunohistochemistry, as well as the staining results, clinical follow-up data and eventual genetic referrals for cell division cycle 73 gene mutational screening. This allowed us to identify the sensitivity and specificity for various clinical and histological inclusion criteria for the detection of atypical adenomas and carcinomas, as well as to analyze the clinical and histological inclusion criteria in relation to immunohistochemical outcomes.
Materials and methods
Cohort description and histological criteria
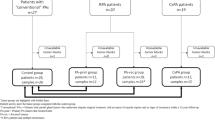
From January 2010 to September 2018, a total of 2019 parathyroid tumors (adenomas, atypical adenomas, and carcinomas) were diagnosed at our institution. Secondary hyperparathyroidism cases (if not developing into tertiary hyperparathyroidism) and parathyroid biopsies were excluded from the analysis. Diagnostic criteria were based on the WHO classification of 2004 [2], and the vast majority of cases (>99%) were diagnosed and scored by two different endocrine pathologists (CCJ and AH), reducing the possibility of interobserver errors. We retrieved a list of all parafibromin stainings ordered at our pathology department by an electronic search function in our patient database. Inclusion criteria for the ordering of the stainings were manually reviewed by reading each pathology report, and the outcomes of the parafibromin and adenomatous polyposis coli immunohistochemistry as well as the clinical implications (genetic screening and patient outcome) were registered. In addition, Ki-67 staining results were reviewed and counted in hot spot areas (at least 2000 cells). The study was conducted with approval of the local ethical committee.
The equivocal inclusion criteria for subsequent parafibromin staining were clinical suspicion of malignancy (severe hypercalcemia, markedly elevated parathyroid hormone levels), known hereditary syndrome in which primary hyperparathyroidism is a feature, young patient age (<30), tumor weight (>1000 mg), trabecular growth, other unusual growth patterns (pseudo-rosettes, insular, micropapillary), marked nuclear pleomorphism, macronucleoli, increased cellular density, presence of mitoses, fibrosis or cysts, and unclear relation between tumor and capsule. The unequivocal (equaling malignancy) inclusion criteria were lymphovascular invasion, capsular invasion, and periparathyroidal tissue invasion. No lesions with clear history of manipulation (e.g., biopsy or ethanol injection) were included in the study, to avoid iatrogenic instigation of atypical histological changes such as fibrosis, hemorrhage, and necrosis [28, 29]. Examples of histological criteria used in this study are illustrated in Fig. 1.
Photomicrographs displaying commonly encountered histomorphological features in our cohort. All images are magnified x400 unless otherwise specified. a Hematoxylin-eosin staining of a parathyroid adenoma displaying fibrosis and a focal trabecular growth pattern, two equivocal histological criteria that motivated immunohistochemical analyses. Magnified x100. b Hematoxylin-eosin staining of an atypical adenoma displaying nuclear pleomorphism and macronucleoli. c Hematoxylin-eosin staining of a parathyroid carcinoma displaying vascular invasion. d The intravascular focus was verified by CD31 immunohistochemistry
In addition to the main cohort, tumors from five additional patients with established cell division cycle 73 gene mutations (diagnosed ahead of the introduction of clinical routine parafibromin immunohistochemical analyses) were retrieved and analyzed as a control cohort for the sensitivity of parafibromin immunohistochemistry to detect cases with germline mutations.
Immunohistochemistry
The immunohistochemistry stainings were performed in an accredited pathology laboratory setting using a Ventana Benchmark Ultra system (Ventana Medical Systems, Tucson, AZ, USA). Four micrometer sections from each tissue sample were deparaffinized using xylene and ethanol. Antigen retrieval was performed using citrate buffer (for parafibromin) or EDTA (for adenomatous polyposis coli) and standardized heating in a microwave oven. Staining was performed using a monoclonal parafibromin antibody (clone 2H1, Santa Cruz Biotechnology, TX, USA) and a monoclonal adenomatous polyposis coli antibody (clone EP701Y, Abcam, Cambridge, UK), and the methodology and antibody clones were consistent throughout the evaluation period.
We defined aberrant parafibromin immunohistochemistry as either total absence of nuclear immunoreactivity (staining in ≤10% of tumor nuclei) or partial loss of nuclear immunoreactivity (staining in 11–89% of tumor nuclei)—the latter a previously reported phenomenon in which a reduction in the amount of positively stained nuclei is observed [6]. The staining was defined as “positive” when ≥90% of tumor nuclei exhibited parafibromin immunoreactivity. Cases with loss of nucleolar immunoreactivity were analyzed separately as there is no consensus as to how this staining should be interpreted— although reports show a correlation between this staining type and cell division cycle 73 gene mutations disrupting the nucleolar localization signal of parafibromin [30,31,32,33]. Loss of nucleolar immunoreactivity was defined as the evident lack of parafibromin immunoreactivity in the tumor nuclei, otherwise positive for the same marker. The phenomenon had to be present in the majority of nucleoli observed.
