Abstract
Fibroblast growth factor (FGF) 21 is an endocrine growth factor mainly secreted by the liver in response to a ketogenic diet and alcohol consumption. FGF21 signaling requires co-receptor β-klotho (KLB) co-acting with FGF receptors, which has pleiotropic metabolic effects, including induced hepatic fatty acid oxidation and ketogenesis, in human and animal models of obesity. We examined the hepatocyte-specific enhancer/promoter of FGF21 expression plasmids in high-fat diet-fed mice for 12 weeks. Hydrodynamic injection for FGF21 delivery every 6 weeks sustained high circulating levels of FGF21, resulting in marked reductions in body weight, epididymal fat mass, insulin resistance, and liver steatosis. FGF21-induced lipolysis in the adipose tissue enabled the liver to be flooded with fat-derived FFAs. The hepatic expression of Glut2 and Bdh1 was upregulated, whereas that of gluconeogenesis-related genes, G6p and Pepck, and lipogenesis-related genes, Srebp-1 and Srebp-2, was significantly suppressed. FGF21 induced the phosphorylation of AMPK at Thr172 and Raptor at ser792 and suppressed that of mTOR at ser2448, which downregulated mTORC1 signaling and reduced IRS-1 phosphorylation at ser1101. Finally, in the skeletal muscle, FGF21 increased Glut4 and Mct2, a membrane protein that acts as a carrier for ketone bodies. Enzymes for ketone body catabolism (Scot) and citrate cycle (Cs, Idh3a), and a marker of regenerating muscle (myogenin) were also upregulated via increased KLB expression. Thus, FGF21-induced lipolysis was continuously induced by a high-fat diet and fat-derived FFAs might cause liver damage. Hepatic fatty acid oxidation and ketone body synthesis may act as hepatic FFAs’ disposal mechanisms and contribute to improved liver steatosis. Liver-derived ketone bodies might be used for energy in the skeletal muscle. The potential FGF21-related crosstalk between the liver and extraliver organs is a promising strategy to prevent and treat metabolic syndrome-related nonalcoholic steatohepatitis.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most common liver diseases worldwide; it is significantly associated with metabolic syndromes, including obesity, dyslipidemia, and insulin resistance1,2. Emerging evidence indicates that obesity-related metabolic inflammation is a key process in the development of NAFLD3,4. Several metabolic and inflammatory pathways and mediators between the liver and various extraliver organs have been reported to associate with NAFLD, leading to type 2 diabetes mellitus, cardiovascular disease, and chronic kidney disease5,6,7. However, currently available pharmacotherapeutic options for the treatment of patients with NAFLD remain limited in the heterogeneous population; hence, novel treatments are needed.
FGF21, which has an increased expression in obesity and NAFLD, has been identified as a promising therapeutic target in obesity-related metabolic disorders8,9. FGF21 is a member of the hormone-like subgroup within the FGF superfamily that also contains FGF19 and FGF23, which are critical for maintaining whole-body homeostasis10,11. FGF21 expression is increased by peroxisome proliferator-activated receptor α and activating transcription factor 4 via the exposure to excess endoplasmic reticulum stress, oxidative stress, and amino acid deprivation12,13. The C-terminus of FGF21 binds with high affinity to KLB, which is present as a complex with FGF receptors (FGFRs) on the surface of cells14,15,16. FGF21 is known to exert antidiabetic, lipid-lowering, and weight-reducing effects when administered at pharmacological concentrations in rodents17. Its functions are deemed vital in the lipolysis of peripheral/abdominal adipose tissue and stimulation of adiponectin production, which, in turn, enhances insulin sensitivity in the liver18,19.
The mechanism through which FGF21 mediates metabolic activity in the liver remains largely unknown. KLB is required for FGF21 to activate two specific FGFR subtypes—FGFR1c and FGFR3c. FGFRs are widely expressed in adult tissues, although their relative levels differ among the various organ systems14,15,16. Under normal conditions, hepatocytes express high levels of KLB and FGFR3c; however, the expression level of FGFR1c is quite low20. Obesity increases the expressions of FGF21, KLB, FGFR1c, and FGFR3c in the liver21,22. Recently, FGF21 has been reported to repress the mammalian target of rapamycin complex 1 (mTORC1) and improve insulin sensitivity and glycogen storage in a hepatocyte-autonomous manner, indicating an autocrine function of FGF21 in the liver23.
Given the numerous metabolic functions of FGF21, FGF21-based pharmacotherapies are currently being explored as potential antiobesity/NAFLD drugs and are under evaluation in clinical trials24,25,26. In addition, recent studies on long-acting FGF21 analogs in obese patients with type 2 diabetes have demonstrated improvements in serum lipid profiles and weight loss. A glyco-engineered long-acting FGF21 variant with optimal pharmaceutical and pharmacokinetic properties enabled weekly to twice-monthly subcutaneous dosing27,28,29.
Because FGF21 stimulates lipolysis in white adipose tissue (WAT) and the oxidation of fatty acids and production of ketone bodies in the liver, the pharmacological effects of sustained FGF21 treatment in humans with NAFLD as well as in rodent NAFLD models include direct and indirect consequences induced by FGF2130,31,32,33,34. In this study, we aimed to determine the beneficial effects of sustained FGF21 levels by the hepatocyte-specific overexpression of FGF21 on the progression or reversal of NAFLD in diet-induced obese mice. High-fat diet (HFD)-fed C57BL/6 mice were intravenously injected with pLIVE plasmid expressing mouse FGF21 under the control of an albumin promoter and α-fetoprotein enhancer35. Hepatocyte-derived FGF21 has a higher physiological activity than recombinant FGF21 from Escherichia coli because the endocrine FGF signaling through FGFRs requires klothos, which are cell-surface proteins that possess tandem glycosidase domains27. In this present study, FGF21 therapy was demonstrated to protect against diet-induced obesity and NAFLD and systemically improve insulin resistance via lipolysis in WAT.
