Abstract
Nonalcoholic steatohepatitis (NASH) is associated with lipotoxic liver injury, leading to insulin resistance, inflammation, and fibrosis. Despite its increased global incidence, very few promising treatments for NASH are available. Pirfenidone is an antifibrotic agent used to treat pulmonary fibrosis; it suppresses the pulmonary influx of T cells and macrophages. Here, we investigated the effect of pirfenidone in a mouse model of lipotoxicity-induced NASH via a high-cholesterol and high-fat diet. After 12 weeks of feeding, pirfenidone administration attenuated excessive hepatic lipid accumulation and peroxidation by reducing the expression of genes related to lipogenesis and fatty acid synthesis and enhancing the expression of those related to fatty acid oxidation. Flow cytometry indicated that pirfenidone reduced the number of total hepatic macrophages, particularly CD11c+CD206–(M1)-type macrophages, increased the number of CD11c–CD206+(M2)-type macrophages, and subsequently reduced T-cell numbers, which helped improve insulin resistance and steatohepatitis. Moreover, pirfenidone downregulated the lipopolysaccharide (LPS)-induced mRNA expression of M1 marker genes and upregulated IL-4-induced M2 marker genes in a dose-dependent manner in RAW264.7 macrophages. Importantly, pirfenidone reversed insulin resistance, hepatic inflammation, and fibrosis in mice with pre-existing NASH. These findings suggest that pirfenidone is a potential candidate for the treatment of NASH.
Similar content being viewed by others
Introduction
A sedentary lifestyle coupled with caloric redundancy has become common in a growing number of regions and can lead to nonalcoholic fatty liver disease (NAFLD) [1]. NAFLD often coexists with obesity and type 2 diabetes mellitus, and increasing data have indicated a rapid increase in the rate of NAFLD over the past three decades [2]. Recently published reports revealed that 20–30% of adults in developed countries have NAFLD, which can progress to nonalcoholic steatohepatitis (NASH) [3, 4]. NAFLD is frequently described as a spectrum of diseases that ranges from simple steatosis to NASH with advanced fibrosis. Previously, we developed a cholesterol- and saturated fatty acid-induced lipotoxic NASH model that successfully mimicked the pathophysiological features of human NASH, and found that excessive hepatic lipid accumulation promoted the activation of macrophages/Kupffer cells to aggravate insulin resistance, hepatic inflammation, and fibrogenesis [5].
Hepatic fibrosis, a major feature of NASH, is a wound-healing process involved in the response of liver tissue to toxic, infectious, or metabolic agents [6]. During liver injury, hepatic stellate cells (HSCs) are activated by fibrogenic cytokines and chemokines or by reactive oxygen molecules produced by macrophages recruited to the site of injury [7]. Compared with agents aimed to ameliorate insulin resistance or oxidative stress, which are considered major causes of NASH progression, few trials have investigated hepatic fibrosis as a therapeutic target. Since there is no standard therapy for NASH, and many recent agents have obtained unsatisfying results, more effective therapies are urgently needed for patients with NASH.
Pirfenidone (5-methyl-1-phenil-2 (1H)-pyridone) is an approved therapy for idiopathic pulmonary fibrosis (IPF) in developed countries, because of its antifibrotic properties [8]. It also suppresses bleomycin-induced increases in the pulmonary influx of T cells and macrophages. In addition, a previous study demonstrated that pirfenidone suppressed the proliferation of HSCs by decreasing hydroxyproline concentrations and increasing the regeneration of active cells [9]. Furthermore, pirfenidone decreased the expression of inflammatory cytokines and inhibited the activation of nuclear factor-κB (NF-κB) in lipopolysaccharide (LPS)-induced liver injury in rats [10]. Pirfenidone also protected against LPS-induced hepatotoxicity by reducing aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels and attenuated oxidative stress in the livers of rats [11].
Multiple factors and pathways are involved in the pathogenesis of NAFLD/NASH, including the tumor necrosis factor-α (TNF-α) and transforming growth factor-β1 (TGF-β1) pathways, which are related to tissue inflammation and fibrosis [12]. Thus, treatment with pirfenidone may prevent progression of NASH by inhibiting hepatic inflammation and fibrosis. In this study, the preventive and therapeutic effects of pirfenidone and the potential mechanisms behind these effects were investigated in a lipotoxic model of NASH. The results indicated that pirfenidone prevented and reversed insulin resistance and steatohepatitis by regulating macrophage and T-cell accumulation, as well as the ratio of CD11c+CD206− (M1) to CD11c–CD206+ (M2) phenotypes of macrophages/Kupffer cells in the livers of mice. Importantly, pirfenidone improved insulin resistance, hepatic inflammation, and fibrosis in mice with pre-existing NASH.
Materials and methods
Mice and diets
Seven-week-old male C57BL/6J mice were obtained from Charles River Laboratories (Yokohama, Japan) and housed in a room under controlled temperature (25 °C), humidity, and light (12/12-h artificial light/dark cycle) conditions after 1 week of adaptation. The mice were divided into four groups and fed for 12 weeks as follows: (1) normal chow (NC) diet containing 10% of calories from fat (CRF-1; Charles River); (2) NC diet with 0.2% pirfenidone (Shionogi & Co., Ltd., Osaka, Japan) (NC+pirfenidone); (3) high-fat, cholesterol, and cholate (CL) diet, containing 60% of calories from fat, 1.25% cholesterol, and 0.5% sodium cholate (Research Diets, Inc., New Brunswick, NJ, USA); and (4) CL diet with 0.2% pirfenidone (CL+pirfenidone). All mice were maintained under a 1212-h light/dark cycle and given free access to food and water. All mouse studies were approved by the Kanazawa University Animal Experiment Committee (42185).