The adenomatous polyposis coli immunoreactivity was defined as positive (>90% of tumor cells displaying cytoplasmic adenomatous polyposis coli expression), reduced (weak staining of subsets of cells or patchy stainings in certain areas), or negative (staining in ≤10% of tumor cells).
Statistical analyses
Statistical analyses were made with IBM SPSS Statistics 24 (IBM, Armonk, NY, USA). Non-normal distribution was assumed for all data, applying Mann–Whitney U test and Fisher’s exact test for comparison between groups. P-values <0.05 were considered as statistically significant. A multiple logistic regression analysis was performed to analyze the association between aberrant parafibromin immunohistochemistry and parathyroid carcinoma. The diagnostic performance ability of parafibromin for the distinction of parathyroid carcinoma from atypical adenomas and adenomas was analyzed with receiver-operating characteristics and displayed as area under the curve.
DNA sequencing and multiplex ligation-dependent probe amplification analyses
All coding exons of the cell division cycle 73 gene were amplified using exon-specific primers (primers and PCR conditions are available upon request). Direct Sanger sequencing was performed on both strands using Big-Dye terminator sequencing (v1.1, Applied Biosystems, CA, USA), and run on an ABI3130XL Genetic Analyzer (Applied Biosystems, CA, USA). The sequencing reactions were carried out according to the manufacturer’s recommendations. Chromatograms were analyzed using SeqScape v3.7 (Applied Biosystems, CA, USA) with the NM_024529 used as a reference sequence.
Multiplex ligation-dependent probe amplification analysis was performed using the available probe set for the cell division cycle 73 gene (P466-A1, MRC-Holland, Amsterdam, Netherlands). Multiplex ligation-dependent probe amplification was carried out according to the provider’s recommendations, with the exception that the PCR reactions were performed in a 25-μl reaction volume. Amplification products were quantified by capillary electrophoresis on an ABI3130XL Genetic Analyzer (Applied Biosystems, CA, USA) and the accompanying software. The tracing data were analyzed in GeneMarker software v1.7 (SoftGenetics LLC State College, PA, USA). The normalized quotients for the different probes were considered as a deletion when below 0.75 and indicative of duplication when above 1.3.
Results
Immunohistochemical outcomes
Out of 2019 parathyroid tumors, parafibromin immunostaining was ordered for a total of 297 parathyroid tumors from 293 patients, representing 14.7% of the total number of tumors at our institution (Table 1). Of these patients with parafibromin-stained tumors, 179 (61%) were female and 114 (39%) were male, and the mean patient age was 58.5 years (median 59). The average tumor weight for cases with mass information available from a single lesion (n = 275 patients) was 2562 mg. An increasing trend among the pathologists for the ordering of parafibromin immunostainings was noted, from 5% of cases in 2010 to 25% of cases in 2018 (data not shown).
For 59 cases, the parafibromin staining was deemed technically suboptimal due to negatively stained internal controls (n = 56) or due to technical artefacts (intense background staining or suspected poor fixation of tissues, n = 3), and no interpretations regarding staining patterns were made. The remaining 238 tumors (182 adenomas, 46 atypical adenomas, and 10 carcinomas) displayed reliable results from a control staining perspective and were therefore included in the subsequent analyses.
The main outcome of the parafibromin stainings is summarized in Table 1, and examples of the staining results are illustrated in Fig. 2. Fifty-one parathyroid tumors from 48 patients out of the 238 tumors with successful parafibromin immunohistochemistry results (21%) displayed aberrant parafibromin immunoreactivity. In total, 7 out of 10 carcinomas in our cohort (70%) displayed either partial loss or total absence of nuclear parafibromin immunoreactivity, whereas the remaining three cases (30%) were positive for this marker. Ten out of 182 informative adenomas (5%) and 27 out of 46 informative atypical adenomas (59%) demonstrated an aberrant nuclear staining. In addition, four adenomas and three atypical adenomas displayed loss of nucleolar immunoreactivity.