Insulin activities in the adipose tissue regulate the delivery of fatty acids from blood to the liver, following the lipolysis of triglyceride in the adipose tissue. Mechanistically, FGF21 reduced intrahepatic lipid accumulation not by enhancing fatty acid oxidation but by inhibiting lipogenesis via the suppression of sterol regulatory element-binding proteins (Srebps). Moreover, FGF21 increased 3-hydroxybutyrate dehydrogenase (Bdh) 1 expression, which catalyzes the interconversion of acetoacetate and 3-hydroxybutyric acid (3-OHBA) during fatty acid catabolism36,37. Despite being the source of ketone bodies, the liver cannot use them for energy. Liver-derived ketone bodies could be used in the skeletal muscle in this NAFLD mouse model. Furthermore, FGF21 overexpression activated adenosine monophosphate-activated protein kinase (AMPK) signaling, suppressed mTOR signaling, and improved insulin sensitivity by decreasing the gluconeogenesis genes, namely, glucose-6-phosphate (G6p) and phosphoenolpyruvate carboxylase (Pepck), in the liver. Our data suggest that the hepatocyte-specific expression of FGF21 prevents and reverses NAFLD. This FGF21-related potential crosstalk between the liver and extraliver organs is a promising strategy for NAFLD therapy.
Materials/subjects and methods
Animals and treatment
A mouse model of diet-induced obesity and NAFLD was used in this study. Eight-week-old male C57/BL6J mice were purchased from Japan Charles River. Eleven mice were fed a control chow diet and ten mice were fed an HFD (D12492, Research Diet Inc., Tokyo, Japan) for 12 weeks. Half of the chow- or HFD-fed mice were hydrodynamically injected with pLIVE-mouse fgf21 plasmids every 6 weeks, whereas the other half of each group were injected with pLIVE-control plasmids. At the end of the treatment, livers, epididymal fats, skeletal muscles, and blood were isolated from each animal. The study protocol was conducted in accordance with the guidelines for the care and use of laboratory animals set by the Kyoto Prefectural University of Medicine (Kyoto, Japan) and was approved by the Committee on the Ethics of Animal Experiments of the same institution. Animals were housed under conventional conditions with controlled temperature, humidity, and light (12-h light–dark cycle), were provided with food and water ad libitum, and were housed in transparent polymer X (TPX) cages (CL-0104-2, CLEA Japan Inc., Tokyo, Japan) with a maximum of eight mice per cage.
Hydrodynamic tail vein injections of fgf21 plasmid
The wild-type mouse fgf21 cDNA constructs obtained by PCR amplification were sub-cloned into the pLIVE vector (Mirus Bio, Madison, WI). Plasmid DNA constructs were delivered by hydrodynamic tail vein injection38. The tail vein injections were performed with 40 µg of each plasmid in a solution of TransIT-EE® according to the manufacturer’s instructions (Mirus Bio LLC, Madison, WI, USA).
Analysis of liver architecture
As previously described, liver sections were stained with hematoxylin and eosin or Oil Red O utilizing standard techniques39.
Two-step real-time polymerase chain reaction
PCR was performed as described below39. Briefly, the total RNA was extracted from whole livers using RNeasy kits (Qiagen, Valencia, CA, USA), which were reverse-transcribed using a random primer and Superscript RNase H-reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Specificity was confirmed for all primer pairs (Supplemental material, Table 1) by sequencing the PCR products. Target gene levels were then presented as a ratio of levels in the treated versus corresponding control groups. Fold changes were determined by using point and interval estimates.
Immunoblot assay
Proteins isolated from whole fats, livers, and muscles were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Membranes were then probed with anti-AMPK, anti-phospho-AMPK at Thr172, anti-insulin receptor substrate (IRS)-1, anti-phospho-IRS-1 at Ser1101, anti-regulatory associated protein of mammalian target of rapamycin (Raptor), anti-phospho-Raptor at Ser792, anti-mTOR, anti-phospho-mTOR at ser2448, anti-p70S6 kinase, anti-phospho-p70S6K at Thr389, anti-S6 ribosomal protein (S6RP), anti-phospho-S6RP at ser235/236, anti-adipose triglyceride lipase (ATGL), anti-hormone-sensitive lipase (HSL), anti-phospho-HSL at ser660 (Cell Signaling Technology Inc., Beverly, MA, USA), anti-myogenin (F5D, Santa Cruz, Dallas, TX, USA. sc-12732), anti-Srebp-1 (C-20, Santa Cruz, Dallas, TX, USA. sc-366) or anti-actin (Sigma-Aldrich, St. Louis, MO, USA), followed by incubation with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG (Invitrogen, Carlsbad, CA, USA). Antigens were then visualized by electrochemiluminescence (GE Healthcare, Chicago, IL, USA). Immunoblots were scanned, and band intensities were quantified by Image J (NIH) densitometry analysis.
