Abstract
Objective
To describe the population to which we administered recombinant erythropoietin and to determine the effectiveness of this treatment as quantified by the change in hematocrit.
Study design
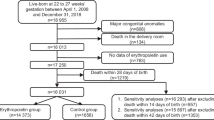
This retrospective chart review study included infants who received erythropoietin for the treatment of anemia of prematurity.
Results
There were 132 infants representing 162 unique treatment courses included in the study. The average duration of therapy was 9 days (±7) and 6 doses (±2). The average change in hematocrit (Hct) was 6.2% (SD 3.9%, p < 0.001). Rise in Hct was associated with a higher number of rEPO doses (p < 0.001) and higher postmenstrual age (p < 0.001). In our small cohort we did not find an association between the number of rEPO doses and retinopathy of prematurity (ROP) requiring treatment.
Conclusion
Erythropoietin is safe and effective at treating anemia of prematurity as evidenced by a clinically and statistically significant increase in Hct from baseline.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Villeneuve A, Arsenault V, Lacroix J, Tucci M. Neonatal red blood cell transfusion. Vox Sang. 2021;116:366–78.
Bell EF. Red cell transfusion thresholds for preterm infants: finally some answers. Arch Dis Child Fetal Neonatal Ed. 2022;107:126–30.
Colobatti R, Sainati L, Trevisanuto D. Anemia and transfusion in the neonate. Semin Fetal Neonatal Med. 2016;21:2–9.
Christensen RD, Baer VL, Lambert DK, Ilstrup SJ, Eggert LD, Henry E. Association, among very-low birthweight neonates, between red blood cell transfusions in the week after birth and severe intraventricular hemorrhage. Transfusion. 2014;54:104–8.
Ohlsson A, Aher SM. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst Rev. 2017;11:CD004863.
Ohlsson A, Aher SM. Late erythropoiesis-stimulating agents to prevent red blood cell transfusion in preterm or low birth weight infants. Cochrane Database Syst Rev. 2020;1:CD004868.
Maier RF, Obladen M, Müller-Hansen I, Kattner E, Merz U, Arlettaz R, et al. Early treatment with erythropoietin β ameliorates anemia and reduces transfusion requirements in infants with birth weights below 1000g. J Pediatr. 2002;141:8–15.
El-Lahony DM, Saleh NY, Habib MS, Shehata MA, El-Hawy MA. The role of recombinant human erythropoietin in neonatal anemia. Hematol Oncol Stem Cell Ther. 2020;13:147–51.
Ohls RK, Osborne KA, Christensen RD. Efficacy and cost analysis of treating very low birth weight infants with erythropoietin during their first two weeks of life: a randomized, placebo-controlled trial. J Pediatr. 1995;126:421–6.
Ohls RK, Harcum J, Schibler KR, Christensen RD. The effect of erythropoietin on the transfusion requirements of preterm infants weighing 750 grams or less: a randomized, double blind, placebo-controlled study. J Pediatr. 1997;131:661–5.
Ohls RK, Ehrenkranz RA, Wright LL, Lemons JA, Korones SB, Stoll BJ, et al. Effects of early erythropoietin therapy on the transfusion requirements of preterm infants below 1250 grams birth weight: a multicenter, randomized, controlled trial. Pediatrics. 2001;108:934–42.
Juul SE, Vu PT, Comstock BA, Wadhawan R, Mayock DE, Courtney SE, et al. Effect of high-dose erythropoietin on blood transfusions in extremely low gestational age neonates: post hoc analysis of a randomized clinical trial. JAMA Pediatr. 2020;174:933–43.
Salsbury DC. Anemia of prematurity. Neonatal Netw. 2001;20:13–20.
Strauss RG. Anemia of prematurity: pathophysiology & treatment. Blood Rev. 2010;24:221–5.
Romagnoli C, Zecca E, Gallini F, Zuppa PGAA. Do recombinant human erythropoietin and iron supplementation increase the risk of retinopathy of prematurity? Eur J Pediatr. 2000;159:627–34.
Fischer HS, Reibel NJ, Bührer C, Dame C. Effect of early erythropoietin on retinopathy of prematurity: a stratified meta-analysis. Neonatology. 2023;120:566–76.
Acknowledgements
M. David Gothard, BS, MS of BioStats of Ohio, Inc. for his support in statistical analysis and power calculations.
Author information
Authors and Affiliations
Contributions
JC was responsible for literature review, project design, data collection, manuscript composition. JDM and JLM were responsible for project design, literature review, manuscript review. RMR was responsible for project design, literature review, data analysis, manuscript review. MLN was responsible for literature review and manuscript review. MN, RD and AS were responsible for literature review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval and Consent to Participate
The protocol for this study was approved by the University Hospitals institutional review board. The need for informed consent was waived due to the retrospective nature of the study. This study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Connolly, J.M., McClary, J.D., Desai, R. et al. Efficacy of recombinant erythropoietin for the late treatment of anemia of prematurity in a level IV neonatal intensive care unit: a retrospective single-center cohort study. J Perinatol (2024). https://doi.org/10.1038/s41372-024-02001-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-024-02001-6