Examples of parafibromin and adenomatous polyposis coli immunohistochemical staining outcomes. All images are magnified x400 unless otherwise specified. a Positive nuclear parafibromin immunoreactivity together with a weak cytoplasmatic staining. This was the predominant staining pattern among the adenomas. b The adenoma was also uniformly positive for cytoplasmic adenomatous polyposis coli. c This parathyroid carcinoma displayed partial loss of nuclear parafibromin immunoreactivity. d Loss of nucleolar parafibromin immunoreactivity magnified x1000. e Total loss of nuclear parafibromin expression in a separate parathyroid carcinoma specimen
Regarding each staining pattern, 38 tumors demonstrated a “partial loss” phenomenon with a reduction in the number of parafibromin-positive nuclei (8 adenomas, 25 atypical adenomas, and 5 carcinomas), seven cases displayed a specific loss of nucleolar parafibromin immunoreactivity (4 adenomas and 3 atypical adenomas), and six tumors stained completely negative for nuclear and nucleolar parafibromin (2 adenomas, 2 atypical adenomas, and 2 carcinomas). The remaining 187 tumors stained positive for nuclear parafibromin.
The adenomatous polyposis coli staining results are summarized in Supplementary Table 1. In all, 283 cases were informatively stained for this marker, including 215 adenomas, 58 atypical adenomas, and 10 carcinomas. A total of 252 tumors were positive, 30 displayed reduced immunoreactivity (either very weak or patchy stainings) and one case was negative. Of the 215 adenomas, 212 (99%) exhibited positive adenomatous polyposis coli staining and 3 cases (1%) displayed reduced immunoreactivity— these three cases also displayed partial loss of nuclear parafibromin immunoreactivity. Of the 58 atypical adenomas, 37 (64%) stained positive for adenomatous polyposis coli, 20 (34%) displayed reduced immunoreactivity, and one case (2%) was entirely negative, this case also displayed negative parafibromin immunohistochemistry. Among the carcinomas, seven out of ten (70%) displayed reduced adenomatous polyposis coli immunoreactivity and correlated perfectly to parafibromin staining outcomes, whereas the remaining three cases (30%) were positive for both adenomatous polyposis coli and parafibromin.
In addition, Ki-67 indexes were available in 295 out of 297 cases (99%) and ranged from 0.1 to 27% (Table 1).
Coupling to histological and clinical parameters
The most common equivocal inclusion criteria for ordering parafibromin staining at our institution are reported in Table 2, and were identified as tumor weight (n = 146 cases; 49%), fibrosis (n = 75; 25%), marked pleomorphism (n = 58; 20%), uncertain relations to the tumor capsule (n = 57; 19%), cysts (n = 49; 16%), and trabecular growth (n = 49; 16%). Other equivocal signs included other unusual growth patterns (n = 33), severe clinical symptoms (n = 30), presence of mitoses (n = 28), increased cellular density (n = 23), macronucleoli (n = 18), suspicion of hereditary syndromes (n = 6), and young patient age (n = 6). The majority of these parameters are listed as equivocal signs of malignancy, according to the 2004 WHO classification [2]. Unequivocal signs of malignancy (such as lymphovascular or perineural invasion, periparathyroidal extension, and distant metastases) were not included in the subsequent analyses, as one or several of these variables per definition are present in the carcinoma cohort, but absent among the atypical adenomas and adenomas.
Patient follow-up and outcome
Clinical follow-up was performed for all parafibromin-aberrant tumors in the cohort, as well as for the remaining atypical adenomas and parathyroid carcinomas irrespectively of their parafibromin status. The follow-up time of the patient cohort with aberrantly stained parafibromin ranged from 0 to 122 months (median 20). There is one persistent and two recurrent hypercalcemic events recorded in the parafibromin-aberrant cohort to this date. Two of these patients exhibit unknown cell division cycle 73 mutational status; a 60-year-old female displayed persistent disease and uremia following surgery for an atypical adenoma, and a 65-year-old male with parathyroid carcinoma with recurrent hypercalcemia was successfully treated for a contralateral adenoma 2 years after surgery. In addition, two siblings with an established cell division cycle 73 mutation are included in the study, the sister was diagnosed with parathyroid carcinoma at 37 years of age and is followed by her local hospital, whereas her brother has undergone three rounds of neck surgery (for three adenomas and one atypical adenoma; the latter tumor included in this study) at our institution followed by debulking surgery of the parathyroid transplant in the left forearm due to recurrent hypercalcemia. He is now well with no biochemical signs of disease to this date.
No recurrences or metastatic disease events have been recorded for any of the remaining parafibromin-aberrant cases, including the ten adenomas. Similarly, no recurrences were noted among the parafibromin-positive carcinomas and atypical adenomas.