Quantitative real-time PCR
Quantitative real-time PCR was performed as described previously [13, 14], and the primers used are shown in Supplementary Table 1.
Histological examination and immunohistochemistry
Mouse livers were removed immediately after killing and weighed, stored in 10% buffered formalin, and embedded in paraffin. Liver sections were stained with hematoxylin and eosin or Sirius Red or were analyzed by immunohistochemical staining of EGF-like module-containing mucin-like hormone receptor-like 1 (F4/80) or α-smooth muscle actin (α-SMA), as described previously [5, 15]. The severity of hepatic histological changes was assessed in H&E and Sirius Red stain and blindly scored by two pathologists according to a NASH activity score [16, 17].
Cell culture
Mouse RAW264.7 macrophages were grown to 70–80% confluence in DMEM supplemented with 10% fetal bovine serum (FBS). After 6 h of serum starvation, the culture media were replaced with fresh media containing 100 ng/mL LPS (Sigma–Aldrich, St. Louis, MO, USA) or 10 ng/mL interleukin (IL)-4 (Sigma–Aldrich) combined with 30, 100, or 300 μg/mL pirfenidone for 16 h [13].
The RI-T rat HSC line was obtained from Health Science Research Resources Bank (JCRB1088, Osaka, Japan) and seeded in RPMI-1640 supplemented with 10% FBS. After 6 h of serum starvation, the cells were co-incubated with 3 ng/mL TGF-β1 (R&D Systems, Minneapolis, MN, USA) and 30, 100, or 300 μg/mL pirfenidone for 16 h [6].
Fluorescence-activated cell-sorting (FACS) analysis
The left lobes of the livers were removed and weighed carefully after perfusion with PBS containing 2% BSA (pH 7.4). Then, the tissues were lysed and digested gently for 20 min at 37 °C using type IV collagenase (Sigma-Aldrich) mixed with type I deoxyribonuclease in PBS containing 2% BSA (pH 7.4). After filtration, the cell suspension was centrifuged at 5000 rpm for 3 min. The sediment was resuspended in PBS containing 2% FBS and incubated with Fc-Block (BD Bioscience, San Jose, CA) followed by fluorochrome-conjugated antibodies (BD Biosciences; Supplementary Table 2). Cells were then analyzed using the FACSAria II system as described previously [13, 14]. Data analyses and compensation were performed using FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analyses
Experimental data are expressed as means ± SEM. P < 0.05 was considered significant. Statistical differences between groups were determined by a two-tailed Student's t-test. An overall difference among more than two groups was determined by one-way ANOVA. If one-way ANOVAs were significant, differences between individual groups were estimated by Tukey post hoc test. All calculations were performed with SPSS version 19.0 statistical software (IBM Corporation, Armonk, NY).
Results
Pirfenidone attenuated hepatic steatosis in diet-induced NASH mice
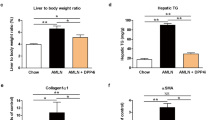
To determine the effect of pirfenidone on diet-induced NASH, mice were fed individually a NC or CL diet with or without 0.2% pirfenidone. After 12 weeks of feeding, bodyweight (Fig. 1a) and epididymal fat weight (Table 1) were similar among the groups. Liver weight was increased significantly by the CL diet but was unaffected by pirfenidone administration. However, pirfenidone decreased serum triglyceride (TG), total cholesterol (TC), nonesterified fatty acid (NEFA), and AST levels in CL mice (Table 1). Histological analysis showed that mice fed with a CL diet exhibited lipid droplet accumulation in hepatocytes, which was reduced remarkably by pirfenidone (Fig. 1b). The severity of liver injury was quantified by NAFLD Activity Score (NAS). Compared with CL diet only, pirfenidone attenuated steatosis and lobular inflammation, following a lower overall NAFLD activity score (NAS) (Table 2). Consistent with the histologic findings, the CL diet also markedly increased hepatic TG, TC, and NEFA levels compared with the NC diet, whereas pirfenidone treatment significantly suppressed the lipid accumulation induced by the CL diet (Fig. 1c). In addition, the level of thiobarbituric acid reactive substances (TBARS) was increased in the livers of mice fed with a CL diet compared with the NC diet, which indicated excessive lipid peroxidation and oxidative stress in NASH mice; these effects were decreased by pirfenidone administration (Fig. 1c). On the other hand, compared with the NC group, expression of antioxidant genes was decreased (Gpx1, Cat, and Sod1), while NADPH oxidase complex (gp91phox, p22phox, p67phox, and p47phox) expression was elevated in the liver of NASH mice (Supplementary Figs. 1a, b). Pirfenidone increased mRNA levels of antioxidant genes and downregulated gene expression of NADPH oxidase complex in NASH (Supplementary Fig. 1a, b).