Tissue and plasma biochemical measurements
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol, triglyceride and FFA levels were measured at SRL, Inc. (Tokyo, Japan). Serum FGF21, adiponectin, leptin, insulin and 3-OHBA were measured using a Mouse FGF21 ELISA Kit (Arigo Biolaboratories Corp., Hsinchu, Taiwan), Mouse Adiponectin/Acrp30 Quantikine ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA), Mouse Leptin AssayMax ELISA Kit (Assaypro, St Charles, MO, USA), Morinaga Mouse/Rat Insulin ELISA Kit (Morinaga, Yokohama, Japan) and β-Hydroxybutyrate, Ketone Body, Assay Kit (Cayman Chemical, Ann Arbor, MI, USA). Meanwhile, tissue triglyceride levels were determined by the commercial assay kits in accordance with the manufacturer’s instructions (Triglycerides- E-test; Wako Pure Chemical Industries, Tokyo, Japan). Homeostasis model assessment-insulin resistance (HOMA-IR) was calculated as (fasting glucose mM × fasting insulin microunits/ml)/22.5.
Statistical analysis
Results are presented as the mean ± SEM. Significance was established using the Student’s t test and analysis of variance, when appropriate. Differences were considered significant if p < 0.05.
Results
Sustained high circulating levels of FGF21 with the hydrodynamic injection system for FGF21 delivery every 6 weeks
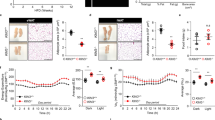
In total, 21 B6 mice that were fed a normal chow (n = 11) or HFD (n = 10) for 12 weeks and were transfected with pLIVE-control or pLIVE-mfgf21 every 6 weeks were examined (Fig. 1A). To confirm that the circulating levels of serum FGF21 were sustained throughout the treatment duration, blood tests measuring the FGF21 level were performed at 4 weeks of treatment (Fig. 1B). Serum FGF21 levels were increased in the HFD-fed mice and were much higher in all mice that were transfected with pLIVE-mfgf21, regardless of the diet (Fig. 1B). At the end of the treatments, serum FGF21 levels and hepatic FGF21 mRNA were assessed. Serum FGF21 levels and hepatic FGF21 expressions were significantly elevated in mice that were transfected with pLIVE-mfgf21 compared with the control mice (Fig. 1B, C). Although the FGF21 treatment had no effect on the expression of hepatic FGFR1 and FGFR3, the hepatic mRNA levels of KLB expression were noted to increase in mice fed with chow and HFD (Fig. 1D).
A In vivo experimental design. B Serum FGF21 levels were determined at 4 weeks and end of the treatment (n = 5–6/group). C Hepatic mRNA levels of FGF21 were determined by quantitative real-time PCR analysis. D Hepatic mRNA levels of KLB, FGFR1 and FGFR3 expression were determined. Results were normalized to glucuronidase expression. Means data are displayed as fold changes relative to chow-fed and pLIVE-control plasmid-injected mice (*p < 0.05, **p < 0.01).
Improved obesity, dyslipidemia, and insulin resistance under FGF21 treatment
We assessed the body weight (BW) during treatment as well as the liver and epididymal fat weight/BW ratio at the end of the 12-week treatment period. Although HFD-fed mice gained BW and became obese, FGF21 treatment reduced BW gains even in chow-fed mice (Fig. 2A). The liver/BW ratio was decreased in both FGF21 treatment groups, and the increased epididymal fat/BW ratio due to the HFD was greatly suppressed by FGF21 treatment despite the average intake of a similar amount of food (Fig. 2B, Supplementary Fig. S2a). Furthermore, feeding the mice with HFD for 12 weeks elevated serum total cholesterol levels but decreased triglyceride levels with no change in FFAs (Fig. 2C). FGF21 treatment decreased the total serum cholesterol levels in both chow- and HFD-fed mice and decreased triglyceride levels only in the chow group (Fig. 2C). Although FGF21 treatment reduced serum FFAs only in chow-fed mice, which was a similar effect as that observed regarding serum triglyceride levels, FGF21 had no effect on these levels in HFD-fed mice (Fig. 2C). HFD-fed mice exhibited high blood glucose, insulin levels, and homeostatic model assessment-insulin resistance index, which were then ameliorated by FGF21 treatment (Fig. 2D). Consequently, FGF21 improved HFD-induced obesity, hypertrophy of epididymal fats, hyperlipidemia, and systemic insulin resistance but had no effect on serum FFA levels.
A Body weight changes during 12-week treatment are plotted. B Liver/body weight (BW) and epididymal fat/BW ratio (%) were assessed at 12 weeks. C Serum total cholesterol, triglyceride, and FFA were determined. D Serum glucose, insulin and HOMA-IR were determined. Means data from each group (n = 5–6/group) are displayed (*p < 0.05, **p < 0.01).
Hepatic steatosis under FGF21 treatment
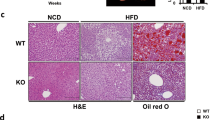
The serum ALT levels were decreased to approximately half in both chow- and HFD-fed mice injected with FGF21 (Fig. 3A). The amelioration of liver steatosis was confirmed by hematoxylin and eosin staining, Oil Red O staining, and the liver triglyceride content measurement (Fig. 3B, C). FGF21-treated mice showed a clear reversal of HFD-induced intrahepatic lipid accumulation and liver triglyceride contents. Thus, FGF21 induced a marked reduction in liver steatosis.
A Serum ALT values were determined. B Hematoxylin and eosin (H&E), and Oil red O staining of liver sections from representative HFD-fed mice injected by pLIVE-control or -mfgf21. C Hepatic triglyceride (TG) contents were measured. Means data from each group (n = 5–6/group) are displayed (*p < 0.05, **p < 0.01).