Statistical analyses
Parathyroid carcinomas more often displayed aberrant parafibromin immunoreactivity than the combined group of atypical adenomas and adenomas (Fisher exact test, p < 0.001), but there was no difference between the carcinomas and atypical adenomas per se (Fisher exact test, p = 0.617). Moreover, atypical adenomas more frequently harbored parafibromin aberrancies than adenomas (Fisher exact test, p < 0.00001).
Male patients displayed aberrant parafibromin immunohistochemistry as well as parathyroid carcinoma more often than female patients (Fisher’s Exact Test, p = 0.034 and p = 0.0044, respectively), and aberrant parafibromin immunohistochemistry cases displayed higher Ki-67 indexes than parafibromin-positive cases (Mann–Whitney U, p < 0.00001). There were no statistically significant differences between the parafibromin-positive and aberrant cohorts in terms of age (Mann–Whitney U; p = 0.569).
The sensitivity, specificity, positive predictive values, and negative predictive values were calculated for various staining outcomes to differ atypical adenoma/carcinoma from adenoma (Table 3). The specificity for parafibromin immunohistochemistry (complete or partial loss of immunoreactivity) to rule out atypical adenoma/carcinoma with a positive nuclear staining was 97%. In contrast, the sensitivity for aberrant immunohistochemistry to detect atypical adenomas/carcinomas was 64%, and the corresponding positive predictive value and negative predictive value were 85 and 90%, respectively. Similarly, the positive predictive value and negative predictive value were 18 and 98%, respectively for the detection of carcinomas versus atypical adenomas/adenomas. There was no equivocal (non-definite) histological parameter that outperformed parafibromin immunohistochemistry in terms of positive predictive value and negative predictive value (Tables 2, 3). The sensitivity and specificity regarding adenomatous polyposis coli immunohistochemistry for the detection of parathyroid carcinoma versus atypical adenomas and adenomas were 70 and 91%, respectively, with a positive predictive value of 23% and a negative predictive value of 99% (Supplementary Table 1).
When performing a multiple logistic regression analysis including gender, adenoma weight, and Ki-67 index, the latter two divided into quartiles, aberrant parafibromin immunohistochemistry remained the only independent variable associated with parathyroid cancer (odds ratio: 8.256; p = 0.029; Table 4). To further analyze the diagnostic performance ability of parafibromin, a receiver-operating characteristics curve was obtained, with an area under curve of 0.755 for the distinction of parathyroid carcinoma from atypical adenomas and adenomas (Fig. 3).
Parafibromin staining correlation to cell division cycle 73 genotypes
Three patients in this cohort have been previously investigated for cell division cycle 73 gene mutations, two siblings part of a familial isolated hyperparathyroidism family, displaying parathyroid carcinoma and atypical adenoma, respectively. Both their tumors displayed partial loss of parafibromin immunoreactivity in this study (Table 5). Of the 46 remaining patients with aberrant parafibromin, one patient was referred for genetic testing, and subsequently found to be cell division cycle 73 wild-type. The remaining patients were lost to follow-up and eventually tested in their corresponding local hospitals, alternatively never referred to the clinical genetics department. As of this, we sequenced tumor DNA from parathyroid carcinomas with available fresh-frozen tissue enlisted in the original cohort (n = 3); all displaying reduced parafibromin immunoreactivity. All three cases were cell division cycle 73 wild-type, and an ensuing multiplex ligation-dependent probe amplification analysis could not detect any gross deletions of the gene (Table 5).
In order to document even more cases with established cell division cycle 73 genotypes, an extensive follow-up of cases diagnosed outside of the study period was performed. By doing so, we identified five additional primary hyperparathyroidism patients with established cell division cycle 73 gene mutations (Table 5). Tumor tissues for these patients were retrieved and stained for parafibromin in the same clinical setting as the main cohort, and all tumors displayed either reduced or completely negative parafibromin stainings. Together with the two cases from our main cohort, parafibromin immunohistochemistry analyses for a total of seven tumors from patients with germline cell division cycle 73 mutations are presented here—of which all (7/7; 100%) displayed aberrant staining.
