Abstract
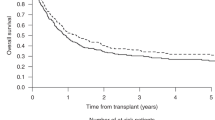
Allogeneic haemopoietic cell transplant (allo-HCT) may be curative in acute myeloid leukaemia (AML) in second complete remission (CR2) but the impact of reduced intensity (RIC) versus myeloablative conditioning (MAC) is uncertain. The Acute Leukaemia Working Party of the European Society for Blood and Bone Marrow Transplantation Registry studied an AML CR2 cohort characterised by age ≥ 18 years, first allo-HCT 2007–2016, available cytogenetic profile at diagnosis, donors who were matched family, volunteer unrelated with HLA antigen match 10/10 or 9/10 or haplo-identical. The 1879 eligible patients included 1010 (54%) MAC allo-HCT recipients. In patients <50 years (y), two year outcomes for MAC vs RIC allo-HCT were equivalent with leukaemia-free survival (LFS) 54% for each, overall survival (OS), 61% vs 62%, non-relapse mortality (NRM) 18% vs 15% and graft versus host disease relapse-free survival (GRFS) 38% vs 42%. In patients ≥50 y, 2 y outcomes for MAC vs RIC allo-HCT were equivalent for LFS 52% vs 49%, OS 58% vs 55% and GRFS 42.4% vs 36%. However, NRM was significantly inferior after MAC allo-HCT, 27% vs 19% (P = 0.01) despite worse cGVHD after RIC-allo (32% vs 39%). These data support the need for ongoing prospective study of conditioning intensity and GVHD mitigation in AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2016;129:424–47.
Ivey A, Hills RK, Simpson MA, Jovanovic JV, Gilkes A, Grech A, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016;374:422–33.
Freeman SD, Hills RK, Virgo P, Khan N, Couzens S, Dillon R, et al. Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J Clin Oncol J Am Soc Clin Oncol. 2018;36:1486–97.
Breems DA, Van Putten WLJ, Huijgens PC, Ossenkoppele GJ, Verhoef GEG, Verdonck LF, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol J Am Soc Clin Oncol. 2005;23:1969–78.
Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhäuser M, Juliusson G, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9:579–90.
Appelbaum FR Indications for allogeneic hematopoietic cell transplantation for acute myeloid leukemia in the genomic era. Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Meet. 2014;34:e327–33.
Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–61.
Cornelissen JJ, van Putten WLJ, Verdonck LF, Theobald M, Jacky E, Daenen SMG, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109:3658–66.
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101.
Burnett A, Wetzler M, Löwenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol J Am Soc Clin Oncol. 2011;29:487–94.
Cornelissen JJ, Versluis J, Passweg JR, van Putten WLJ, Manz MG, Maertens J, et al. Comparative therapeutic value of post-remission approaches in patients with acute myeloid leukemia aged 40–60 years. Leukemia. 2015;29:1041–50.
Gorin N-C, Giebel S, Labopin M, Savani BN, Mohty M, Nagler A. Autologous stem cell transplantation for adult acute leukemia in 2015: time to rethink? Present status and future prospects. Bone Marrow Transpl. 2015;50:1495–502.
Forman SJ, Rowe JM. The myth of the second remission of acute leukemia in the adult. Blood. 2013;121:1077–82. Feb 14.
Gale RP, Lazarus HM. Correcting 2 more myths regarding transplants for AML in second remission. Blood. 2014;123:794.
Gale RP, Wiernik PH, Lazarus HM. Should persons with acute myeloid leukemia have a transplant in first remission? Leukemia. 2014;28:1949–52.
Ganzel C, Sun Z, Cripe LD, Fernandez HF, Douer D, Rowe JM, et al. Very poor long-term survival in past and more recent studies for relapsed AML patients: the ECOG-ACRIN experience. Am J Hematol. 2018;93:1074–81.
Burnett AK, Goldstone A, Hills RK, Milligan D, Prentice A, Yin J, et al. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol J Am Soc Clin Oncol. 2013;31:1293–301.
Kurosawa S, Yamaguchi T, Miyawaki S, Uchida N, Sakura T, Kanamori H, et al. Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica. 2010;95:1857–64.