Autocrine activities in the liver under FGF21 treatment
To explore the molecular mechanisms underlying the FGF21 effect in the regulation of insulin and glucose homeostasis in the liver, we assessed hepatic gluconeogenesis-, lipogenesis-, fatty acid oxidation- and ketogenesis-related gene expressions and insulin signaling-related molecules such as AMPK and mTORC1. When hepatic gluconeogenesis-related genes (G6p and Pepck), hepatic lipogenesis-related genes (Srebp-1 and -2), and hepatic fatty acid transporter CD36 were underexpressed in HFD-fed mice, FGF21 treatment significantly increased the mRNA levels of hepatic Glut2 and Bdh1 (Fig. 4A, B). FGF21 treatment reduced the protein levels of both the Srebp-1 precursor (molecular weight, 125 kDa) and mature soluble fragment (molecular weight, 68 kDa) in the nucleus, which were induced by HFD (Fig. 4D, Supplementary Fig. S2e). Microsomal triglyceride transfer protein 1 and, unexpectedly, hepatic mitochondrial β-oxidation-related genes, carnitine palmitoyltransferase-1, -2, and long-chain acyl-CoA dehydrogenase were not upregulated (Supplementary Fig. S2b). We also evaluated the mRNA levels of hepatic bile acid synthesis-related genes (cytochrome P (Cyp)7a1, Cyp8b1, Cyp27a1, Cyp7b1, farnesoid X receptor, and small heterodimer partner) and transport genes (bile salt export pump, sodium taurocholate cotransporting polypeptide, ATP binding cassette subfamily B member (Abcb) 4, Abcg5, and Abcg8) (Supplementary Figs. S2c, d). Although bile acid transport-related genes (both import and export) were mostly enhanced in HFD-fed mice, FGF21 treatment had minimal effect on hepatic bile acid synthesis for example cyp7a1, which was consistent with the findings of previous studies40,41. However, the effects of short- or long-term FGF21 treatment on bile acid synthesis have not been fully elucidated yet. These results revealed that the effects of FGF21 on the liver suppress gluconeogenesis and lipid synthesis without obvious increases in fatty acid oxidation and partly induce the synthesis of ketone bodies for the disposal of excess acetyl-CoA derived from FFAs. Slightly increased serum levels of 3-OHBA due to HFD (p = 0.056) did not differ between the control and FGF21-treated mice (Fig. 4C). Furthermore, in HFD-fed mouse livers, FGF21 upregulated the phosphorylation of Raptor at ser792 via AMPK activation (Fig. 4D) and directly downregulated the tuberous sclerosis complex 1/2-mediated phosphorylation of mTOR at ser2448 (Fig. 4E). As a result, p70S6 kinase at thr389, S6 ribosomal protein at ser235/236, and IRS-1 at ser1101 were clearly suppressed by FGF21 treatment (Fig. 4E). Consistent with the findings of a previous study23, these observations indicated that FGF21 restored insulin signaling by suppressing the mTOR signaling-mediated phosphorylation of IRS-1 at ser1101.
A Hepatic mRNA levels of G6p, Pepck, Glut2, Bdh1, CD36 B Srebp-1, -2 and fatty acid synthase (Fas) were determined by quantitative real-time PCR analysis (n = 5–6/group). C Serum 3-OHBA levels were determined in each group (n = 5-6/group). Means data from each group (n = 5-6/group) are displayed (*p < 0.05, **p < 0.01). D Srebp-1 (precursor and mature), p-AMPK, AMPK, p-Raptor and Raptor levels were evaluated by immunoblot analysis. To control for loading, the blot was stripped and re-probed for β-actin, a housekeeping gene. E p-mTOR, mTOR, p-p70S6K, p70S6K, p-S6RP, S6RP, p-IRS, and IRS-1 levels were evaluated by immunoblot analysis of livers from three mice/group. Immunoblots were scanned, and band intensities were quantified by Image J (NIH) densitometry analysis. Each ratio was also expressed as fold change of controls. (*p < 0.05, **p < 0.01).
FGF21-induced lipolysis in the adipose tissue
Despite an increase in the serum adiponectin levels of chow-fed mice, which was inconsistent with previous study findings, FGF21 treatment had little effect on these levels of HFD-fed mice (Fig. 5A). On the other hand, serum leptin levels that would have been elevated by HFD were clearly decreased in mice transfected with pLIVE-mfgf21. FGF21 treatment suppressed the mRNA expression levels of leptin, but not adiponectin, in both groups of FGF21-treated mice (Fig. 5B). Although FGF21 improved liver steatosis with a marked loss of epididymal fats, serum FFA levels were found to be similar among the mice in HFD-fed groups (Figs. 2B, C, and 3). To confirm the mechanism underlying fat loss and blood FFAs, we examined lipolysis-related molecules and found that the phosphorylation of ATGL and HSL at ser660 was upregulated by FGF21 treatment (Fig. 5C). The lipolysis of the adipose tissue by FGF21 produces FFAs. Therefore, continuous lipolysis during FGF21 treatment may lead to hepatic steatosis, but the disposal system of FFAs can overcome this phenomenon.