Discussion
The advent of parafibromin immunohistochemistry has provided endocrine pathologists with a much-needed adjunct tool to pinpoint cases with potential for future malignant behavior, in addition to detect patients with underlying constitutional cell division cycle 73 mutations [6, 7, 11, 15]. From a screening perspective, the specificity is generally more important than sensitivity when the disease (parathyroid carcinoma) is so uncommonly found compared with adenomas in unselected materials. In this aspect, parafibromin exhibits a stout sensitivity and specificity, but since the low prevalence of parathyroid carcinoma demands almost near-perfect specificity, the marker could probably not be employed as a general screening tool for all parathyroid tumors. To potentially overcome this, we have analyzed data from >2000 parathyroid tumors, in which a clinical or histological suspicion of malignancy had to be fulfilled a priori in order to proceed with the parafibromin staining. By doing so, we hoped to improve the specificity of the method even further. Indeed, the specificity for parafibromin immunohistochemistry to rule out atypical adenoma/carcinoma with a positive staining was 97% with a corresponding negative predictive value of 90%, suggesting that a pre-selection of cases eligible for parafibromin immunohistochemistry would yield a low number of false positive cases (adenomas with aberrant staining).
In our material, parafibromin immunohistochemistry was an independent predictor of parathyroid carcinoma irrespectively of tumor weight, proliferation counts, and patient gender, indicating that the analysis is not biased by confounding factors regularly overrepresented in parathyroid carcinomas. Moreover, our results indicate that the negative predictive value of parafibromin is remarkably high (98%) in terms of ruling out parathyroid carcinoma in the presence of a positive staining, and parafibromin in this context was much more reliable than absence of any of the six most common equivocal histological parameters (Table 2).
When focusing on the carcinomas, seven out of ten cases (70%) displayed an aberrant parafibromin staining, and these cases also displayed reduced adenomatous polyposis coli immunoreactivity. The remaining three cases displayed retained parafibromin and adenomatous polyposis coli immunoreactivity and fairly low Ki-67 indexes (<1, 2.2, and 2.8%, respectively). From a histological standpoint, these three cases displayed lymphovascular and capsular invasion as well as a trabecular growth pattern. Although based on few cases, our numbers in this aspect mirrors the one from previous publications, indication that approximately one-third of parathyroid carcinomas arise without cell division cycle 73 gene and/or parafibromin immunohistochemical aberrancies [4,5,6,7, 9, 13, 34].
The notion that equivocal histological parameters can indicate malignant parathyroid lesions has been long known, and has recently been reinforced by recent advances in the field indicating that there might be additional histological features associated to parafibromin-negative parathyroid tumors [2, 35]. Interestingly, several histological parameters also showed high-negative predictive values for atypical adenomas and carcinomas, specifically the presence of a trabecular growth pattern (83%) and fibrosis (82%). Even so, the parafibromin immunohistochemistry provided superior positive and negative predictive values to all equivocal histological parameters, suggesting that histology alone is not optimal when assessing parathyroid tumors in the clinic.
In our study, a large proportion of atypical adenomas displayed aberrant parafibromin stainings and rather high Ki-67 indexes, nevertheless only a single case displayed persistent disease postoperatively, and no cases with distant metastases have been reported. This indicates that most atypical adenomas are indolent and display low recurrence rates, which is in line with previous findings [2, 15]. Aberrant parafibromin staining in an atypical adenoma seems to be a poor predictor for future recurrences for the individual patient, which further validates that the true strength of parafibromin staining in a clinical high-volume setting is to rule out malignancy with positive staining. Even so, a patient displaying a noninvasive tumor with aberrant parafibromin immunohistochemistry should probably be subject to prolonged follow-up, as subsets of these tumors might recur as malignant, alternatively they are carriers of germline cell division cycle 73 gene mutations with an increased risk of developing additional tumors [15, 35]. The lack of long-term follow-up for our cohort could imply that several high-proliferative and parafibromin-aberrant cases designated as atypical adenomas will recur in the future, which is in line with the slow-growing properties of parathyroid tumors. Indeed, a significant delay in time from original diagnosis to the first biochemical or radiological evidence of recurrence is not uncommonly encountered in the clinical setting.
In this study, we chose to include loss of nucleolar immunoreactivity as a parameter, as this staining pattern is aberrant and has previously been coupled to mutations disrupting the nucleolar localization signal of parafibromin [30,31,32,33]. However, we have reported this staining pattern separately across the paper, with nuclear scoring (“negative” and “partial loss”) as the main feature—since the latter two staining patterns are the only two that are significantly reproduced by independent groups. As only seven samples in our cohort exhibited loss of nucleolar parafibromin (four adenomas and three atypical adenomas), in addition to the fact that none of the samples with negative nucleolar staining have been cell division cycle 73 gene sequenced, we are in no position to determine whether this staining pattern is of relevance in the clinical setting or not.