Pirfenidone reduced diet-induced hepatic lipid accumulation and prevented the development of hepatic steatosis in vivo. a Weight gain in mice fed with different diets for 12 weeks. b Representative hematoxylin- and eosin-stained liver sections. Scale bar, 100 μm. c Hepatic TG, TC, NEFA, and TBARS levels. d The mRNA expression of lipogenesis- and fatty acid synthesis-related genes in the livers of mice. e The mRNA expression of fatty acid oxidation-related genes in the livers of mice. n = 5–8. *P < 0.05, **P < 0.01 vs. NC diet; #P < 0.05, ##P < 0.01 vs. CL diet
In the livers of CL diet-fed mice, the expression of genes related to lipogenesis and fatty acid synthesis, including Srebp1c, Chrebp, Fasn, and Scd1, was upregulated markedly compared with NC-fed mice. However, the expression of these lipogenic genes was downregulated by pirfenidone treatment (Fig. 1d). In contrast, the expression of genes related to fatty acid oxidation, such as Acc, Ppara, Cpt1a, and Lcad, was increased significantly by pirfenidone in the livers of CL diet-fed mice (Fig. 1e). These results suggest that pirfenidone reduced lipid accumulation in the livers of NASH mice by suppressing lipogenesis and enhancing fatty acid oxidation.
Pirfenidone improved diet-induced glucose intolerance and insulin resistance
To assess the effect of pirfenidone on glucose metabolism, glucose tolerance tests and insulin tolerance tests were performed. Blood glucose levels were not affected by pirfenidone in NC-fed mice, whereas CL diet-induced glucose intolerance and insulin resistance were improved significantly by pirfenidone (Fig. 2a, b). Pirfenidone treatment also decreased plasma insulin levels in both the fasting and fed states compared with the CL diet-fed mice (Fig. 2c). Immunoblotting showed that compared with NC diet only, the hepatic tyrosine-phosphorylated IR-β and serine-phosphorylated Akt were slightly decreased in the NC+ PFD group but significantly reduced in the CL group after insulin stimulation. Treatment with pirfenidone restored the levels of these insulin signaling-related proteins in the livers of CL mice (Fig. 2d). Therefore, the preventive effects of pirfenidone on steatohepatitis were associated with protection against CL diet-induced glucose intolerance and insulin resistance.
Pirfenidone alleviated diet-induced glucose intolerance and hepatic insulin resistance. a Glucose tolerance test (2 g/kg body weight). b Insulin tolerance test in NASH mice (0.5 U/kg body weight). c Plasma insulin levels. n = 5–8. d Immunoblotting of phospho(Tyr1146)-insulin receptor-β (p-IRβ), IRβ, phospho(Ser473)-Akt (p-Akt), and Akt in the livers of mice; the levels of p-IRβ and p-Akt were normalized to those of IRβ and Akt, respectively. n = 4. *P < 0.05, **P < 0.01 vs. NC diet; #P < 0.05, ##P < 0.01 vs. CL diet
Pirfenidone attenuated inflammation in the livers of NASH mice
In this lipotoxic NASH model, the number of hepatic F4/80+ macrophages/Kupffer cells was increased markedly by CL diet feeding, indicating that the CL diet not only induces hepatic steatosis and insulin resistance, but also causes liver inflammation [5]. Immunostaining and mRNA expression analyses showed that pirfenidone decreased the number of F4/80+ cells in the livers of CL mice (Fig. 3a, b). Pirfenidone also markedly downregulated the expression of the proinflammatory genes that were increased by a CL diet, including Tnfa, Il6, and Il1b (Fig. 3b). These findings were further associated with the phosphorylation of inflammatory signals, such as NF-κB p65, p38 mitogen-activated protein kinase (MAPK), and extracellular signal-regulated kinase (ERK) 1/2, which were activated by the CL diet and suppressed significantly by pirfenidone (Fig. 3c).
Pirfenidone ameliorated inflammation in NASH livers and RAW264.7 cells. a Representative F4/80-immunostained liver sections. Scale bar, 100 μm. b mRNA expression of F4/80 and inflammatory cytokines in mouse livers. c Immunoblotting and quantification of phosphorylated NF-κB p65 (p-NF-κB p65), phosphorylated p38 MAPK (p-p38MAPK), and phosphorylated ERK1/2 (p-ERK1/2) levels in mouse livers. n = 5–8. *P < 0.05, ** P < 0.01 vs. NC diet; #P < 0.05, ##P < 0.01 vs. CL diet. d Inflammatory cytokine mRNA expression in RAW264.7 cells. e Immunoblotting and quantification of p-NF-κB p65, p-p38 MAPK, and p-ERK1/2 levels in RAW264.7 cells. n = 6. *P < 0.05, **P < 0.01 vs. no-treatment (NT) group; #P < 0.05, ##P < 0.01 vs. LPS-stimulated group
Previous studies showed that pirfenidone suppressed iNOS and NF-κB activation induced by interleukin-1β in hepatocytes, or prevented hepatocytes from TNF-α-induced apoptosis, suggesting that pirfenidone plays a preventive role in hepatocytes with inflammatory stimulation [18, 19]. Thus, we investigated its anti-inflammatory effects in vitro with RAW264.7 macrophages. After LPS stimulation, the expression of genes related to proinflammatory cytokines and chemokines, Tnfa, Il1b, Mcp-1, and Il6, was notably upregulated in RAW264.7 cells. Co-incubation with pirfenidone (30–300 μg/mL) inhibited the increased expression of these genes in a dose-dependent manner compared with the LPS group (Fig. 3d). Consistent with this, pirfenidone significantly reduced the LPS-induced phosphorylation of NF-κB p65, p38 MAPK, and ERK1/2 in RAW264.7 cells (Fig. 3e). Therefore, pirfenidone attenuated inflammation in diet-induced NASH mice and LPS-stimulated RAW264.7 cells by suppressing inflammatory signals.