A Serum adiponectin and leptin levels were determined in each group (n = 5-6/group). Data are presented as Means. B mRNA levels of adiponectin and leptin in fat tissues were determined by quantitative real-time PCR analysis. Results were normalized to GUS expression and then expressed as fold changes relative to gene expression in control plasmid-injected and chow-fed mice. Means data from each group (n = 5–6/group) (*p < 0.05, **p < 0.01). C ATGL, p-HSL at Ser660 and HSL levels were evaluated by immunoblot analysis of fat tissues from three mice/group. Immunoblots were scanned, and band intensities were quantified by Image J (NIH) densitometry analysis. Each ratio was also expressed as fold change of controls. (*p < 0.05).
FGF21-induced glucose and ketone body uptake for energy in the skeletal muscle
FGF21 treatment decreased gluconeogenesis and lipogenesis and had negligible effect on fatty acid oxidation and the export of very-low-density lipoproteins in the liver (Supplementary Fig. S2b). To explore the disposal system for excess FFAs derived from lipolysis, we assessed the role of skeletal muscle in glucose and ketone body metabolism. FGF21 treatment increased the KLB mRNA level in the skeletal muscle of both chow- and HFD-fed mice (Fig. 6A). Interestingly, FGFR3 was significantly upregulated by FGF21 in HFD-fed mice (Fig. 6A). In these mice, the mRNA levels of Glut4, which is a transporter for glucose uptake, and monocarboxylate transporter 2 (Mct2), which is essential for ketone body uptake, were significantly elevated after FGF21 treatment (Fig. 6B). Moreover, to confirm the optimal utilization of acetyl-CoA from glucose and ketone bodies, a key enzyme for ketone body catabolism, succinyl-CoA: 3-ketoacid CoA transferase (Scot), and the enzymes of the TCA cycle, citrate synthetase (Cs), aconitase 2, and isocitrate dehydrogenase (NAD+) 3 catalytic subunit alpha (Idh3α) were examined. The mRNA levels of these TCA cycle enzymes, which were significantly decreased by HFD, were increased in HFD-fed mice under FGF21 treatment. Finally, we analyzed the expression of muscle regeneration-related genes, such as myoblast determination protein 1, myogenic factor 5, and myogenin. The mRNA and protein levels of myogenin expression suppressed by HFD were restored by FGF21 treatment (Fig. 6D, E).
A mRNA levels of KLB, FGFR1, FGFR3 (B) Glut4, Mct1, Mct2 (C) Scot, Cs, Aco2, Idh3α, D Myogenic, Myoid and Myf5 in skeletal muscles were determined by quantitative real-time PCR analysis (n = 5-6/group). E Myogenin levels were evaluated by immunoblot analysis of skeletal muscles from three mice/group. Immunoblots were scanned, and band intensities were quantified by Image J (NIH) densitometry analysis. Each ratio was also expressed as fold change of controls. (*p < 0.05).
Discussion
Our data suggest that the hepatocyte-specific expression of FGF21 mediated by a nonviral vector can achieve long-term sustained levels of FGF21 in blood and potential FGF21-related crosstalk between the liver and extraliver organs, which makes it an attractive target for obesity-related NAFLD therapy. Because liver processes were continuously induced by HFD- and fat-derived FFAs because of FGF21-induced lipolysis, hepatic fatty acid oxidation and ketone body synthesis could act as a hepatic FFAs disposal system and improve liver steatosis. Moreover, liver-derived ketone bodies could be utilized for energy in the skeletal muscle in this model.
The levels of hepatic FGF21 mRNA and serum FGF21 sustained for a long period (>3 months) were not harmful and did not worsen the levels of serum AST, ALT, and other biomarkers. Preliminarily, we confirmed that hepatic FGF21 was rapidly released into the culture medium from Hep3B and HepG2 cells transfected with FGF21 expression plasmids and was not detected in the immunoblotting experiment with these cell lysates (Supplementary Fig. S1a–c). Owing to the low cell binding affinity of FGF21, FGF21 signaling was regulated by the expression of its receptor complex, FGFR1, FGFR3, and in particular, KLB in the target organ14,15,16. Technically, intravascular hydrodynamic gene delivery is a safe and efficient in vivo procedure for gene transfer in small animal models. The clinical translation of this approach requires the prevention of hemodynamic side effects. Direct injection of plasmid DNA into the target liver segment using a catheter with a balloon has been recently demonstrated to restrict the perfusion area38. FGF21, a secreted protein that can be expressed long-term using the pLIVE vector, is a suitable candidate for this purpose. On the other hand, various long-acting FGF21 analogs, including LY2405319, PF-05231023, and AKR-001, have already been tested in clinical trials for people with obesity26,28,29,42. Moreover, pegbelfermin (BMS-986036) is a PEGylated FGF21 analog that is currently being investigated for the treatment of nonalcoholic steatohepatitis43. In a phase IIa trial, the subcutaneous injection of 10-mg pegbelfermin once a day or 20-mg pegbelfermin once a week has shown promising improvements in several nonalcoholic steatohepatitis-related outcomes. Therefore, gene transfer using pLIVE vectors is an excellent experimental model, which is, however, somewhat invasive from the perspective of clinical applicability.
FGF21 not only stimulates lipolysis in WAT but also activates brown adipose tissue and induces the browning of WAT44. The hallmarks of browning include the appearance of uncoupling protein-1-expressing multilocular and mitochondria-rich adipocytes in WAT. However, we found that the mRNA levels of uncoupling protein-1 expression did not increase by FGF21 treatment (data not shown), whereas lipolysis was induced (Fig. 5C). The lipolysis of the adipose tissue produces glycerol and FFAs that serve as energy sources during the fasting state. This lipolysis is tightly regulated under normal conditions. Conversely, in the context of obesity and insulin resistance, excessive lipolysis causes hepatic steatosis as FFAs released from peripheral adipose depots constitute a major source of triglycerides in the liver of patients with NAFLD36.
