Abstract
Broadband near-infrared (NIR)-emitting phosphors are key for next-generation smart NIR light sources based on blue LEDs. To achieve excellent NIR phosphors, we propose a strategy of enhancing the crystallinity, modifying the micromorphology, and maintaining the valence state of Cr3+ in Ca3Sc2Si3O12 garnet (CSSG). By adding fluxes and sintering in a reducing atmosphere, the internal quantum efficiency (IQE) is greatly enhanced to 92.3%. The optimized CSSG:6%Cr3+ exhibits excellent thermal stability. At 150 °C, 97.4% of the NIR emission at room temperature can be maintained. The fabricated NIR-LED device emits a high optical power of 109.9 mW at 520 mA. The performances of both the achieved phosphor and the NIR-LED are almost the best results until now. The mechanism for the optimization is investigated. An application of the NIR-LED light source is demonstrated.
Similar content being viewed by others
Introduction
NIR spectroscopy has good penetration for organic matter, and it has drawn attention for application in monitoring foods and medicines, bioimaging, and night vision1,2,3,4,5,6. Smart NIR light sources, an emerging field, are proposed to be combined with smart phones to achieve convenient and fast applications7,8,9. In contrast to traditional tungsten filament lamps and halogen lamps, only light-emitting diodes (LEDs) that have a solid state and a small size are suitable for smart NIR devices. However, NIR-LED chips can only give narrow NIR emissions, which limits their applications10,11,12. Broad NIR-emitting phosphor-converted (pc) LEDs, adopting the technology of pc-white LEDs13,14,15,16,17,18,19, are believed to be the best solution. White LEDs are commonly based on blue-LED chips. Thus, how to achieve broad NIR phosphors that can be efficiently excited by blue light is one of the most important challenges20.
Recently, a number of NIR phosphors were realized21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44. Among them, Cr3+ usually manifests a high efficiency, and the IQE can reach 58–75%31,32,33,34,35,36,37. The radiant power is 14.7–54.29 mW when driven at 100–130 mA34,35,36,37,38,39,40. A high radiant power is beneficial for monitoring and detection. Liu et al made great progress in improving the radiant power, which was enhanced from 7–18.2 mW42,43 to 65.2 mW44 when driven at 350 mA. To achieve high radiance, a high-power LED chip operating at a high current is usually needed. In this situation, the large amount of heat will result in a high temperature on the surface of the chip. Thus, the greatest challenge is how to make NIR phosphors have excellent thermal stability to overcome the thermal quenching effect, in addition to a high QE for NIR phosphors.
We note that Cr3+ has a high QE in garnets34,35. The silicate garnet Ca3Sc2Si3O12 (CSSG) is a promising host for Ce3+ due to its excellent thermal stability and high QE45,46,47. Fortunately, Cr3+ can be excited by blue light and show broadband NIR emission in CSSG39. Unfortunately, the reported luminescence (IQE: 12.8%) and thermal stability were low because the Cr3+ luminescence suffered from impurities and oxidation of Cr4+ when the material was sintered in air39. Based on our previous experience, we propose a strategy to optimize CSSG:Cr3+ by enhancing the crystallinity, modifying the micromorphology, and maintaining the valence state of Cr3+. By adding fluxes and sintering in a CO reducing atmosphere, the IQE is greatly enhanced to 92.3%. At 150 °C, 97.4% of the NIR emission at room temperature can be maintained, indicating excellent thermal stability. When combined with a high-power 460 nm blue chip, the estimated radiant power of the fabricated pc-LED even reaches 109.9 mW when driven at 520 mA. The properties of both the optimized CSSG:Cr3+ and the achieved NIR-LED are almost the best results to date as far as we know. Benefiting from the high radiant power, the pc-NIR-LED device has good application potential in night-vision technology.
In this work, mechanisms for optimization were investigated. The electron–phonon coupling (EPC) mechanism in CSSG that usually determines the Cr3+ luminescence is revealed for the first time. Many types of Cr3+-doped NIR phosphors have been discovered. We believe that this work provides an effective strategy to optimize and discover more Cr3+-doped NIR phosphors using only a simple method. Moreover, this work will advance the development and application of next-generation smart NIR light sources.