Pirfenidone reduced the numbers of M1-type macrophages and T cells and increased the number of M2-type macrophages in NASH mouse livers
To further understand the mechanism by which pirfenidone ameliorates chronic inflammation and insulin resistance in the livers of NASH mice, FACS analysis was performed to quantify hepatic macrophage subsets. Consistent with the immunohistochemical findings, the total number of hepatic macrophages, number of M1-type macrophages, and proportion of M1-type macrophages were decreased by 67.2%, 80.9%, and 38.1%, respectively, whereas the proportion of M2-type macrophages was increased significantly after pirfenidone administration by 62.7% compared with the CL diet group. In addition, the M1/M2 ratio was decreased by 39.2% after pirfenidone treatment (Fig. 4a, b). Consistent with the M1/M2 macrophage polarization status observed by FACS analysis, the liver mRNA expression of M1 macrophage markers (Cd11c, iNOS, Mcp-1, and Ccr2) was suppressed, whereas that of M2 macrophage markers (Cd209a, Chi3l3, Mgl1, and Mrc2) was increased by pirfenidone (Supplementary Fig. 2a). Moreover, FACS analysis revealed that pirfenidone reduced the numbers of hepatic CD3+, CD4+, and CD8+ T cells by 40%, 55%, and 41%, respectively (Fig. 4c, d). In parallel with in vitro experiments, pirfenidone augmented the mRNA expression of IL-4-induced M2 markers (Mrc2, Cd206, and Mgl1) in a dose-dependent manner (Supplementary Fig. 2b), suggesting that not only hepatocytes but also macrophages may be a direct target of pirfenidone. These results indicate that pirfenidone induced a dynamic shift toward an M2-dominant macrophage phenotype and subsequently reduced the accumulation of T cells in the liver to ameliorate hepatic insulin resistance and inflammation.
Pirfenidone reduced the numbers of M1-type macrophages and T cells and increased the number of M2-type macrophages in the livers of NASH mice. a, b A representative plot and quantitation of total and M1/M2 macrophages in the livers of mice. c, d A representative plot of CD3+ T cells and quantitation of CD3+, CD4+, and CD8+ T cells in the livers of mice. n = 8. *P < 0.05, **P < 0.01 vs. CL diet
Pirfenidone improved fibrosis in NASH livers and RI-T cells
Histological staining with Sirius Red and immunostaining with α-SMA demonstrated that the CL diet alone induced HSC activation, thereby causing severe fibrosis, compared with the NC diet. However, pirfenidone administration mitigated these changes (Fig. 5a). Hydroxyproline is a biochemical marker of the hepatic collagen level and is elevated by CL diet feeding. Pirfenidone markedly decreased the elevated hydroxyproline level induced by the CL diet (Fig. 5b). In addition, compared with the CL group, pirfenidone treatment suppressed the expression of the profibrotic genes MMP-12 and tissue inhibitor of metalloproteinase (TIMP)-1 (Fig. 5c). The observations were verified by analyzing the expression of fibrogenic genes and performing α-SMA immunoblotting (Fig. 5d, e).
Pirfenidone mitigated fibrosis in NASH livers and RI-T cells. a Representative Sirius Red staining and α-SMA immunostaining in liver sections. Scale bar, 100 μm. b Hydroxyproline level in mouse livers. c mRNA expression of MMP genes in mouse livers. d mRNA expression of fibrogenic genes in mouse livers. e Immunoblotting and quantification of α-SMA levels in mouse livers. n = 8. **P < 0.01 vs. NC diet; #P < 0.05 vs. CL diet. f mRNA expression of fibrogenic genes in RI-T cells. g Immunoblotting and quantification of α-SMA expression in RI-T cells. n = 6. **P < 0.01 vs. NT group; #P < 0.05, ##P < 0.01 vs. TGF-β1-stimulated group
To examine the antifibrogenic effect of pirfenidone in vitro, RI-T cells were cocultured with or without 30–300 μg/mL pirfenidone in the presence of TGF-β1. The expression levels of the HSC activation marker a-SMA and the extracellular matrix (ECM) synthesis markers collagen type I alpha 1 (Col1a1) and fibronectin, were increased by stimulation with TGF-β1. However, pirfenidone treatment significantly inhibited the elevated expression of these genes in a dose-dependent manner (Fig. 5f). These findings were confirmed by immunoblotting (Fig. 5g). Together, these in vivo and in vitro results suggest that pirfenidone attenuated fibrosis by suppressing HSC activation.