The observed rate of failure for our parafibromin stainings is also worth discussing. In this study, 59 cases were deemed unsatisfactorily stained due to negative internal controls (n = 56) or technical artifacts (intense background staining or suspected poor fixation of tissues, n = 3). Interestingly, all 59 cases have been previously stained for Ki-67, in addition to adenomatous polyposis coli (56 of the cases; 95%) and parathyroid hormone (25 of the cases; 42%). All cases investigated displayed adequate stainings with interpretable internal controls for at least one of these markers, suggesting that the immunoreactivity was retained for the vast majority of samples that failed parafibromin analysis. The reason for the rather high rate of technical failure is therefore thought to stem from physical properties of the parafibromin antibody rather than a generalized phenomenon of poor fixation. Our data are also in line with our previous reports on the subject, in which the parafibromin staining could be interpreted differently for the same cases when adjusting different immunohistochemistry parameters slightly [6].
The notion that parafibromin correctly pinpoints cell division cycle 73 mutated cases in the clinic is an additional advantage of this staining in addition to the rule out ability of malignancy. A limitation to the current study is the lack of genetic data in terms of cell division cycle 73 genotypes, which is partly due to the fact that large subsets of our patient material are patients affiliated to local hospitals outside of Karolinska. Once operated and diagnosed, the local endocrinologist or general practitioner is often responsible for follow-up and eventual genetic referrals. Even so, there were surprisingly few patients with aberrant parafibromin stainings who actually underwent clinical screening even at our department. Given the fact that an eventual aberrant parafibromin staining was specifically mentioned in the pathology reports, often joined by a recommendation of further genetic screening, our patient cohort could in theory consist of several cases with undetermined constitutional cell division cycle 73 gene mutations, alternatively; subset with verified mutations—but lost to follow-up. Even so, by including a number of patients diagnosed outside of the defined study period, we demonstrate aberrant parafibromin immunostainings in all seven patients with germline cell division cycle 73 mutations investigated—thereby indicating that our staining method is highly sensitive for the detection of constitutional cell division cycle 73 gene mutations. In addition, we did not detect somatic cell division cycle 73 gene mutations or gross deletions in three parafibromin-aberrant parathyroid carcinomas from our cohort, which was fairly unexpected given the stout coupling between mutations and reduction of parafibromin immunoreactivity [8, 9, 35]. Although only based on three samples, the results indicate that our parafibromin immunohistochemistry might display reduced specificity in terms of detecting cell division cycle 73 gene mutations. Indeed, aberrant parafibromin immunoreactivity has been found also for cell division cycle 73 wild-type tumors, which in theory could stem from additional, unidentified somatic events regulating parafibromin expression besides mutations and gene deletions [36].
For adenomatous polyposis coli immunohistochemistry, the sensitivity and specificity for the recognition of parathyroid carcinoma versus atypical adenomas and adenomas were 70 and 91%, respectively (with a positive predictive value of 23% and a negative predictive value of 99%). This indicates that adenomatous polyposis coli, in addition to parafibromin, holds great value as a rule out marker of malignancy, given the low prevalence of parathyroid carcinoma compared with atypical adenomas and adenomas. Even so, as the power of parafibromin lies in its specificity, a marker with superior sensitivity might be a more adequate adjunct in the clinical setting. One example of a marker with superior sensitivity for the detection of parathyroid malignancy is protein gene product 9.5, a protein that also complements parafibromin by being a positive marker, as compared to parafibromin and adenomatous polyposis coli, in which negativity is counted as aberrant [25].
Our data collectively suggest that the main strength of parafibromin immunohistochemistry is the ability to function as a rule out marker of malignancy, as positive immunoreactivity very strongly argues against carcinoma, which should be the most pressing clinical issue in our opinion when assessing parathyroid tumors with equivocal histological findings. We also acknowledge the rule-in properties of an aberrant staining, as it indicates the presence of either an atypical adenoma or a carcinoma.
References
Quinn CE, Healy J, Lebastchi AH, Brown TC, Stein JE, Prasad ML, et al. Modern experience with aggressive parathyroid tumors in a high-volume New England referral center. J Am Coll Surg. 2015;220:1054–62.
Bondeson L, Grimelius L, DeLellis RA, Lloyd R, Åkerström G, Larsson C, et al. Parathyroid carcinoma and parathyroid adenoma. In: DeLellis RA, Lloyd RV, Heitz PU, et al., editors. Pathology and genetics of tumours of endocrine organs. Lyon: International Agency for Research on Cancer Press, 2004. p. 124–32.
DeLellis RA, Arnold A, Bilezikian JP, Eng C, Larsson C, Lloyd RV, et al. Tumours of the parathyroid glands. In: Lloyd RV, Osamura RY, Klöppel G et al., editors. WHO classification of tumours of endocrine organs. Fourth edition. Lyon: International Agency for Research on Cancer, 2017. p. 146–58.