Results
Optimization of CSSG:Cr3+
CSSG belongs to a cubic crystal system with the space group of Iα3d (Fig. S1). Ca, Sc, and Si are coordinated with 8, 6, and 4 oxygen atoms, respectively45,46,47. Considering the effective ionic radii of Ca2+ (1.12 Å), Sc3+ (0.745 Å), Si4+ (0.26 Å), and Cr3+ (r = 0.615 Å), it is believed that Cr3+ occupies the Sc3+ site due to the close radii and same ionic valence39. Thus, Cr3+ suffers from a weak crystal field (CF) environment in the ScO6 octahedron (Fig. 1a). The spin-allowed transitions of 4A2g → 4T1g(4F) and 4A2g → 4T2g(4F) lead to two excitation bands centered at 460 and 640 nm, respectively (Fig. 1b). The spin-forbidden transition of 4A2g → 2Eg(2G) (R-line) at ~701 nm is also detected. Under 460 nm excitation, CSSG:Cr3+ shows a broad NIR emission peaking at ~770 nm with a full-width at half maximum (FWHM) value of ~1750 cm−1 (~110 nm), arising from the 4T2g(4F) to 4A2 transition of Cr3+ in the weak CF.
a Coordination of (Sc/Cr)O6 in CSSG. b Photoluminescence (PL) and photoluminescence excitation (PLE) spectra of CSSG:3%Cr3+. c Relative PL intensities of the samples with and without fluxes sintered in air and a CO atmosphere. d PL intensities of CSSG:3%Cr3+, xwt% Li2CO3 (x = 0.5, 0.8, 1, 1.5, 2, 3, 4, 5, 6). e PL intensities of CSSG:y%Cr3+, 1 wt% Li2CO3 (y = 1, 2, 3, 4, 5, 6).
CSSG:Cr3+ synthesized in an air atmosphere exhibits a weak NIR emission (Figs. S2–5), which could be attributed to a lower crystallinity and oxidation of Cr3+ 39. For the optimized Cr3+ concentration, that is, CSSG:3%Cr3+, its IQE and external quantum efficiency (EQE) are quite low, only ~12.8% and 4.8%, respectively (Fig. S6). To enhance the luminescence by improving the crystallinity, fluxes of NH4F, CaF2, H3BO3, LiF, and Li2CO3 were added during the synthesis under the air condition. The NIR emission of the flux-free sample was set as the normalized standard. As Fig. 1c shows, H3BO3, LiF, and Li2CO3 enhance the luminescence, while NH4F and CaF2 decrease the luminescence. Thus, H3BO3, LiF, and Li2CO3 were selected, and CSSG:3%Cr3+ was sintered in a CO reducing atmosphere to further maintain the state of Cr3+. It is noted that the luminescence is greatly enhanced by 2~3 times. Li2CO3 has the best effect, and the optimal amount is 1 wt% (Fig. 1d). Correspondingly, the IQE and EQE of CSSG:3%Cr3+ reach ~77.8% and 15.5%, respectively. Moreover, by adding 1 wt% Li2CO3 and sintering in CO, the Cr3+ concentration is optimized to 6% (Fig. 1e). Then, the achieved EQE of CSSG:6%Cr3+ even reaches 21.5%. Here, the QE is the measured result. Due to the limitation of the measurement, up to 850 nm, the results are smaller than the actual values, which will be illustrated later. Therefore, the actual QE is almost the best value among NIR phosphors as far as we know.
Crystal structures and micromorphology
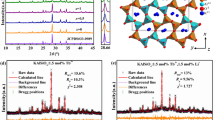
To clarify the enhancement of the optimized CSSG:Cr3+ compared with the initial phosphor, the crystal structures and micromorphology were studied. For CSSG:3%Cr3+ sintered in air (Fig. 2a), the X-ray diffraction (XRD) pattern mainly shows a garnet phase of CSSG (PDF # 72-1969), but a few impurity phases of SiO2 and Sc2O3 are also observed. These impurity phases can also be observed in the SEM-EDS mapping images. Ca, Sc, Si, O, and Cr are inhomogeneously distributed in the square region, in which Sc is rich and Si and Ca are absent.