Therapeutic effects of pirfenidone on advanced NASH in mice
Considering the promising results on preventing the progression of NASH, we next investigated whether pirfenidone reversed pre-existing NASH. A NASH model was developed by feeding mice a CL diet for 12 weeks, followed by a CL diet with or without pirfenidone for another 12 weeks (Supplementary Fig. 3a). In comparison with 12 weeks of CL diet feeding, 24 weeks of CL diet further deteriorated hepatic steatosis, lobular inflammation, and hepatocyte ballooning with higher NAS. Changing diet with pirfenidone prevented the deterioration by reducing steatosis and lobular inflammation, resulting in maintaining NAS of 12 weeks of CL feeding (Table 2). Pirfenidone treatment decreased the plasma levels of TG, NEFA, AST, and ALT without affecting body, liver, or epididymal fat weights (Supplementary Table 3). Hepatic lipid accumulation (TG, TC, and NEFA) and peroxidation (TBARS) were also markedly suppressed by pirfenidone (Fig. 6a). Pirfenidone upregulated hepatic mRNA expression of antioxidant genes, whereas it reduced gene expression of NADPH oxidase complex (Supplementary Fig. 3d, e). In addition, pirfenidone ameliorated pre-existing hepatic steatosis (Fig. 6b) and significantly improved glucose intolerance and insulin resistance (Supplementary Fig. 3b, c). Histologically, pirfenidone treatment significantly reduced macrophage/Kupffer cell infiltration (Fig. 6b). Potent hepatic inflammation, as characterized by increased stress or inflammatory signaling and upregulated inflammatory gene expression, was attenuated significantly by pirfenidone (Supplementary Fig. 3f, g). Importantly, pirfenidone reduced HSC activation, decreased the hydroxyproline level in the liver (Fig. 6b, c), and suppressed fibrogenic gene expression (Fig. 6d). Furthermore, pirfenidone significantly decreased MMP-12 and TIMP-1 expression in the liver (Fig. 6e). Taken together, these results suggest that pirfenidone reversed advanced NASH.
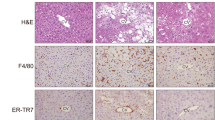
Pirfenidone reversed advanced NASH in mice. a Hepatic TG, TC, NEFA, and TBARS levels. b Representative hematoxylin- and eosin staining, F4/80 immunostaining, Sirius Red staining, and α-SMA immunostaining in liver sections. Scale bars, 100 µm. c Hydroxyproline level and immunoblotting and quantification of α-SMA expression in mouse livers. d mRNA expression of fibrogenic genes in mouse livers. e mRNA expression of MMP genes in mouse livers. n = 8. *P < 0.05, **P < 0.01 vs. CL diet
Discussion
In this study, we investigated the preventive and therapeutic effects of pirfenidone in a diet-induced lipotoxic NASH model and revealed the potential mechanism behind the results. The data indicated that pirfenidone alleviated excessive hepatic lipid accumulation and peroxidation and increased insulin signaling in NASH. In addition, pirfenidone reduced the accumulation of M1-type macrophages/Kupffer cells and T cells in the liver, leading to attenuation of hepatic inflammation and insulin resistance. Moreover, pirfenidone suppressed the activation of HSCs and fibrogenesis in the livers of CL diet-fed mice. Finally, pirfenidone reversed insulin resistance, hepatic inflammation, and fibrosis in mice with pre-existing NASH.
Previous reports showed that pirfenidone ameliorated the hepatotoxicity caused by increased ALT and AST levels and oxidative stress by reducing malondialdehyde concentrations in rats [9, 11]. In this study, feeding mice a CL diet for 12 weeks successfully induced NASH manifestations, including lipid accumulation in the liver and hepatic steatosis. Importantly, treatment with pirfenidone improved hepatic lipid accumulation and dyslipidemia that was not secondary to a decrease in weight or adiposity. Increased hepatic fatty acid transport and/or de novo lipogenesis induce oxidative stress, which subsequently affects lipid accumulation and peroxidation and causes insulin resistance, inflammation, and fibrosis in the liver [5]. Pirfenidone both suppressed the expression of genes related to lipogenesis and fatty acid synthesis and upregulated those related to fatty acid oxidation in the livers of NASH mice. Combined with the elevation of antioxidant gene expression and the suppression of NADPH oxidase complex, the reduction leads to increased oxidative stress in the liver (Fig. 1c–e; Supplementary Figs. 1a, b and 3d, e) [20, 21]. These findings suggest that pirfenidone attenuates lipid accumulation and the subsequent development of insulin resistance, inflammation, and fibrosis, partly by reducing the oxidative stress in the liver induced by a CL diet.
Oxidative stress and insulin resistance are considered the main causes of progression to NASH [22]. A clinical study by Sharma et al. demonstrated that standard therapy with oral pirfenidone improved diabetic nephropathy [12]. In this study, the representative features of insulin resistance, metabolic syndrome, and type 2 diabetes (increased TG secretion and hepatic steatosis [23]) were ameliorated by pirfenidone. In addition, pirfenidone stimulated hepatic insulin signaling by inducing Tyr phosphorylation of IR-β and Ser phosphorylation of Akt. Therefore, pirfenidone decreased TG secretion and enhanced insulin signaling in the liver, which contributed to the improved glucose intolerance and insulin sensitivity.