Mitochondrial β-oxidation is determined as the dominant oxidative pathway for the disposal of FFAs in liver45. Fatty acid β-oxidation is the process of breaking down long-chain acyl-CoA into acetyl-CoA, which is used in a myriad of biochemical pathways. For example, it may be used as a starting material for the biosynthesis of lipids such as triglycerides, phospholipids, or cholesterol36. Most importantly, for energy generation, acetyl-CoA may enter the citric acid cycle and be oxidized to produce energy, if oxygen is available. Acetyl-CoA also enters the ketogenic pathway to form 3-OHBA. Ketones cannot be oxidized in the liver but are exported and utilized by peripheral tissues, with little impact on hepatic energetics in a normal state46. In people with obesity as well as the murine model used in the present study, the liver is continuously exposed to the postabsorptive and fat-derived circulating FFAs. Although acetyl-CoA disposal is primarily associated with the TCA cycle, ketone synthesis plays a critical role in the removal of excess acetyl-CoA and is impaired in human fatty liver with increased acetyl-CoA and hyperglycemia46. Bdh1 is the last enzyme of hepatic ketogenesis and the first enzyme of ketolysis in extraliver organs. Bdh1 deficiency is well-tolerated under normal diet conditions but is exacerbated during fasting, with a marked increase in the AcAc/3HB ratio and hepatic steatosis47. As has been recently reported, FGF21 regulates lipolysis in adipocytes in response to the metabolic state but is not required for ketogenesis48. At least, the phenotypes of fgf21 transgenic mice demonstrate that FGF21 stimulates hepatic ketogenesis12,37. In our diet-induced obesity model, the high levels of serum FGF21 induced lipolysis and FFAs production from the peripheral adipose tissue (Fig. 5C). Ketone body production as a secondary system for hepatic FFA disposal might improve liver steatosis.
Ketone bodies are a major oxidative fuel source for muscles during starvation and in diabetes and attenuate wasting in models of atrophy49. These are important vectors of energy transfer. The relative capacity of tissues to utilize ketone bodies for energy is considered to be determined by the levels of the ketolytic enzyme Scot, which is often expressed in the myocardium, brain, kidney, and skeletal muscle50. Although fatty acids, glucose, and 3-OHBA may compete for acetyl-CoA in the TCA cycle, 3-OHBA is utilized for energy by diabetic muscles under FGF21 treatment51. We found that the transporter of ketone bodies, Mct2 and Scot, and the TCA cycle enzymes Cs and Idh3α were upregulated by FGF21 treatment (Fig. 6A–C). Furthermore, as recently reported concerning the metabolic functions of FGF21 in extraliver organs, FGF21 enhances the neuronal ability to use ketone bodies by upregulating the expression of MCTs and inducing myogenic differentiation in C2C12 cells52,53,54,55. We found that FGF21 treatment increased the mRNA and protein levels of myogenin expression, thereby indicating the protective function of FGF21 in muscle atrophy in people with obesity.
Recently, the sodium/glucose transporter (SGLT) 2 inhibitor has been reported to induce transcriptional reprogramming to activate catabolic pathways, increase fatty acid oxidation, reduce hepatic steatosis, and increase hepatic and plasma levels of FGF2151. SGLT2 inhibitors are antidiabetic drugs that induce urinary glucose loss. As a result, plasma glucose levels reduce and overall glycemic control improves51. Intriguingly, SGLT2 inhibitors reduce the risk of cardiovascular disease and mortality in type-2 diabetes and may improve hepatic steatosis and NAFLD56. SGLT2 inhibitors act in an insulin-independent manner, which leads to modest weight loss and induced fatty acid oxidation and ketogenesis57. FGF21 is an important coordinator of fasting-induced metabolic responses and reduction in adiposity by increasing lipolysis, hepatic fatty acid oxidation, and ketogenesis. FGF21 is not essential for a metabolic switch to a fasting-like catabolic state but is required to induce lipolysis and reduction in adiposity in response to SGLT2 inhibitors57.
The present study has several limitations. First, tail vein injection was performed using 40 µg plasmid DNA but the amount of DNA could have been reduced. The serum FGF21 levels were much higher than the controls because of the long-term, continuous expression of exogenous FGF21 in the liver due to the pLIVE vector. These levels were sufficient compared to the previous in vivo FGF21 administration model to produce an effect58. Therefore, a direct injection of the plasmid DNA to a small liver segment may be enough to achieve sustained serum levels of FGF21. Second, the study findings were not sufficient in explaining FGF21-induced ketone production and utility. Ketones are produced as a fuel source when glucose is not available to cells. This condition is most frequently noted in patients with uncontrolled type 1 diabetes and can lead to a medical emergency. In the present study, the administration of FGF21 did not increase serum 3-OHBA levels in HFD-fed mice, suggesting that the produced 3-OHBA was immediately utilized and compensated for by the muscles and brain. Further studies using ketogenic diet models and describing a comparison with single-injection models of FGF21 analogs are warranted. Considering the results of the present study and those of previous studies, we conclude that FGF21 treatment can prevent the progression of obesity-related NAFLD via FGF21-related potential crosstalk and direct/indirect consequences between the liver and extraliver organs. Although FGFs, including FGF19, induce cell proliferation, FGF21 is required to limit the progression from NAFLD to hepatocellular carcinoma in response to prolonged exposure to an obesogenic diet59. The induction of hepatic FGF21 in response to the high-fat, high-sucrose obesogenic diet plays an important role in limiting the progression of liver pathology from NAFLD to hepatocellular carcinoma60,61. The lack of FGF21 induces nonalcoholic steatohepatitis–hepatocellular carcinoma transition via hepatocyte-TLR4-IL-17A signaling62. To determine an effective treatment for nonalcoholic steatohepatitis–hepatocellular carcinoma transition and obesity-related complications such as sarcopenia, future studies on the therapeutic use of FGF21, including their related drugs, are needed.