For CSSG:3%Cr3+ sintered in CO with 1 wt% Li2CO3 (Fig. 2b), the SiO2 and Sc2O3 impurities are quite low in the XRD pattern. The distributions of elements are more homogeneous. In addition, the diffraction peak intensity of the CSSG phase increases (Fig. 2c). These results demonstrate that the enhanced crystallinity of the CSSG phase is one of the reasons for the improvement of the Cr3+ NIR emissions.
The CSSG:3%Cr3+ phosphor has a particle size of ~10 μm (Fig. 2d). The particle displays a small variation in the cathodoluminescence (CL), as the CL image shows. The bright region (point I) and the dark region (point II) are only different in CL intensity. Their normalized CL spectra are almost the same, having an emission peak at ~760 nm and an FWHM of 1583–1590 cm−1 (93–94 nm), similar to the photoluminescence (PL) spectrum in Figs. S2–S5.
Diagnosis of the valence state of Cr3+
To diagnose the change in the ionic valence of Cr, X-ray photoelectron spectroscopy (XPS), diffuse reflection (DR), and electron paramagnetic resonance (EPR) results for CSSG:3%Cr3+ sintered in air and CO are given in Fig. 3. The binding energies at 99, 344, 399, and 528 eV are from Si-2p, Ca-2p, Sc-2p, and O-1s, respectively. The binding energy at 40 eV for Cr-3p is detected in the two samples48. The changes in Cr are not apparent in the XPS spectra. However, in the DR spectra (Fig. 3c), the absorption band of Cr4+ (at ~1140 nm) is clearly identified for the sample sintered in air in addition to the absorption band of Cr3+ (at ~460 and 640 nm)34,35. This means that some Cr3+ ions are oxidized into Cr4+ even though Cr2O3 is used as the raw material. For the sample sintered in CO, the Cr4+ absorption band almost disappears, and only the Cr3+ absorption band is observed. This means that Cr3+ is well maintained in the CO reducing atmosphere.
In the 3d3 electronic configuration of Cr3+, three electrons occupy the d orbitals and give rise to a total spin of S = 3/2. 4A2g(F) is the ground state for Cr3+. In an octahedral crystal field, the 4F state splits into a singlet orbital 4A2g and two orbitals 4T1g and 4T2g, thus causing EPR signals49. As Fig. 3d shows, the sharp peaks at g = 3.8–3.9 belong to the isolated Cr3+ ion, and the peaks at approximately g = 2 represent the first neighbor Cr3+ –Cr3+ pair42,43,44,49. The EPR intensity for the sample sintered in CO is stronger than that for the sample sintered in air, demonstrating an increased ratio of Cr3+. Combining the DR and EPR results, it is claimed that Cr3+ can be maintained and increased in the reducing atmosphere, which is another reason for the improvement of the Cr3+ NIR luminescence.
Temperature-dependent NIR emissions
Figure 4a–c shows the temperature-dependent luminescence of CSSG:6%Cr3+. The integrated PL intensity at 25 °C is set as the normalized standard, and 97.4% can still be maintained at 150 °C for the phosphor sintered in CO, whereas 85.6% can be maintained for the phosphor sintered in air. The temperature dependence of the emission intensity can be well fitted by the Arrhenius formula, and the activation energy is calculated to be ∆E = 0.336 eV for the optimized sample, compared with ∆E = 0.220 eV for the initial sample (Fig. 4b). When the temperature increases from 25 to 300 °C, the peak position redshifts from ~783 to ~807 nm (Fig. 4d), attributed to the decreased CF caused by lattice expansion. The FWHM increases from 1483 to 1551 cm−1 (92.3 to 100.2 nm), attributed to the strengthened EPC effect that will be discussed in the following.