Hepatic inflammation is a major feature of NASH, suggesting a significant influence of the innate immune system on the progression to NASH. An overload of lipid intake or exogenous endotoxins activate Toll-like receptors, which are present in Kupffer cells in the liver and in recruited bone marrow-derived macrophages, and activation of Toll-like receptors increases the production of inflammatory mediators in NASH [24]. Previous reports showed that pirfenidone inhibited the secretion of TNF-α and IFN-γ, as well as the activation of NF-κB, following LPS treatment in rats [10, 11, 25]. Oku et al. found that pirfenidone prevented the increase in inflammatory mediators, such as IL-1β, IL-6, and MCP-1 in mice with bleomycin-induced pulmonary fibrosis [26]. A recent study indicated that pirfenidone prevents TNF-α-induced liver injury by reducing liver cell apoptosis [19]. In this study, pirfenidone inhibited the activation of Kupffer cells in the livers of NASH mice and reduced the elevated expression of TNF-α and other inflammatory mediators caused by excessive lipid accumulation. Oxidative stress and reactive oxygen species induce lipid peroxidation, which triggers the generation of aldehyde by-products, such as malondialdehyde, and TNF-α-induced liver damage [27]. Oxidant-sensitive transcription factors, such as NF-κB, are also activated by reactive oxygen species, and these transcription factors upregulate the expression of proinflammatory cytokines [28]. The suppression of oxidative stress by pirfenidone contributed to the reduced proinflammatory gene expression and NF-κB p65 phosphorylation observed in this study.
Growing evidence has suggested that activation of NF-κB, p38, ERK1/2, and cytokines is central to preventing insulin resistance by inhibiting insulin receptor signaling and suppressing organ insulin sensitivity [29,30,31]. This study showed that pirfenidone treatment enhanced insulin receptor signaling, perhaps by inhibiting the phosphorylation of inflammatory signaling proteins. Thus, pirfenidone reduced the expression of proinflammatory mediators and the phosphorylation of inflammatory signals by suppressing oxidative stress, which attenuated insulin resistance in the liver.
Macrophages, which are the major cells that produce inflammatory cytokines and chemokines during NAFLD-induced systemic glucose intolerance and fibrosis in the liver, can be functionally divided into two heterogeneous phenotypes (classical [M1] and alternative [M2]) [32]. M1 macrophages can be stimulated by proinflammatory factors, such as LPS, to promote inflammatory cytokine production that results in strong microbicidal and tumoricidal activities. M2 macrophages, which can be activated by IL-4, participate in tissue remodeling and immunoregulatory progression [33]. Under normal circumstances, the balance between the M1 and M2 phenotypes is maintained by regulatory genes; however, chronic inflammation, obesity, and comorbidities, such as insulin resistance and NAFLD, alter this balance and increase the M1/M2 ratio [34,35,36]. A recent study suggested that M2 macrophages protect against alcohol- and high-fat-induced inflammation and hepatocyte injury by promoting M1 macrophage apoptosis [37]. Thus, agents that adjust macrophage polarization by limiting M1 functions and/or improving M2 activation might be novel therapies for inflammation, insulin resistance, and fibrosis in NASH [32]. In this study, FACS data showed that pirfenidone significantly reduced the numbers of total and M1 macrophages and simultaneously increased the proportion of M2 macrophages in mice fed with a CL diet (Fig. 4a, b). In addition, the in vitro results indicated that pirfenidone suppressed LPS-stimulated M1 macrophages and increased the alternative activation of IL-4-induced M2 macrophages in RAW264.7 cells (Fig. 3d, Supplementary Fig. 1b). These results suggest that pirfenidone induced a dynamic shift from M1-type to M2-type macrophages to increase the M1/M2 ratio and attenuate hepatic inflammation and insulin resistance during NASH progression.
In response to oxidative stress, hepatic inflammation in NASH is related to both the accumulation of M1-polarized macrophages and T cells. Tiemessen et al. found that coculturing macrophages with T cells induced the differentiation of monocytes/macrophages toward an alternatively activated phenotype and decreased the LPS-induced production of proinflammatory mediators, including IL-1β, IL-6, TNF-α, and NF-κB [38]. Recent studies indicated that the number of CD3+ T cells in adipose tissue was associated with obesity-related metabolic diseases, such as type 2 diabetes mellitus. In addition, the hepatic recruitment of CD4+ and CD8+ T cells was increased in response to the immune responses provoked by NASH, which further triggered M1 macrophage responses [39,40,41]. In this study, FACS analysis showed that the activation of hepatic CD3+, CD4+, and CD8+ T cells was complemented in response to the inflammatory effects caused by a CL diet. Notably, pirfenidone decreased the numbers of these T cells by inhibiting the activation of polarized M1 macrophages in the liver (Fig. 4c, d). Thus, pirfenidone reduced the accumulation of helper and cytotoxic T cells in the NASH liver. Since insulin resistance may be reversed by immunotherapy via ameliorated macrophage infiltration and tissue inflammation [36, 42], the current findings suggest that pirfenidone decreased T-cell recruitment to suppress the activation of M1 macrophages and finally attenuate NASH-induced inflammation and glucose intolerance.
