Graphical Abstract

Similar content being viewed by others
To the Editor:
Recent studies have identified mutational genomic signatures introduced by Apolipoprotein B mRNA-Editing Catalytic Polypeptide-like (APOBEC) deaminases as well as inflammatory processes as being pivotal for MM onset and progression [1,2,3,4]. Although these recent insights provide a better understanding of MM pathogenesis, they have not yet been translated into clinical applications such as MM risk stratification. The current standards for MM patient risk classification are the International Staging System (ISS), the Revised ISS (R-ISS) and the second revision of the R-ISS (R2-ISS) introduced between 2005 and 2022, respectively [5]. All scores are based on clinical parameters reflecting tumor burden, and the newer R-ISS and R2-ISS further incorporate high-risk cytogenetics [5]. Considering that most risk-defining chromosomal abnormalities reflect early events in MM cells [6], we concluded that tumor burden and/or cytogenetics-based classifiers might not accurately reflect the dynamics of disease progression in MM patients. Therefore, we hypothesized that a predictive score which reflects molecular mechanisms that drive MM progression, can improve the accuracy of current MM risk classifiers. To test this hypothesis, we constructed and validated a proof-of-principle risk classifier called Editor/Inflammation- or EI-score, which combines mRNA levels of survival-associated APOBEC genes, pro/anti-inflammatory genes as well as clinical markers for MM disease burden.
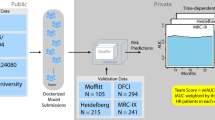
Data from 1143 patients with newly diagnosed MM (NDMM) and available survival information was obtained through the CoMMpass database version IA14, which was generated as part of the Multiple Myeloma Research Foundation (MMRF) Personalized Medicine Initiatives (www.themmrf.org). ISS, R-ISS and R2-ISS staging information was available for 1113, 694, and 694 patients, respectively. For 599 patients, information on both blood parameters and RNA-seq was available. As an independent validation cohort, we analyzed clinical, cytogenetic, and RNA-seq data from 263 NDMM patients treated as part of the IFM/DFCI 2009 trial (ClinicalTrials.gov identifier: NCT01191060) [7]. IFM/DFCI patients were treated with Bortezomib, Lenalidomide and Dexamethasone (VRD) alone or with VRD+autologous stem cell transplantation (ASCT). All patient baseline characteristics (CoMMpass and IFM/DFCI) are summarized in Table S1. A stepwise workflow for the evaluation and selection of individual features and multivariate models in the MMRF CoMMpass dataset is shown in Fig. S1 and described in detail in the Supplementary Methods.
To translate recent whole genome- and RNA-sequencing findings into a predictive score, we pre-selected 163 features, including demographic, clinical, genomic, and cytogenetic information, as well as inflammatory signaling and nucleotide editing-associated mRNA covariates from the MMRF CoMMpass dataset (Fig. S1). Of the 163 tested variables, 25 for overall survival (OS) and 21 for progression-free survival (PFS) showed significant time-to-event outcomes. Notably, only one out of five cytogenetic features, namely +1q/amp1q (Fig. S2), passed our stringent selection criteria in 599 NDMM patients. In line with our hypothesis, we found that mRNA levels of individual APOBEC genes as well as APOBEC-induced genomic mutational signatures (calculated in form of both COSMIC single-base substitution (SBS) signature and APOBEC mutation enrichment score [8, 9]) were associated with inferior OS and PFS (Fig. S2). As the rationale of this study was not to provide a score for immediate clinical application but rather to determine if combining APOBEC and inflammation-associated gene expression variables holds prognostic merit for MM patients, we reduced our feature set to only the most significant variables that were associated with both OS and PFS. We then combined all age- and treatment-independent prognostic variables that passed our selection criteria (and for RNA parameters, showed a median expression >5 fragments per kilobase per million) into multivariate CoxPH models, excluding patient cytogenetics and mutational signatures. This included the following parameters: ß2M, Creatinine, Hemoglobin, LDH, APOBEC2, APOBEC3A, APOBEC3B, APOBEC3C, APOBEC3D, APOBEC3F, APOBEC3G, IL10, IL11, IL17C, IL27, IFNG, TGFB1, TGFB3, IL22RA1, IL2RA, TGFBR3, CXCL13. Patient age >75 y was excluded due to the inclusion criteria of the IFM/DFCI2009 study (18–65 y). The multivariate model with the highest predictive performance while retaining as few parameters as possible included the following seven features: ß2M, LDH, APOBEC2, APOBEC3B, IL11, TGFB1, TGFB3. Based on these seven parameters, we devised a streamlined scoring formula that relies on maximally selected rank statistics established cut-offs and incorporates weights derived from the rounded integer multivariate CoxPH z-score of each parameter. Although we detected strong correlation among expression levels of most members of the APOBEC family, there was no significant positive correlation between APOBEC2 and APOBEC3B (Pearson’s R = 0.039), which are both part of the EI-score (Fig. S3). The distribution of each expressed EI-score gene in the different MMRF CoMMpass cytogenetic and age groups is shown in Fig. S4.