Figure 4e shows the PLE and PL spectra of CSSG:6%Cr3+ at 77 K with a step size of 0.05 nm. The transitions from 4A2g to 4T2g(4F) and 4T1g(4F) are centered at 15,670 cm−1 (~636 nm) and 22,050 cm−1 (~453 nm), respectively. The R-line is only detected in the PLE spectrum at 698.3 nm (~14,320 cm−1). The peak at ~713 nm (~14,030 cm−1) is observed in both the PL and PLE spectra, which is assigned to the zero-phonon line (ZPL) for the 4T2(4F) and 4A2g transition (Fig. S7). The energy gap between 2Eg(2G) and the 4T2g(4F) ZPL was evaluated to be ~290 cm−1, indicating a strong spin–orbit coupling (SOC) of the 2Eg(2G) and 4T2g(4F) states50. Thus, the fluorescent decay curve shows a biexponential model with a lifetime of ~190 µs (Fig. S8–S9).
The PL spectrum has a maximum peak at ~12,980 cm−1 (~770 nm) with a FWHM of 1560 cm−1 (~93 nm). The corresponding Stokes shift is ~2700 cm−1. The peak at 730 nm (~13,700 cm−1) is the phonon satellite of the ZPL. The energy difference between the ZPL and its phonon satellite is determined to be ~330 cm−1, which corresponds to one of the vibrational modes (ħw) that couple with the 4T2g(4F) → 4A2g transition (Fig. S7).
A Stokes shift has a relationship of (2S + 1)ħw, where S is the Huang–Rhys parameter; thus, S is determined to be between 3 and 4, which is smaller than the value (~6) for LSGG:Cr3+ 31. Both the broadband emissive characteristic and the large Stokes shift indicate a stronger EPC for the 4T2g(4F) → 4A2g transition in CSSG:Cr3+. A stronger EPC leads to a larger S value28,29,30,31. At a low temperature (77 K), the EPC effect is weak. Thus, the FWHM value at 77 K decreases by ~190 cm−1 compared with the FWHM of 1750 cm−1 (~110 nm) at RT.
It is worth noting that the ratio in the range of 650–725 nm increases and the emission shows a blueshift with increasing temperature. The small energy gap (~ 290 cm−1) between 2Eg and the 4T2g ZPL leads to mixing of the 2Eg(2G) and 4T2g(4F) states. When the temperature increases, electronic transfer from the 4T2g state to the 2Eg state is strengthened with the assistance of EPC. Thus, the radiative transitions from the 2Eg(2G) state increase, and blueshifts are observed.
Performance of the fabricated NIR-LED device
The CSSG:6%Cr3+ phosphor shows a green color (Fig. 5a). Based on the optimized phosphor and a high-power blue chip, an NIR-LED device was fabricated and is displayed in Fig. 5b–d. The electroluminescence (EL) spectra, optical powers, and conversion efficiencies of the device depending on the driving current (I) are given in Fig. 5e–h. The strong emission peak at ~460 nm comes from the blue chip. The broad NIR emission band comes from CSSG:Cr3+. The optical powers of both the total radiance and NIR light increase with increasing current until reaching maxima of 97.8 and 64.7 mW, respectively, at 520 mA.
a Body color of the CSSG:6%Cr3+ phosphor under sunlight. b NIR-LED device fabricated using the optimized phosphor and a 460 nm blue LED. c Working state of the NIR-LED taken without a filter and d with a longpass filter at 650 nm. e EL spectra, f output optical powers, and g conversion efficiencies of the NIR-LED depending on the driving current. h EL spectrum fitted by the Gaussian formula.
The conversion efficiency from the emitted blue light to NIR light (ηNIR/blue light) drops from 33.5 to 12.3% when the driving current increases from 100 to 600 mA. Correspondingly, the conversion efficiency from the input electronic power to NIR emissions (ηNIR/input) decreases from 7.2 to 2.3%. ηNIR/input is much lower than ηNIR/blue light. The lower photoelectric conversion efficiency (ηblue light/input) from the input electronic power to blue light should be responsible for this phenomenon because the ηblue light/input of the used blue chip, ranging from 31.9 to 19.4% at 100–600 mA, is not very high (Fig. S9). If the used blue chip is efficient, then ηNIR/input can be greatly enhanced further.