Pirfenidone is known for its antifibrotic functions and the ability to protect against progressive fibrotic disorders [43]. The therapeutic effects of pirfenidone on IPF have been investigated widely and supported by clinical studies [44]. Liver biopsy showed that pirfenidone treatment reduced steatosis and fibrosis and improved inflammation and liver cell regeneration in patients chronically infected with hepatitis C virus [45]. One goal of this study was to identify and evaluate the antifibrotic effects of pirfenidone in diet-induced NASH fibrosis. On a molecular level, the TGF-β1 signaling pathway in HSCs has the most potent effect on fibrosis development during liver injury. Elevated TGF-β1 signaling leads to phosphorylation of Smad3, which mediates the transcription of fibrogenic genes, such as a-SMA and Col1a1, and induces extracellular matrix secretion for tissue repair [46]. Consistent with previous reports [9, 19, 47], this study found that pirfenidone reduced liver fibrosis by suppressing the transcription of these key genes and reducing α-SMA and hydroxyproline levels in both in vivo and in vitro experiments (Fig. 5, Fig. 6b–d). In addition, pirfenidone reduced TGF-β1 expression by modulating MMPs and their endogenous inhibitors in some experimental models [47, 48]. Some MMPs, such as MMP-12 and TIMP-1, exert profibrotic effects by promoting inflammation and myofibroblast activation. The current data suggest that pirfenidone reduced MMP-12 and TIMP-1 expression in the mouse liver. Thus, pirfenidone reduced collagen accumulation and suppressed HSC activation to alleviate hepatic fibrosis.
The effects of TGF-β1 are important for the movement of inflammatory immune cells in inflamed tissues [49]. During the NF-κB-driven inflammatory response, Smad2/3 signaling in the TGF-β1 pathway induces local monocyte infiltration [50, 51]. Under diabetic conditions, Smad2/3 can also be activated via an ERK/p38 MAP kinase-dependent mechanism [52, 53]. In this study, pirfenidone inhibited MAPK (p38 and ERK1/2) activation and NF-κB phosphorylation, which suppressed Smad signaling in the TGF-β1 pathway (Fig. 3c, e; Supplementary Fig. 2e). Thus, pirfenidone inactivated the TGF-β1 pathway, which reduced inflammation and the accumulation of T cells and macrophages.
In summary, this study demonstrated that pirfenidone, an FDA-approved antifibrotic drug for IPF, limited and reversed hepatic steatosis and insulin resistance in a diet-induced lipotoxic model of NASH by alleviating lipid accumulation and oxidative stress. The accompanying liver inflammation was suppressed by pirfenidone, which was associated with reduced hepatic T-cell and macrophage recruitment, as well as with induction of M2-dominant polarized macrophages/Kupffer cells. Both in vitro and in vivo results indicated that pirfenidone also attenuated hepatic fibrosis by suppressing the TGF-β pathway and stellate cell activation. These data potentially implicate pirfenidone as a therapeutic target for NASH (Fig. 7).
Theoretical schematic of pirfenidone in lipotoxicity-induced NASH. Hepatic lipid accumulation caused lipid peroxidation, leading to insulin resistance, liver inflammation, and further fibrosis. Pirfenidone limited lipid accumulation and oxidative stress in the liver and improved insulin sensitivity. Liver inflammation was attenuated by pirfenidone via decreasing hepatic T-cell and macrophage recruitment, as well as inducting of M2-dominant polarized macrophages/Kupffer cells. Pirfenidone also ameliorated hepatic fibrosis by suppressing the TGF-β1 pathway and stellate cell activation
References
Li J, Cordero P, Nguyen V, Oben JA. The role of vitamins in the pathogenesis of non-alcoholic fatty liver disease. Integr Med Insights. 2016;11:19–25.
Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90.
Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23.
Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis. 2010;28:162–8.
Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–403.
Nakano S, Nagasawa T, Ijiro T, Inada Y, Tamura T, Maruyama K, et al. Bezafibrate prevents hepatic stellate cell activation and fibrogenesis in a murine steatohepatitis model, and suppresses fibrogenic response induced by transforming growth factor-beta1 in a cultured stellate cell line. Hepatol Res. 2008;38:1026–39.
Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306.
Lancaster L, Morrison L, Auais A, Ding B, Iqbal A, Polman B, et al. Safety of pirfenidone in patients with idiopathic pulmonary fibrosis: experience from 92 sites in an open-label US expanded access program. Pulm Ther. 2017;3:317–325.
Garcı́a L, Hernández I, Sandoval A, Salazar A, Garcia J, Vera J, et al. Pirfenidone effectively reverses experimental liver fibrosis. J Hepatol. 2002;37:797–805.
Tsuchiya H, Kaibori M, Yanagida H, Yokoigawa N, Kwon AH, Okumura T, et al. Pirfenidone prevents endotoxin-induced liver injury after partial hepatectomy in rats. J Hepatol. 2004;40:94–101.
Wang F, Wen T, Chen XY, Wu H. Protective effects of pirfenidone on D-galactosamine and lipopolysaccharide-induced acute hepatotoxicity in rats. Inflamm Res. 2008;57:183–8.
Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, et al. Pirfenidone for diabetic nephropathy. J Am Soc Nephrol. 2011;22:1144–51.
Ni Y, Nagashimada M, Zhan L, Nagata N, Kobori M, Sugiura M, et al. Prevention and reversal of lipotoxicity-induced hepatic insulin resistance and steatohepatitis in mice by an antioxidant carotenoid, beta-cryptoxanthin. Endocrinology. 2015;156:987–99.
Ni Y, Nagashimada M, Zhuge F, Zhan L, Nagata N, Tsutsui A, et al. Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: a comparison with vitamin E. Sci Rep. 2015;5:17192.