To evaluate the prognostic accuracy of the EI-score compared to ISS, R-ISS, R2ISS, and mSMARTcyto (a reduced version of the Mayo clinic mSMART score: https://www.msmart.org, based on the presence of t(4;14), t(14;16), t(14;20), +1q and/or del(17p)), we computed performance metrics for the outcome prediction of each score in MMRF CoMMpass patients (Table 1, Fig. S5). The EI-score achieved the best performance for OS and PFS prediction (n = 599; Concordance index (Ci) 0.7 and 0.69, respectively), followed by R2-ISS (n = 694; Ci 0.66 and 0.61), ISS (n = 1113; Ci 0.66 and 0.6), R-ISS (n = 690; Ci 0.64 and 0.6), and mSMARTcyto (n = 823; Ci 0.58 and 0.54). We then successfully validated the EI-score in the IFM/DFCI2009 NDMM cohort (n = 263) (Fig. S5), representing a homogeneously treated patient collective. Notably, addition of EI-score gene expression information to ISS, R-ISS, R2-ISS (Fig. 1A, Table 1, Table S2), and mSMARTcyto, improved the performance of each classifier significantly. Moreover, applying the EI-score exclusively to MM patient subgroups with del(17p), +1q, and t(4;14) allowed to identify previously unrecognized favorable risk patients with adverse risk cytogenetics in the MMRF CoMMpass (Fig. 1B, Fig. S6) as well as in the IFM/DFCI cohort (Fig. 1C). In line, we found that del(17p), +1q, and t(4;14) patients with a high EI-score, displayed an enrichment of APOBEC-induced genomic mutations compared to low/intermediate EI-score patients (Fig. S7). These results demonstrate that the integration of APOBEC and inflammatory cytokine mRNA levels improve the prognostic capacity of chromosomal abnormalities, which are currently viewed as risk class defining. To adjust for the heterogeneous treatment protocols of patients included in the MMRF CoMMpass dataset, we also conducted a sub-analysis of MM patients receiving Cyclophosphamide, Bortezomib, Dexamethasone (CyBorD) or VRD ± ASCT (Fig. S8) and a sub-analysis of MM patients receiving VRD ± ASCT + maintenance therapy (Fig. S9), in which the EI-score also outperformed ISS, R-ISS, and R2-ISS. A possible explanation why APOBEC family members have so far not been part of probe-based mRNA classifiers such as EMC-92 [10] and UAMS-70 [11] is likely due to their high sequence similarity resulting in probe cross-hybridization and multimapping to several APOBEC members [12]. The high hazard ratio and predictive performance of APOBEC3B expression for adverse PFS and OS which appears to be independent from that of APOBEC-induced mutational signatures, likely reflects APOBEC3B’s additional involvement in MM pathogenesis through immune editing, viral and retroelement restriction, DNA demethylation, and tissue homeostasis [13]. Although APOBEC3B-induced C-to-U lesions are typically resolved by DNA repair response mechanisms, they can promote chronic replication stress and thus contribute to MM development, which could be a reason for the high predictive value we observed for APOBEC mRNA levels with MM patient outcomes. The MM microenvironment is characterized by a desynchronized cytokine milieu, with imbalanced pro- and anti-inflammatory factors that impact on MM and niche cells. Besides their general role in inflammatory processes, IL-11 as well as TGF-ß have both been implicated in the growth and differentiation block of osteoblasts [14], which in turn modulates MM cell activity. Likewise, APOBEC3B and APOBEC2 upregulation has been linked to systemic inflammation [13], suggesting that a pro-inflammatory microenvironment in MM cells could drive APOBEC2 and APOBEC3B expression. However, the precise regulation and function of APOBEC2 and APOBEC3B in MM cells still needs to be defined.
Shown are graphical representations of OS Kaplan–Meier estimates based on the application of the EI-score[OS] to (A) MMRF CoMMpass patients who were stratified into ISS and R-ISS stage II and III as well as into R2-ISS low intermediate, high intermediate, and high risk groups. B MMRF CoMMpass patients carrying del(17p), t(4;14), or +1q, and (C) IFM/DFCI patients carrying del(17p), t(4;14), or +1q reclassified by the EI-score.
In this study, we have developed the EI-score which serves as an important proof-of-concept, demonstrating that inclusion of molecular markers that reflect disease progression can improve MM risk assessment. Although our data highlights the limitations of cytogenetics-based risk stratifiers, ISS, R-ISS and R2-ISS represent the current clinical standard due to their accessibility. Eventually, the development of more contemporary stratification systems will be necessary to improve risk- and treatment stratifications of MM patients.