On the other hand, only 84.3% of the NIR emission can be detected due to the limitation of the measurement range, up to 850 nm (Fig. 5h). If the 15.7% unmeasured part is taken into account, then the actual NIR optical power should be 76.8 mW at 520 mA. Thus, the total optical power even reaches 109.9 mW, which is much higher than the performances reported until now44.
The QEs discussed above are the measured results. The QE and radiant power were measured by using the same spectrometer. If the unmeasured part is also taken into account, then the IQE and EQE can actually reach 92.3% and 25.5%, respectively, which are almost the best results as far as we know up to now.
Applications in night vision
Figure 6 shows the application of the NIR-LED for night vision. Visible images taken by a visible camera are colorful when water, milk, and cups are illuminated by either fluorescent light or NIR light. The logo is clear under fluorescent light. However, only black-and-white images are captured by an NIR camera. When the NIR-LED is off, nothing can be captured. When the NIR-LED is on, the logo is much clearer when it is taken by the NIR camera than when it is taken by the visible camera, especially the logo on the surface of the glass filled with transparent water. These results indicate that the achieved CSSG:Cr3+ phosphor enables the NIR-LED to have good application in night-vision technology. Potential applications in monitoring foods and medicines are also expected for such NIR phosphors and NIR-LED light sources.
Discussion
In conclusion, CSSG:Cr3+ exhibits broad NIR emission from 700 to 900 nm under blue light excitation. The previously reported CSSG:Cr3+ synthesized in air has low IQE (12.8%) and EQE (4.8%), limiting its performance in pc-NIR-LED devices. By adding fluxes and synthesizing in a CO reducing atmosphere, the IQE and EQE are greatly improved to 77.8% and 21.5%, respectively. If the unmeasured part is taken into account, then the actual IQE and EQE should reach 92.3% and 25.5%, respectively, which is almost the best result among the NIR phosphors developed until now. Investigation of the crystal structures and micromorphology demonstrated that the improvement arises from the modification of the crystallinity and the maintenance of Cr3+. The achieved CSSG:6%Cr3+ exhibits excellent thermal stability, and 97.4% of the emission intensity at room temperature can still be maintained at 150 °C. Thus, when it was used in a high-power blue chip, the fabricated NIR-LED showed a high optical power of nearly 110 mW at 520 mA, which is almost the best performance among NIR-LED light sources.
Among the reported NIR phosphors, Cr3+ is an important activator, and its luminescence is determined by both the selected host and the synthesis technology. We believe that this work provides an effective strategy to optimize NIR phosphors using only a simple but the best method. Thus, it will inspire more researchers to achieve much better performance of known NIR phosphors and advance development of next-generation smart NIR-LED light sources.
Materials and methods
Synthesis
Samples with the nominal composition of Ca3Sc2-xSi3O12:yCr3+, xwt% flux were synthesized by a high-temperature solid-state reaction. The starting materials of CaCO3 (99.9%), Sc2O3 (99.9%), SiO2 (AR), and Cr2O3 (99.95%) and fluxes of NH4F, CaF2, H3BO3, LiF, and Li2CO3 were weighed according to the nominal composition and then ground in an agate mortar for 30 min. After that, the powders were sintered at 1450 °C for 3 h in air and a CO atmosphere.
Fabrication of pc-NIR-LEDs
NIR-LEDs were fabricated using the optimized NIR phosphor CSSG:6%Cr3+ and high-power blue-LED chips (460 nm). The phosphors were thoroughly mixed with epoxy resin and then coated on the chips.