Carpten JD, Robbins CM, Villablanca A, Forsberg L, Presciuttini S, Bailey-Wilson J, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet. 2002;32:676–80.
Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349:1722–9.
Juhlin CC, Villablanca A, Sandelin K, Haglund F, Nordenström J, Forsberg L, et al. Parafibromin immunoreactivity: its use as an additional diagnostic marker for parathyroid tumor classification. Endocr Relat Cancer. 2007;14:501–12.
Gill AJ, Clarkson A, Gimm O, Keil J, Dralle H, Howell VM, et al. Loss of nuclear expression of parafibromin distinguishes parathyroid carcinomas and hyperparathyroidism-jaw tumor (HPT-JT) syndrome-related adenomas from sporadic parathyroid adenomas and hyperplasias. Am J Surg Pathol. 2006;30:1140–9.
Juhlin C, Larsson C, Yakoleva T, Leibiger I, Leibiger B, Alimov A, et al. Loss of parafibromin expression in a subset of parathyroid adenomas. Endocr Relat Cancer. 2006;13:509–23.
Cetani F, Ambrogini E, Viacava P, Pardi E, Fanelli G, Naccarato AG, et al. Should parafibromin staining replace HRTP2 gene analysis as an additional tool for histologic diagnosis of parathyroid carcinoma? Eur J Endocrinol. 2007;156:547–54.
Tan M-H, Morrison C, Wang P, Yang X, Haven CJ, Zhang C, et al. Loss of parafibromin immunoreactivity is a distinguishing feature of parathyroid carcinoma. Clin Cancer Res. 2004;10:6629–37.
Marcocci C, Cetani F. Parafibromin as a tool for the diagnosis of parathyroid tumors. Adv Anat Pathol. 2008;15:179. author reply179-180.
Juhlin CC, Höög A. Parafibromin as a diagnostic instrument for parathyroid carcinoma-lone ranger or part of the posse? Int J Endocrinol. 2010;2010:324964.
Hu Y, Liao Q, Cao S, Gao X, Zhao Y. Diagnostic performance of parafibromin immunohistochemical staining for sporadic parathyroid carcinoma: a meta-analysis. Endocrine. 2016;54:612–9.
Juhlin CC, Nilsson I-L, Johansson K, Haglund F, Villablanca A, Höög A, et al. Parafibromin and APC as screening markers for malignant potential in atypical parathyroid adenomas. Endocr Pathol. 2010;21:166–77.
Kruijff S, Sidhu SB, Sywak MS, Gill AJ, Delbridge LW. Negative parafibromin staining predicts malignant behavior in atypical parathyroid adenomas. Ann Surg Oncol. 2014;21:426–33.
Yang Y-J, Han J-W, Youn H-D, Cho E-J. The tumor suppressor, parafibromin, mediates histone H3 K9 methylation for cyclin D1 repression. Nucleic Acids Res. 2010;38:382–90.
Woodard GE, Lin L, Zhang J-H, Agarwal SK, Marx SJ, Simonds WF. Parafibromin, product of the hyperparathyroidism-jaw tumor syndrome gene HRPT2, regulates cyclin D1/PRAD1 expression. Oncogene. 2005;24:1272–6.
Takahashi A, Tsutsumi R, Kikuchi I, Obuse C, Saito Y, Seidi A, et al. SHP2 tyrosine phosphatase converts parafibromin/Cdc73 from a tumor suppressor to an oncogenic driver. Mol Cell. 2011;43:45–56.
Mosimann C, Hausmann G, Basler K. Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/Armadillo. Cell. 2006;125:327–41.
Kikuchi I, Takahashi-Kanemitsu A, Sakiyama N, Tang C, Tang P-J, Noda S, et al. Dephosphorylated parafibromin is a transcriptional coactivator of the Wnt/Hedgehog/Notch pathways. Nat Commun. 2016;7:12887.
Juhlin CC, Haglund F, Villablanca A, Forsberg L, Sandelin K, Bränström R, et al. Loss of expression for the Wnt pathway components adenomatous polyposis coli and glycogen synthase kinase 3-beta in parathyroid carcinomas. Int J Oncol. 2009;34:481–92.
Witteveen JE, Hamdy NA, Dekkers OM, Kievit J, van Wezel T, Teh BT, et al. Downregulation of CASR expression and global loss of parafibromin staining are strong negative determinants of prognosis in parathyroid carcinoma. Mod Pathol. 2011;24:688–97.
