Abstract
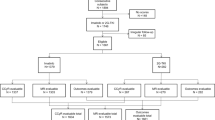
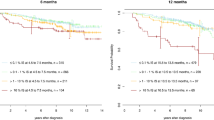
We interrogated data from 278 consecutive subjects with chronic myeloid leukaemia (CML) presenting in accelerated phase diagnosed by European LeukemiaNet (ELN) criteria receiving initial imatinib (n = 187) or a 2nd-generation tyrosine kinase-inhibitor (2G-TKI; n = 91). In multi-variable analyses, blood and/or bone marrow blasts ≥15% (Hazard ratio [HR] = 3.7 [1.6, 8.5], p = 0.003) and blood basophils <3% (HR = 4.6 [2.0, 10.7], p < 0.001) were significantly-associated with worse transformation-free survival (TFS). Age ≥60 years (HR = 4.3 [1.7, 11.4], p = 0.003), platelet concentration <230 × 10E + 9/L (HR = 4.7 [2.0, 10.7], p < 0.001) and blood and/or bone marrow blasts ≥9% (HR = 3.9 [1.7, 8.7], p = 0.001) were significantly-associated with worse survival. Based on number of adverse prognostic co-variates of TFS and survival, respectively, subjects were classified into the low- (none), intermediate- (one) and high-risk (≥2) cohorts with significant difference in TFS and survival (all p < 0.001). In propensity-score matching analysis subjects initially receiving a 2G-TKI had higher cumulative incidences of cytogenetic and molecular responses but similar TFS and survival to those receiving imatinib. Our data should help inform physicians treating person with CML initially presenting in accelerated phase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hoffmann VS, Baccarani M, Hasford J, Lindoerfer D, Burgstaller S, Sertic D, et al. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia. 2015;29:1336–1343.
Lauseker M, Bachl K, Turkina A, Faber E, Prejzner W, Olsson-Strömberg U, et al. Prognosis of patients with chronic myeloid leukemia presenting in advanced phase is defined mainly by blast count, but also by age, chromosomal aberrations and hemoglobin. Am J Hematol. 2019;94:1236–1243.
Ohanian M, Kantarjian HM, Shoukier M, Dellasala S, Musaelyan A, Nogueras Gonzalez GM, et al. The clinical impact of time to response in de novo accelerated-phase chronic myeloid leukemia. Am J Hematol. 2020;10.1002/ajh.25907. https://doi.org/10.1002/ajh.25907 [published online ahead of print].
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–6051.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884.
Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820.
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–984.
Deininger MW, Shah NP, Altman JK, Berman E, Bhatia R, Bhatnagar B, et al. Chronic myeloid leukemia, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2020;18:1385–1415.
Qin YZ, Jiang Q, Jiang H, Lai YY, Shi HX, Chen WM, et al. Prevalence and outcomes of uncommon BCR-ABL1 fusion transcripts in patients with chronic myeloid leukaemia: data from a single centre. Br J Haematol. 2018;182:693–700.
Qin YZ, Jiang Q, Jiang H, Li JL, Li LD, Zhu HH, et al. Which method better evaluates the molecular response in newly diagnosed chronic phase chronic myeloid leukemia patients with imatinib treatment, BCR-ABL(IS) or log reduction from the baseline level? Leuk Res. 2013;37:1035–1040.
Guilhot F, Apperley J, Kim DW, Bullorsky EO, Baccarani M, Roboz GJ, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–4150.
Mnatzaganian G, Davidson DC, Hiller JE, Ryan P. Propensity score matching and randomization. J Clin Epidemiol. 2015;68:760–768.
Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–1294.
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259.
Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–2405. Blood 2016 Jul 21; 128(3): 462-463.
Kantarjian HM, Dixon D, Keating MJ, Talpaz M, Walters RS, McCredie KB, et al. Characteristics of accelerated disease in chronic myelogenous leukemia. Cancer. 1988;61:1441–1446.
Sokal JE, Baccarani M, Russo D, Tura S. Staging and prognosis in chronic myelogenous leukemia. Semin Hematol. 1988;25:49–61.
Acknowledgements
We thank medical staff and patients’ participants. RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme. Funded, in part, by the National Nature Science Foundation of China (No. 81970140) and CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2019-I2M-5-034).
Author information
Authors and Affiliations
Contributions
QJ designed the study. QJ, SY and X-SZ collected and analyzed the data. QJ, SY, X-SZ, RPG and X-JH prepared the typescript. All authors approved the final typescript, take responsibility for the content and agreed to submit for publication.
Corresponding authors
Ethics declarations
Competing interests
RPG is a consultant to BeiGene Ltd., Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Parmaceuticals Inc. and CStone Pharmaceuticals; advisor to Antegene Biotech LLC, Medical Director, FFF Enterprises Inc.; partner, AZAC Inc.; Board of Directors, Russian Foundation for Cancer Research Support; and Scientific Advisory Board: StemRad Ltd.
Ethics approval
Approve by the Ethics Committee of People’s Hospital Beijing compliant with precepts the Helsinki Declaration.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yang, S., Zhang, Xs., Gale, R.P. et al. Co-variates associated with outcomes of tyrosine kinase-inhibitor therapy in persons with chronic myeloid leukaemia initially presenting in accelerated phase. Leukemia 36, 1818–1824 (2022). https://doi.org/10.1038/s41375-022-01583-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01583-4
This article is cited by
-
Allogeneic stem cell transplantation is still a highly curative therapy in adults with philadelphia chromosome–positive acute lymphoblastic leukaemia
Annals of Hematology (2024)
-
Pathogenesis and management of accelerated and blast phases of chronic myeloid leukemia
Leukemia (2023)
-
Patients with chronic myeloid leukemia and coronavirus disease 2019 in the Omicron era
Annals of Hematology (2023)