Characterization
XRD patterns were measured by a Bruker D8 X-ray diffractometer with Cu Kα radiation (λ = 1.54056 Å) at 40 kV and 40 mA. DR spectra were measured by a LAMBDA 950. PL and PLE spectra at RT-300 °C were characterized by a Hitachi F-4600. PL and PLE spectra at 77 K were measured by a Horiba FL-311 by dipping the sample in liquid nitrogen. EPR data were recorded on a Bruker E500 with the X-band frequencies (≈9.845 GHz) and a microwave power of 0.63 mW. XPS was performed on a Kratos Axis Ultra DLD. IQEs and EQEs were recorded by an Otsuka Photal Electronics QE-2100. A field-emission scanning electron microscope (FE-SEM, Hitachi S-4800) equipped with an energy dispersive X-ray spectroscopy (EDS) system and a CL system (MonoCL4, Gatan) was used to measure the morphology. EL spectra and performances of fabricated pc-NIR-LED devices were measured by an integrating sphere (Labsphere), and data were collected by a multichannel photodetector (MCPD-9800, Otsuka Photal Electronics). Visible images and NIR images were taken by a visible camera (SONY ILCE-7M2K) and an NIR camera (Work Power UC-500M), respectively.
References
Tang, X. et al. Dual-band infrared imaging using stacked colloidal quantum dot photodiodes. Nat. Photonics 13, 277–282 (2019).
Gu, Y. Y. et al. High-sensitivity imaging of time-domain near-infrared light transducer. Nat. Photonics 13, 525–531 (2019).
Tuong Ly, K. et al. Near-infrared organic light-emitting diodes with very high external quantum efficiency and radiance. Nat. Photonics 11, 63–68 (2017).
Pan, Z. W. et al. Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates. Nat. Mater. 11, 58–63 (2011).
Zeng, B. B. et al. Hybrid graphene metasurfaces for high-speed mid-infrared light modulation and single-pixel imaging. Light.: Sci. Appl. 7, 51 (2018).
Liang, Y. J. et al. New function of the Yb3+ ion as an efficient emitter of persistent luminescence in the short-wave infrared. Light.: Sci. Appl. 5, e16124 (2016).
Dincer, C. et al. Disposable sensors in diagnostics, food, and environmental monitoring. Adv. Mater. 31, 1806739 (2019).
Zampetti, A., Minotto, A. & Cacialli, F. Near-infrared (NIR) organic light-emitting diodes (OLEDs): challenges and opportunities. Adv. Funct. Mater. 29, 1807623 (2019).
Tessler, N. et al. Efficient near-infrared polymer nanocrystal light-emitting diodes. Science 295, 1506–1508 (2002).
Zhao, X. F. & Tan, Z. K. Large-area near-infrared perovskite light-emitting diodes. Nat. Photonics 14, 215–218 (2020).
Lukovic, M. et al. LED-based Vis-NIR spectrally tunable light source—the optimization algorithm. J. Eur. Optical Soc.-Rapid Publ. 12, 19 (2016).
Filippo, R., Taralli, E. & Rajteri, M. LEDs: sources and intrinsically bandwidth-limited detectors. Sensors 17, 1673 (2017).
Yao, Q. et al. YAG: Ce3+ transparent ceramic phosphors brighten the next-generation laser-driven lighting. Adv. Mater. https://doi.org/10.1002/adma.201907888 (2020).
Liu, Y. F. et al. An excellent cyan-emitting orthosilicate phosphor for NUV-pumped white LED application. J. Mater. Chem. C. 5, 12365–12377 (2017).
Liu, Y. F. et al. Ba9Lu2Si6O24:Ce3+: an efficient green phosphor with high thermal and radiation stability for solid-state lighting. Adv. Optical Mater. 3, 1096–1101 (2015).
Wei, Y. et al. New strategy for designing orangish-red-emitting phosphor via oxygen-vacancy-induced electronic localization. Light.: Sci. Appl. 8, 15 (2019).
Zhao, M. et al. Emerging ultra-narrow-band cyan-emitting phosphor for white LEDs with enhanced color rendition. Light; Sci. Appl. 8, 38 (2019).
Senden, T. et al. Quenching of the red Mn4+ luminescence in Mn4+-doped fluoride LED phosphors. Light.: Sci. Appl. 7, 8 (2018).
Dai, P. P. et al. A single Eu2+-activated high-color-rendering oxychloride white-light phosphor for white-light-emitting diodes. Light.: Sci. Appl. 5, e16024 (2016).
De Guzman, G. N. A. et al. Near-infrared phosphors and their full potential: a review on practical applications and future perspectives. J. Lumin. 219, 116944 (2020).