Data availability
The data that support the findings of this study are available from the corresponding author, Kya, upon reasonable request.
References
Ludwig, J., Viggiano, T. R., McGill, D. B. & Oh, B. J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 55, 434–438 (1980).
Kahn, S. E., Hull, R. L. & Utzschneider, K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006).
Tilg, H. & Moschen, A. R. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 14, 222–231 (2008).
Maachi, M. et al. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int. J. Obes. Relat. Metab. Disord. 28, 993–997 (2004).
Targher, G. et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 30, 2119–2121 (2007).
Targher, G. et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 54, 3541–3546 (2005).
Pais, R. & Bourron, O. Fatty liver and renal function impairment - time for awareness? J. Hepatol. 68, 13–15 (2017).
Dushay, J. et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 139, 456–463 (2010).
Kharitonenkov, A. et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 115, 1627–1635 (2005).
Guan, D., Zhao, L., Chen, D., Yu, B. & Yu, J. Regulation of fibroblast growth factor 15/19 and 21 on metabolism: in the fed or fasted state. J. Transl. Med. 14, 63 (2016).
Owen, B. M., Mangelsdorf, D. J. & Kliewer, S. A. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends. Endocrinol. Metab. 26, 22–29 (2015).
Inagaki, T. et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 5, 415–425 (2007).
Maruyama, R., Shimizu, M., Li, J., Inoue, J. & Sato, R. Fibroblast growth factor 21 induction by activating transcription factor 4 is regulated through three amino acid response elements in its promoter region. Biosci. Biotechnol. Biochem. 80, 929–934 (2016).
Suzuki, M. et al. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol. Endocrinol. 22, 1006–1014 (2008).
Kurosu, H. et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 282, 26687–26695 (2007).
Agrawal, A. et al. Molecular elements in FGF19 and FGF21 defining KLB/FGFR activity and specificity. Mol. Metab. 13, 45–55 (2018).
Muise, E. S. et al. Downstream signaling pathways in mouse adipose tissues following acute in vivo administration of fibroblast growth factor 21. PLoS ONE 8, e73011 (2013).
Lin, X., Liu, Y. B. & Hu, H. Metabolic role of fibroblast growth factor 21 in liver, adipose and nervous system tissues. Biomed. Rep. 6, 495–502 (2017).
Jimenez, V. et al. FGF21 gene therapy as treatment for obesity and insulin resistance. EMBO Mol. Med. 10, e8791 (2018).
Hughes, S. E. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J. Histochem. Cytochem. 45, 1005–1019 (1997).
Gallego-Escuredo, J. M. et al. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int. J. Obes. 39, 121–129 (2015).
Tillman, E. J. & Rolph, T. FGF21: an emerging therapeutic target for non-alcoholic steatohepatitis and related metabolic diseases. Front. Endocrinol. 11, 601290 (2020).
Gong, Q. et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology 64, 425–438 (2016).
Barb, D., Bril, F., Kalavalapalli, S. & Cusi, K. Plasma fibroblast growth factor 21 is associated with severity of nonalcoholic steatohepatitis in patients with obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. 104, 3327–3336 (2019).
Gimeno, R. E. & Moller, D. E. FGF21-based pharmacotherapy−potential utility for metabolic disorders. Trends Endocrinol. Metab. 25, 303–311 (2014).
Kaufman, A., Abuqayyas, L., Denney, W. S., Tillman, E. J. & Rolph, T. AKR-001, an Fc-FGF21 analog, showed sustained pharmacodynamic effects on insulin sensitivity and lipid metabolism in type 2 diabetes patients. Cell Rep. Med. 1, 100057 (2020).
Lee, S. et al. Structures of β-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 553, 501–505 (2018).
Le, C. T., Nguyen, G., Park, S. Y., Choi, D. H. & Cho, E. H. LY2405319, an analog of fibroblast growth factor 21 ameliorates α-smooth muscle actin production through inhibition of the succinate-G-protein couple receptor 91 (GPR91) pathway in mice. PLoS One 13, e0192146 (2018).
Gaich, G. et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340 (2013).
Zhao, C. et al. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. J. Lipid Res. 56, 1481–1491 (2015).
Liu, X. et al. Lack of fibroblast growth factor 21 accelerates metabolic liver injury characterized by steatohepatitis in mice. Am. J. Cancer Res. 6, 1011–1025 (2016).
Coskun, T. et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027 (2008).
Fisher, F. M. et al. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology 147, 1073–1083.e1076 (2014).
BonDurant, L. D. et al. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 25, 935–944.e934 (2017).
Woolsey, S. J. et al. A fibroblast growth factor 21-pregnane X receptor pathway downregulates hepatic CYP3A4 in nonalcoholic fatty liver disease. Mol. Pharmacol. 90, 437–446 (2016).
Bernardo, B. et al. FGF21 does not require interscapular brown adipose tissue and improves liver metabolic profile in animal models of obesity and insulin-resistance. Sci. Rep. 5, 11382 (2015).