Kitade H, Sawamoto K, Nagashimada M, Inoue H, Yamamoto Y, Sai Y, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012;61:1680–90.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Liang W, Menke AL, Driessen A, Koek GH, Lindeman JH, Stoop R, et al. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PloS one. 2014;9:e115922.
Nakanishi H, Kaibori M, Teshima S, Yoshida H, Kwon A-H, Kamiyama Y, et al. Pirfenidone inhibits the induction of iNOS stimulated by interleukin-1β at a step of NF-κB DNA binding in hepatocytes. J Hepatol. 2004;41:730–6.
Komiya C, Tanaka M, Tsuchiya K, Shimazu N, Mori K, Furuke S, et al. Antifibrotic effect of pirfenidone in a mouse model of human nonalcoholic steatohepatitis. Sci Rep. 2017;7:44754.
Ji X, Naito Y, Weng H, Ma X, Endo K, Kito N, et al. Renoprotective mechanisms of pirfenidone in hypertension-induced renal injury: through anti-fibrotic and anti-oxidative stress pathways. Biomed Res. 2013;34:309–19.
Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2013;14:20704–28.
Kita Y, Takamura T, Misu H, Ota T, Kurita S, Takeshita Y, et al. Metformin prevents and reverses inflammation in a non-diabetic mouse model of nonalcoholic steatohepatitis. PloS One. 2012;7:e43056.
Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–32.
Kobori M, Ni Y, Takahashi Y, Watanabe N, Sugiura M, Ogawa K, et al. β-Cryptoxanthin alleviates diet-induced nonalcoholic steatohepatitis by suppressing inflammatory gene expression in mice. PloS One. 2014;9:e98294.
Grattendick KJ, Nakashima JM, Feng L, Giri SN, Margolin SB. Effects of three anti-TNF-alpha drugs: etanercept, infliximab and pirfenidone on release of TNF-alpha in medium and TNF-alpha associated with the cell in vitro. Int Immunopharmacol. 2008;8:679–87.
Oku H, Shimizu T, Kawabata T, Nagira M, Hikita I, Ueyama A, et al. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol. 2008;590:400–8.
Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, et al. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–98.
Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–76.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7.
Liu Q, Bengmark S, Qu S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2010;9:1.
Berra E, Diaz-Meco MaT, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem. 1998;273:10792–7.
Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–42.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84.
Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83.
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86.
Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20.
Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130–42.
Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–51.
Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–16.
Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, et al. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 2014;59:886–97.
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20.
Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9.
Raghu G, Selman M. Nintedanib and pirfenidone. New antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. Am J Respir Crit Care Med. 2015;191:252–4.
King TE, Jr., Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92.
Marks DJ, Rahman FZ, Novelli M, Yu RC, McCartney S, Bloom S, et al. An exuberant inflammatory response to E coli: implications for the pathogenesis of ulcerative colitis and pyoderma gangrenosum. Gut. 2006;55:1662–3.
Yang Y, Kim B, Park YK, Koo SI, Lee JY. Astaxanthin prevents TGFβ1-induced pro-fibrogenic gene expression by inhibiting Smad3 activation in hepatic stellate cells. Biochim Biophys Acta. 2015;1850:178–85.
Di Sario A, Bendia E, Macarri G, Candelaresi C, Taffetani S, Marzioni M, et al. The anti-fibrotic effect of pirfenidone in rat liver fibrosis is mediated by downregulation of procollagen α1(I), TIMP-1 and MMP-2. Dig Liver Dis. 2004;36:744–51.
Corbel M, Lanchou J, Germain N, Malledant Y, Boichot E, Lagente V. Modulation of airway remodeling-associated mediators by the antifibrotic compound, pirfenidone, and the matrix metalloproteinase inhibitor, batimastat, during acute lung injury in mice. Eur J Pharmacol. 2001;426:113–21.
Bujak M, Frangogiannis NG. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–95.
Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JJ, Mizel DE, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–6.
Lan HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056–67.
Li JH, Huang XR, Zhu HJ, Oldfield M, Cooper M, Truong LD, et al. Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB J. 2004;18:176–8.
Chung AC, Zhang H, Kong YZ, Tan JJ, Huang XR, Kopp JB, et al. Advanced glycation end-products induce tubular CTGF via TGF-β-independent Smad3 signaling. J Am Soc Nephrol. 2010;21:249–60.
Acknowledgements
The authors thank M. Nakayama and K. Hara (Kanazawa University, Japan) for the technical assistance and animal care.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (B) (25282017) and for Challenging Exploratory Research (15K12698) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Japan Diabetes Foundation (TO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, G., Ni, Y., Nagata, N. et al. Pirfenidone prevents and reverses hepatic insulin resistance and steatohepatitis by polarizing M2 macrophages. Lab Invest 99, 1335–1348 (2019). https://doi.org/10.1038/s41374-019-0255-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-019-0255-4
This article is cited by
-
Pirfenidone ameliorates liver steatosis by targeting the STAT3-SCD1 axis
Inflammation Research (2023)
-
Pirfenidone modifies hepatic miRNAs expression in a model of MAFLD/NASH
Scientific Reports (2021)
-
Prolonged-release pirfenidone prevents obesity-induced cardiac steatosis and fibrosis in a mouse NASH model
Cardiovascular Drugs and Therapy (2021)
-
The role of stress kinases in metabolic disease
Nature Reviews Endocrinology (2020)