Du, J. R. & Poelman, D. Identifying near‐infrared persistent luminescence in Cr3+‐doped magnesium gallogermanates featuring afterglow emission at extremely low temperature. Adv. Optical Mater. 8, 1901848 (2020).
Lyu, T. S. & Dorenbos, P. Designing thermally stimulated 1.06 µm Nd3+ emission for the second bio-imaging window demonstrated by energy transfer from Bi3+ in La-, Gd-, Y-, and LuPO4. Chem. Eng. J. 372, 978–991 (2019).
Song, E. H. et al. Heavy Mn2+ doped MgAl2O4 phosphor for high-efficient near-infrared light-emitting diode and the night-vision application. Adv. Optical Mater. 7, 1901105 (2019).
Qiao, J. W. et al. Divalent europium-doped near-infrared-emitting phosphor for light-emitting diodes. Nat. Commun. 10, 5267 (2019).
Zhao, F. Y. et al. Double perovskite Cs2AgInCl6:Cr3+: broadband and near-infrared luminescent materials. Inorg. Chem. Front. 6, 3621–3628 (2019).
Wang, C. P. et al. An ultra-broadband near-infrared Cr3+-activated gallogermanate Mg3Ga2GeO8 phosphor as light sources for food analysis. ACS Appl. Electron. Mater. 1, 1046–1053 (2019).
Dai, D. J. et al. Broad band emission near-infrared material Mg3Ga2GeO8:Cr3+: substitution of Ga-In, structural modification, luminescence property and application for high efficiency LED. J. Alloy. Compd. 806, 926–938 (2019).
Malysa, B., Meijerink, A. & Jüstel, T. Temperature dependent luminescence Cr3+-doped GdAl3(BO3)4 and YAl3(BO3)4. J. Lumin. 171, 246–253 (2016).
Malysa, B. et al. On the influence of calcium substitution to the optical properties of Cr3+ doped SrSc2O4. J. Lumin. 190, 234–241 (2017).
Malysa, B., Meijerink, A. & Jüstel, T. Temperature dependent photoluminescence of Cr3+ doped Sr8MgLa(PO4)7. Optical Mater. 85, 341–348 (2018).
Malysa, B., Meijerink, A. & Jüstel, T. Temperature dependent Cr3+ photoluminescence in garnets of the type X3Sc2Ga3O12 (X=Lu, Y, Gd, La). J. Lumin. 202, 523–531 (2018).
Zeng, H. T. et al. Two-site occupation for exploring ultra-broadband near-infrared phosphor - double-perovskite La2MgZrO6:Cr3+. Chem. Mater. 31, 5245–5253 (2019).
Yu, D. C. et al. Non-rare-earth Na3AlF6:Cr3+ phosphors for far-red light-emitting diodes. ACS Appl. Electron. Mater. 1, 2325–2333 (2019).
Zhang, L. L. et al. Cr3+-doped broadband NIR garnet phosphor with enhanced luminescence and its application in NIR spectroscopy. Adv. Optical Mater. 7, 1900185 (2019).
Zhang, L. L. et al. A high efficiency broad-band near-infrared Ca2LuZr2Al3O12:Cr3+ garnet phosphor for blue LED chips. J. Mater. Chem. C. 6, 4967–4976 (2018).
Shao, Q. Y. et al. Photoluminescence properties of a ScBO3:Cr3+ phosphor and its applications for broadband near-infrared LEDs. RSC Adv. 8, 12035–12042 (2018).
Xu, X. X. et al. Highly efficient and thermally stable Cr3+-activated silicate phosphors for broadband near-infrared LED applications. Chem. Eng. J. 383, 123108 (2020).
Shao, Q. Y. et al. Broadband near-infrared light source derived from Cr3+-doped phosphors and a blue LED chip. Opt. Lett. 43, 5251–5254 (2018).
Yao, L. Q. et al. Broadband emission of single-phase Ca3Sc2Si3O12:Cr3+/Ln3+ (Ln = Nd, Yb, Ce) phosphors for novel solid-state light sources with visible to near-infrared light output. Ceram. Int. 45, 14249–14255 (2019).