Hospital M-A, Prebet T, Bertoli S, Thomas X, Tavernier E, Braun T, et al. Core-binding factor acute myeloid leukemia in first relapse: a retrospective study from the French AML Intergroup. Blood. 2014;124:1312–9.
Weisdorf DJ, Millard HR, Horowitz MM, Hyare PS, Champlin R, Ho V, et al. Allogeneic transplantation for advanced acute myeloid leukemia: the value of complete remission. Cancer. 2017;123:2025–34.
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075–83.
European Society for Blood and Marrow Transplantation. http://www.ebmt.org. Accessed 30 Mar 2018.
Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–65.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2009;15:1628–33.
Shimoni A, Labopin M, Savani B, Hamladji R-M, Beelen D, Mufti G, et al. Intravenous busulfan compared with treosulfan-based conditioning for allogeneic stem cell transplantation in acute myeloid leukemia: a study on behalf of the acute leukemia working party of European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2018;24:751–7.
Saraceni F, Labopin M, Hamladji R-M, Mufti G, Socié G, Shimoni A, et al. Thiotepa-busulfan-fludarabine compared to busulfan-fludarabine for sibling and unrelated donor transplant in acute myeloid leukemia in first remission. Oncotarget. 2018;9:3379–93.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995;15:825–8.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transpl. 2016;51:610–1.
Hougaard P. Frailty models for survival data. Lifetime Data Anal. 1995;1:255–73.
Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–500.
Michelis FV, Gupta V, Zhang M-J, Wang H-L, Aljurf M, Bacher U, et al. Cytogenetic risk determines outcomes after allogeneic transplantation in older patients with acute myeloid leukemia in their second complete remission: a Center for International Blood and Marrow Transplant Research cohort analysis. Cancer. 2017;123:2035–42.
Michelis FV, Messner HA, Atenafu EG, Kim DD, Kuruvilla J, Lipton JH, et al. Benefit of allogeneic transplantation in patients age ≥ 60 years with acute myeloid leukemia is limited to those in first complete remission at time of transplant. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2014;20:474–9.
Michelis FV, Atenafu EG, Couban S, Frazer J, Shivakumar S, Hogge DE, et al. Duration of first remission and hematopoietic cell transplantation-specific comorbidity index but not age predict survival of patients with AML transplanted in CR2: a retrospective multicenter study. Bone Marrow Transpl. 2016;51:1019–21.
Gyurkocza B, Storb R, Storer BE, Chauncey TR, Lange T, Shizuru JA, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol J Am Soc Clin Oncol. 2010;28:2859–67.
Yanada M, Mori J, Aoki J, Harada K, Mizuno S, Uchida N, et al. Effect of cytogenetic risk status on outcomes for patients with acute myeloid leukemia undergoing various types of allogeneic hematopoietic cell transplantation: an analysis of 7812 patients. Leuk Lymphoma. 2018;59:601–9.
Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol J Am Soc Clin Oncol. 2006;24:5695–702.
Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–83.
Tiercy J-M, Villard J, Roosnek E. Selection of unrelated bone marrow donors by serology, molecular typing and cellular assays. Transpl Immunol. 2002;10:215–21.
Chang Y-J, Wang Y, Liu Y-R, Xu L-P, Zhang X-H, Chen H, et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol OncolJ Hematol Oncol. 2017;10:134.
Eapen, M. Unrelated donor transplantation: peripheral blood or bone marrow—does it matter? Best Pract Res Clin Haematol. 27:278–82.
Gilleece MH, Labopin M, Yakoub-Agha I, Volin L, Socié G, Ljungman P, et al. Measurable residual disease, conditioning regimen intensity, and age predict outcome of allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first remission: a registry analysis of 2292 patients by the Acute Leukemia Working Party European Society of Blood and Marrow Transplantation. Am J Hematol. 2018;93:1142–52.
Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol J Am Soc Clin Oncol. 2017;35:1154–61.
Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108:1092–9.