Li, Y. et al. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology 146, 539–549.e537 (2014).
Huang, M., Sun, R., Huang, Q. & Tian, Z. Technical improvement and application of hydrodynamic gene delivery in study of liver diseases. Front. Pharmacol. 8, 591 (2017).
Yamaguchi, K. et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 45, 1366–1374 (2007).
Chen, M. M., Hale, C., Stanislaus, S., Xu, J. & Véniant, M. M. FGF21 acts as a negative regulator of bile acid synthesis. J. Endocrinol. 237, 139–152 (2018).
Zhang, J. et al. Chronic over-expression of fibroblast growth factor 21 increases bile acid biosynthesis by opposing FGF15/19 action. EBioMedicine 15, 173–183 (2017).
Talukdar, S. et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 23, 427–440 (2016).
Sanyal, A. et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 392, 2705–2717 (2019).
Véniant, M. M. et al. Pharmacologic effects of FGF21 are independent of the “browning” of white adipose tissue. Cell Metab. 21, 731–738 (2015).
Houten, S. M. & Wanders, R. J. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 33, 469–477 (2010).
Fletcher, J. A. et al. Impaired ketogenesis and increased acetyl-CoA oxidation promote hyperglycemia in human fatty liver. JCI Insight 5, e127737 (2019).
Otsuka, H. et al. Deficiency of 3-hydroxybutyrate dehydrogenase (BDH1) in mice causes low ketone body levels and fatty liver during fasting. J. Inherit. Metab. Dis. 43, 960–968 (2020).
Hotta, Y. et al. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology 150, 4625–4633 (2009).
Svensson, K., Albert, V., Cardel, B., Salatino, S. & Handschin, C. Skeletal muscle PGC-1α modulates systemic ketone body homeostasis and ameliorates diabetic hyperketonemia in mice. FASEB J. 30, 1976–1986 (2016).
Davidsohn, N. et al. A single combination gene therapy treats multiple age-related diseases. Proc. Natl Acad. Sci. USA 116, 23505–23511 (2019).
Hattori, Y. Insulin resistance and heart failure during treatment with sodium glucose cotransporter 2 inhibitors: proposed role of ketone utilization. Heart Fail. Rev. 25, 403–408 (2020).
Katsu-Jiménez, Y. & Giménez-Cassina, A. Fibroblast growth Factor-21 promotes ketone body utilization in neurons through activation of AMP-dependent kinase. FGF21 enhances the ability of neurons to use ketone bodies. Mol. Cell. Neurosci. 101, 103415 (2019).
Sun, Y. et al. Modulation of the astrocyte-neuron lactate shuttle system contributes to neuroprotective action of fibroblast growth factor 21. Theranostics 10, 8430–8445 (2020).
Liu, X., Wang, Y., Zhao, S. & Li, X. Fibroblast growth factor 21 promotes C2C12 cells myogenic differentiation by enhancing cell cycle exit. Biomed. Res. Int. 2017, 1648715 (2017).
Liu, X., Wang, Y., Hou, L., Xuong, Y. & Zhao, S. Fibroblast growth factor 21 (FGF21) promotes formation of aerobic myofibers via the FGF21-SIRT1-AMPK-PGC1α pathway. J. Cell. Physiol. 232, 1893–1906 (2017).
Kuchay, M. S. et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care 41, 1801–1808 (2018).
Osataphan, S. et al. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight 4, e123130 (2019).
Larson, K. R., Chaffin, A. T., Goodson, M. L., Fang, Y. & Ryan, K. K. Fibroblast growth factor-21 controls dietary protein intake in male mice. Endocrinology 160, 1069–1080 (2019).
Singhal, G. et al. Deficiency of fibroblast growth factor 21 (FGF21) promotes hepatocellular carcinoma (HCC) in mice on a long-term obesogenic diet. Mol. Metab. 13, 56–66 (2018).
Yang, C. et al. Activation of Liver FGF21 in hepatocarcinogenesis and during hepatic stress. BMC Gastroenterol. 13, 67 (2013).
Huang, X. et al. Forced expression of hepatocyte-specific fibroblast growth factor 21 delays initiation of chemically induced hepatocarcinogenesis. Mol. Carcinog. 45, 934–942 (2006).
Zheng, Q. et al. Lack of FGF21 promotes NASH-HCC transition via hepatocyte-TLR4-IL-17A signaling. Theranostics 10, 9923–9936 (2020).
Acknowledgements
The authors would like to thanks Enugu for the English language review. This study was supported by Grant-in-Aid for Scientific Research (C) (KAKENHI) from the Japan Society for the Program of Science under Grant Number JP19K08473.
Author information
Authors and Affiliations
Contributions
Kyan, Kya and Y.I. planned experiments. Kyan, Kya and Y.I. wrote the manuscript. Kyan and Kya performed experiments. N.T., S.O., and Y.L. assisted with the animal experiments. Kyan and Kya performed the histological experiments. Kyan, Kya, S.O., A.T., S.K., K.O., Y.S., A.U., M.M., T.O. and Y.I. read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
Y.I. received a lecture fee and research grants from Merck & Co., Inc., and a consultation fee and research grants from Novo Nordisk A/S. Rest of the authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yano, K., Yamaguchi, K., Seko, Y. et al. Hepatocyte-specific fibroblast growth factor 21 overexpression ameliorates high-fat diet-induced obesity and liver steatosis in mice. Lab Invest 102, 281–289 (2022). https://doi.org/10.1038/s41374-021-00680-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-021-00680-9