Hayashi, D. et al. A broadband LED source in visible to short-wave-infrared wavelengths for spectral tumor diagnostics. Appl. Phys. Lett. 110, 233701 (2017).
Huang, W. T. et al. Broadband Cr3+, Sn4+-doped oxide nanophosphors for infrared mini light-emitting diodes. Angew. Chem. Int. Ed. 58, 2069–2072 (2019).
Lee, C. et al. Chromium(III)-doped fluoride phosphors with broadband infrared emission for light-emitting diodes. Inorg. Chem. 59, 376–385 (2020).
Rajendran, V. et al. Super broadband near-infrared phosphors with high radiant flux as future light sources for spectroscopy applications. ACS Energy Lett. 3, 2679–2684 (2018).
Rajendran, V. et al. Ultra-broadband phosphors converted near-infrared light emitting diode with efficient radiant power for spectroscopy applications. ACS Photonics 6, 3215–3224 (2019).
Shimomura, Y. et al. Photoluminescence and crystal structure of green-emitting Ca3Sc2Si3O12:Ce3+ phosphor for white light emitting diodes. J. Electrochem. Soc. 154, J35–J38 (2007).
Liu, Y. F. et al. Generation of broadband emission by incorporating N3− into Ca3Sc2Si3O12:Ce3+ garnet for high rendering white LEDs. J. Mater. Chem. 21, 6354–6358 (2011).
Liu, Y. F. et al. Tunable full-color-emitting Ca3Sc2Si3O12:Ce3+, Mn2+ phosphor via charge compensation and energy transfer. Chem. Commun. 47, 10677–10679 (2011).
Scierka, S. J. et al. Determination of the distribution of chromium oxidation states in reduced Cr/Al2O3 catalysts from XPS by factor analysis and curve fitting. Surf. Interface Anal. 20, 901–908 (1993).
Edgar, A. & Hutton, D. R. Exchange-coupled pairs of Cr3+ ions in emerald. J. Phys. C: Solid State Phys. 11, 5051–5063 (1978).
Struve, B. & Huber, G. The effect of the crystal field strength on the optical spectra of Cr3+ in gallium garnet laser crystals. Appl. Phys. B 36, 195–201 (1985).
Acknowledgements
This work is financially supported by the National Key Research and Development Program of China (2016YFC0104502, 2017YFC0111602), Fujian Institute of Innovation, Chinese Academy of Sciences (FJCXY18040203), Public Projects of Zhejiang Province (LGG18E020007), Science and Techology Major Project of Ningbo Municipality (2017C110028), and Natural Science Foundation of Shanxi Province (201801D121020, 201801D221132).
Author information
Authors and Affiliations
Contributions
Y.L. and L.W. initiated the research. The design of the experiments took shape with input from all the authors. Z.J. and C.Y. performed the experiments and measurement with support from P.S. and prepared the draft of the paper. X.W., H.J., and J.J. discussed the paper. All authors assisted in the editing of the final paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jia, Z., Yuan, C., Liu, Y. et al. Strategies to approach high performance in Cr3+-doped phosphors for high-power NIR-LED light sources. Light Sci Appl 9, 86 (2020). https://doi.org/10.1038/s41377-020-0326-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41377-020-0326-8
This article is cited by
-
A highly thermal stable cubic-phase Sc(PO3)3:Cr3+ phosphor with emitting peak at 875 nm
Science China Materials (2024)
-
Broadband near-infrared luminescence properties of fluoride phosphor K3AlF6:Cr3+
Journal of Materials Science: Materials in Electronics (2024)
-
Intervalence charge transfer of Cr3+-Cr3+ aggregation for NIR-II luminescence
Light: Science & Applications (2023)
-
Valence conversion and site reconstruction in near-infrared-emitting chromium-activated garnet for simultaneous enhancement of quantum efficiency and thermal stability
Light: Science & Applications (2023)
-
Energy transfer induced colour tunable photoluminescence performance of thermally stable Sm3+/Eu3+ co-doped Ba3MoTiO8 phosphors for white LED applications
Journal of Materials Science: Materials in Electronics (2023)