Data availability
MMRF sequencing data is available through the CoMMpass database version IA14 (www.themmrf.org). DFM/DFCI 2009 sequencing data can be requested through Nikhil_munshi@dfci.harvard.edu.
References
Rustad EH, Yellapantula V, Leongamornlert D, Bolli N, Ledergor G, Nadeu F, et al. Timing the initiation of multiple myeloma. Nat Commun. 2020;11:1917.
Maura F, Petljak M, Lionetti M, Cifola I, Liang W, Pinatel E, et al. Biological and prognostic impact of APOBEC-induced mutations in the spectrum of plasma cell dyscrasias and multiple myeloma cell lines. Leukemia. 2018;32:1044–8.
Bolli N, Biancon G, Moarii M, Gimondi S, Li Y, de Philippis C, et al. Analysis of the genomic landscape of multiple myeloma highlights novel prognostic markers and disease subgroups. Leukemia. 2018;32:2604–16.
Walker BA, Mavrommatis K, Wardell CP, Ashby TC, Bauer M, Davies FE, et al. Identification of novel mutational drivers reveals oncogene dependencies in multiple myeloma. Blood. 2018;132:587–97.
D’Agostino M, Cairns DA, Lahuerta JJ, Wester R, Bertsch U, Waage A, et al. Second revision of the international staging system (R2-ISS) for overall survival in multiple myeloma: a European Myeloma Network (EMN) report within the HARMONY project. J Clin Oncol. 2022;40:3406–18.
Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer. 2002;2:175–87.
Samur MK, Aktas Samur A, Fulciniti M, Szalat R, Han T, Shammas M, et al. Genome-wide somatic alterations in multiple myeloma reveal a superior outcome group. J Clin Oncol. 2020;38:3107–18.
Jarvis MC, Ebrahimi D, Temiz NA, Harris RS. Mutation signatures including APOBEC in cancer cell lines. JNCI Cancer Spectr. 2018;2:pky002.
Roberts SA, Lawrence MS, Klimczak LJ, Grimm SA, Fargo D, Stojanov P, et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet. 2013;45:970–6.
Kuiper R, Broyl A, de Knegt Y, van Vliet MH, van Beers EH, van der Holt B, et al. A gene expression signature for high-risk multiple myeloma. Leukemia. 2012;26:2406–13.
Shaughnessy JD Jr., Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–84.
Burns MB, Leonard B, Harris RS. APOBEC3B: pathological consequences of an innate immune DNA mutator. Biomed J. 2015;38:102–10.
Cervantes-Gracia K, Gramalla-Schmitz A, Weischedel J, Chahwan R. APOBECs orchestrate genomic and epigenomic editing across health and disease. Trends Genet. 2021;37:1028–43.
Giuliani N, Ferretti M, Bolzoni M, Storti P, Lazzaretti M, Dalla Palma B, et al. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia. 2012;26:1391–401.
Acknowledgements
We thank the MMRF for kindly providing access to the data from the CoMMpass study. This work was supported by the BC Cancer Foundation and the Park, Armstrong, McKeen, Hungerford, Carruthers and DeLasalle families as well as through the International Myeloma Society (IMS) and a Paula and Rodger Riney Foundation Translational Research Grant and the LLSC. SG was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation – project 446251518), the Michael Smith Foundation for Health Research (MSFHR) and the Lotte & John Hecht Memorial Foundation (project RT-2020-0578). AP was supported by Myeloma Canada Dr. Belch MEET Grant, 4-year UBC doctoral fellowship (#6569) and an Echoridge Educational Foundation Scholarship (VGH & UBC Hospital Foundation). Cancer studies in the Harris lab are supported by NCI P01 CA234228 and a Recruitment of Established Investigators Award from the Cancer Prevention and Research Institute of Texas (CPRIT RR220053). R.S.H. is an investigator of the Howard Hughes Medical Institute and the Ewing Halsell President’s Council Distinguished Chair. ML received support through LLSC and CIHR grants (MOP111132). FB was supported by Weyerhaeuser Grad Schol (MBB) scholarship from Simon Fraser University.
Author information
Authors and Affiliations
Contributions
This study was designed by FK, AR, SG, AP, and supervised by FK, AR, KS, and ML. Data analysis was carried out by SG, AP, and MJ. NM, MS, and HA provided (IFM/DFCI 2009) patient RNA sequencing and clinical data. The manuscript was written by FK, SG, and AP, and revised by FK, AR, KS, FB, ML, RH, NM, and MS. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grasedieck, S., Panahi, A., Jarvis, M.C. et al. Redefining high risk multiple myeloma with an APOBEC/Inflammation-based classifier. Leukemia (2024). https://doi.org/10.1038/s41375-024-02210-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41375-024-02210-0