Holtick U, Shimabukuro-Vornhagen A, Chakupurakal G, Theurich S, Leitzke S, Burst A, et al. FLAMSA reduced-intensity conditioning is equally effective in AML patients with primary induction failure as well as in first or second complete remission. Eur J Haematol. 2016;96:475–82.
Malard F, Labopin M, Stuhler G, Bittenbring J, Ganser A, Tischer J, et al. Sequential intensified conditioning regimen allogeneic hematopoietic stem cell transplantation in adult patients with intermediate- or high-risk acute myeloid leukemia in complete remission: a study from the acute leukemia working party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2017;23:278–84.
Khwaja A, Bjorkholm M, Gale RE, Levine RL, Jordan CT, Ehninger G, et al. Acute myeloid leukaemia. Nat Rev Dis Prim. 2016;2:16010.
Kadia TM, Ravandi F, Cortes J, Kantarjian H. New drugs in acute myeloid leukemia. Ann Oncol J Eur Soc Med Oncol. 2016;27:770–8.
Sallman DA, Lancet JE. What are the most promising new agents in acute myeloid leukemia? Curr Opin Hematol. 2017;24:99–107.
Stone RM. Which new agents will be incorporated into frontline therapy in acute myeloid leukemia? Best Pr Res Clin Haematol. 2017;30:312–6.
Yang D, Zhang X, Zhang X, Xu Y. The progress and current status of immunotherapy in acute myeloid leukemia. Ann Hematol. 2017;96:1965–82.
Michelis FV, Atenafu EG, Gupta V, Kim DD, Kuruvilla J, Lambie A, et al. Duration of first remission, hematopoietic cell transplantation-specific comorbidity index and patient age predict survival of patients with AML transplanted in second CR. Bone Marrow Transpl. 2013;48:1450–5.
Michelis FV, Messner HA, Atenafu EG, McGillis L, Lambie A, Uhm J, et al. Patient age, remission status and HCT-CI in a combined score are prognostic for patients with AML undergoing allogeneic hematopoietic cell transplantation in CR1 and CR2. Bone Marrow Transpl. 2015;50:1405–10.
Sorror ML, Logan BR, Zhu X, Rizzo JD, Cooke KR, McCarthy PL, et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: a center for international blood and marrow transplant research study. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2015;21:1479–87.
Acknowledgements
We thank all European Group for Blood and Marrow Transplantation (EBMT) centres and national registries for contributing patients to the study and data managers for their superb work. The study was supported by the European Blood & Marrow Transplantation funded by annual subscription from the constituent transplant centres.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MHG—Travel grant, advisory board and speaker fees—Jazz. BS—Honoraria from JAZZ Pharmaceuticals and Therakos. JB—advisory board and speaker fees—Pfizer, Novartis and Jazz. EF—Travel grant—Neovil; Scientific expert—Novartis. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gilleece, M.H., Labopin, M., Savani, B.N. et al. Allogeneic haemopoietic transplantation for acute myeloid leukaemia in second complete remission: a registry report by the Acute Leukaemia Working Party of the EBMT. Leukemia 34, 87–99 (2020). https://doi.org/10.1038/s41375-019-0527-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0527-4
This article is cited by
-
Identifying the optimal conditioning intensity for stem cell transplantation in patients with myelodysplastic syndrome: a machine learning analysis
Bone Marrow Transplantation (2023)
-
Comparison of outcomes for patients with acute myeloid leukemia undergoing haploidentical stem cell transplantation in first and second complete remission
Annals of Hematology (2023)
-
Allogeneic stem cell transplantation for patients with acute myeloid leukemia (AML) in second complete remission (CR2) transplanted from unrelated donors with post-transplant cyclophosphamide (PTCy). A study on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation
Bone Marrow Transplantation (2023)
-
Matched related versus unrelated versus haploidentical donors for allogeneic transplantation in AML patients achieving first complete remission after two induction courses: a study from the ALWP/EBMT
Bone Marrow Transplantation (2023)
-
Feasibility and efficacy of salvage allogeneic stem cell transplantation in AML patients relapsing after autologous stem cell transplantation
Bone Marrow Transplantation (2022)