Truran PP, Johnson SJ, Bliss RD, Lennard TW, Aspinall SR. Parafibromin, galectin-3, PGP9.5, Ki67, and cyclin D1: using an immunohistochemical panel to aid in the diagnosis of parathyroid cancer. World J Surg. 2014;38:2845–54.
Hosny Mohammed K, Siddiqui MT, Willis BC, Zaharieva Tsvetkova D, Mohamed A, Patel S, et al. Parafibromin, APC, and MIB-1 are useful markers for distinguishing parathyroid carcinomas from adenomas. Appl Immunohistochem Mol Morphol. 2017;25:731–5.
Howell VM, Gill A, Clarkson A, Nelson AE, Dunne R, Delbridge LW, et al. Accuracy of combined protein gene product 9.5 and parafibromin markers for immunohistochemical diagnosis of parathyroid carcinoma. J Clin Endocrinol Metab. 2009;94:434–41.
Juhlin CC, Kiss NB, Villablanca A, Haglund F, Nordenström J, Höög A, et al. Frequent promoter hypermethylation of the APC and RASSF1A tumour suppressors in parathyroid tumours. PLoS ONE. 2010;5:e9472.
Svedlund J, Aurén M, Sundström M, Dralle H, Åkerström G, Björklund P, et al. Aberrant WNT/β-catenin signaling in parathyroid carcinoma. Mol Cancer. 2010;9:294.
Alwaheeb S, Rambaldini G, Boerner S, Coiré C, Fiser J, Asa S. Worrisome histologic alterations following fine-needle aspiration of the parathyroid. J Clin Pathol. 2006;59:1094–6.
Kim J, Horowitz G, Hong M, Orsini M, Asa SL, Higgins K. The dangers of parathyroid biopsy. J Otolaryngol Head Neck Surg. 2017;46:4.
Hahn MA, Marsh DJ. Nucleolar localization of parafibromin is mediated by three nucleolar localization signals. FEBS Lett. 2007;581:5070–4.
Masi G, Iacobone M, Sinigaglia A, Mantelli B, Pennelli G, Castagliuolo I, et al. Characterization of a new CDC73 missense mutation that impairs Parafibromin expression and nucleolar localization. PLoS ONE. 2014;9:e97994.
Panicker LM, Zhang J-H, Dagur PK, Gastinger MJ, Simonds WF. Defective nucleolar localization and dominant interfering properties of a parafibromin L95P missense mutant causing the hyperparathyroidism-jaw tumor syndrome. Endocr Relat Cancer. 2010;17:513–24.
Juhlin CC, Haglund F, Obara T, Arnold A, Larsson C, Höög A. Absence of nucleolar parafibromin immunoreactivity in subsets of parathyroid malignant tumours. Virchows Arch. 2011;459:47–53.
Gill AJ. Understanding the genetic basis of parathyroid carcinoma. Endocr Pathol. 2014;25:30–34.
Gill AJ, Lim G, Cheung VKY, Andrici J, Perry-Keene JL, Paik J, et al. Parafibromin-deficient (HPT-JT Type, CDC73 mutated) parathyroid tumors demonstrate distinctive morphologic features. Am J Surg Pathol. 2019;43:35–46.
Guarnieri V, Battista C, Muscarella LA, Bisceglia M, de Martino D, Baorda F, et al. CDC73 mutations and parafibromin immunohistochemistry in parathyroid tumors: clinical correlations in a single-centre patient cohort. Cell Oncol (Dordr). 2012;35:411–22.
Acknowledgements
The authors are indebted to Ms. Lisa Ånfalk for acquisition of subsets of the clinical data as well as tumor samples for genetic analysis. The study was supported by grants provided from the Swedish Cancer Society and The Swedish Society for Medical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Juhlin, C.C., Nilsson, IL., Lagerstedt-Robinson, K. et al. Parafibromin immunostainings of parathyroid tumors in clinical routine: a near-decade experience from a tertiary center. Mod Pathol 32, 1082–1094 (2019). https://doi.org/10.1038/s41379-019-0252-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0252-6
This article is cited by
-
Reticular fibre structure in the differential diagnosis of parathyroid neoplasms
Diagnostic Pathology (2023)
-
Overview of the 2022 WHO Classification of Familial Endocrine Tumor Syndromes
Endocrine Pathology (2022)
-
Overview of the 2022 WHO Classification of Parathyroid Tumors
Endocrine Pathology (2022)
-
Genomics and Epigenomics in Parathyroid Neoplasia: from Bench to Surgical Pathology Practice
Endocrine Pathology (2021)
-
Lipoadenoma of the Parathyroid Gland: Characterization of an Institutional Series Spanning 28 Years
Endocrine Pathology (2020)