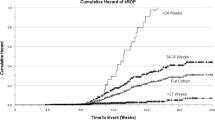
Abstract
Anemia of prematurity (AOP) is a common condition with a well-described chronology, nadir hemoglobin levels, and timeline of recovery. However, the underlying pathophysiology and impact of prolonged exposure of the developing infant to low levels of hemoglobin remains unclear. Phlebotomy losses exacerbate the gradual decline of hemoglobin levels which is insidious in presentation, often without any clinical signs. Progressive anemia in preterm infants is associated with poor weight gain, inability to take oral feeds, tachycardia and exacerbation of apneic, and bradycardic events. There remains a lack of consensus on treatment thresholds for RBC transfusion which vary considerably. This review elaborates on the current state of the problem, its implication for the premature infant including association with subphysiologic cerebral tissue oxygenation, necrotizing enterocolitis, and retinopathy of prematurity. It outlines the impact of prophylaxis and treatment of anemia of prematurity and offers suggestions on improving monitoring and management of the condition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lokeshwar MR, Singhal T, Shah N. Anemia in the newborn. Indian J Pediatr. 2003;70:893–902.
Luban NL. Management of anemia in the newborn. Early Hum Dev. 2008;84:493–8.
Obladen M, Sachsenweger M, Stahnke M. Blood sampling in very low birth weight infants receiving different levels of intensive care. Eur J Pediatr. 1988;147:399–404.
Ringer SA, Richardson DK, Sacher RA, Keszler M, Churchill WH. Variations in transfusion practice in neonatal intensive care. Pediatrics. 1998;101:194–200.
Alagappan A, Shattuck KE, Malloy MH. Impact of transfusion guidelines on neonatal transfusions. J Perinatol. 1998;18:92–97.
Widness JA. Pathophysiology of anemia during the neonatal period, including anemia of prematurity. Neoreviews. 2008;9:e520.
Raffaeli G, Manzoni F, Cortesi V, Cavallaro G, Mosca F, Ghirardello S. Iron homeostasis disruption and oxidative stress in preterm newborns. Nutrients. 2020;12:1554.
Wang Y, Wu Y, Li T, Wang X, Zhu C. Iron metabolism and brain development in premature infants. Front Physiol. 2019;10:463.
Tiker F, Celik B, Tarcan A, Kilicdag H, Ozbek N, Gurakan B. Serum pro-hepcidin levels and relationships with iron parameters in healthy preterm and term newborns. Pediatr Hematol Oncol. 2006;23:293–7.
Maier RJ, Sonntag J, Walka MM, Liu G, Metze BC, Obladen M. Changing practices of red blood cell transfusions in infants with birth weights less than 1000 g. J Pediatr. 2000;136:220–4.
Bowen JR, Patterson JA, Roberts CL, Isbister JP, Irving DO, Ford JB. Red cell and platelet transfusions in neonates: a population-based study. Arch Dis Child Fetal Neonatal Ed. 2015;100:F411–5.
King PJ. Iron nutrition, erythrocytes, and erythropoietin in the NICU: erythropoietic and neuroprotective effects. NeoReviews. 2020;21:e80–8.
Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009;123:e333–7.
King PJ, Coe CL. Iron homeostasis in pregnancy, the fetus and the neonate. NeoReviews. 2016;17:e657–64.
Lönnerdal B, Georgieff MK, Hernell O. Developmental physiology of iron absorption, homeostasis, and metabolism in the healthy term infant. J Pediatr. 2015;167:S8–14.
Domellöf M. Meeting the iron needs of low and very low birth weight infants. Ann Nutr Metab. 2017;71:16–23.
Rao RB, Georgieff MK. Iron therapy for preterm infants. Clin Perinatol. 2009;36:27–42.
Herzlich J, Litmanovitz I, Regev R, Bauer S, Sirota G, Steiner Z, et al. Iron homeostasis after blood transfusion in stable preterm infants—an observational study. J Perinat Med. 2016;44:919–23.
Stripeli F, Kapetanakis J, Gourgiotis D, Drakatos A, Tsolia M, Kossiva L. Post-transfusion changes in serum hepcidin and iron parameters in preterm infants. Pediatr Int. 2018;60:148–52.
Widness JA, Veng-Pedersen P, Peters C, Pereira LM, Schmidt RL, Lowe LS. Erythropoietin pharmacokinetics in premature infants: developmental, nonlinearity, and treatment effects. J Appl Physiol. 1996;80:140–8.
Stockman JA 3rd, Graeber JE, Clark DA, McClellan K, Garcia JF, Kavey RE. Anemia of prematurity: determinants of the erythropoietin response. J Pediatr. 1984;105:786–92.
Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA. 2016;315:889–97.
Yoxall CW, Weindling AM. The measurement of peripheral venous oxyhemoglobin saturation in newborn infants by near infrared spectroscopy with venous occlusion. Pediatr Res. 1996;39:1103–6.
Liu G, Yan Y, Shi B, Huang J, Mu H, Li C, et al. Benefits of progesterone on brain immaturity and white matter injury induced by chronic hypoxia in neonatal rats. J Thorac Cardiovasc Surg. 2020;160:e55–66.
Malhotra A, Sepehrizadeh T, Dhollander T, Wright D, Castillo-Melendez M, Sutherland AE, et al. Advanced MRI analysis to detect white matter brain injury in growth restricted newborn lambs. Neuroimage Clin. 2019;24:101991.
Bailey SM, Hendricks-Munoz KD, Mally P. Cerebral, renal, and splanchnic tissue oxygen saturation values in healthy term newborns. Am J Perinatol. 2014;31:339–44.
Vretzakis G, Georgopoulou S, Stamoulis K, Stamatiou G, Tsakiridis K, Zarogoulidis P, et al. Cerebral oximetry in cardiac anesthesia. J Thorac Dis. 2014;6:S60–69.
Sood BG, McLaughlin K, Cortez J. Near-infrared spectroscopy: applications in neonates. Semin Fetal Neonatal Med. 2015;20:164–72.
Whitehead HV, Vesoulis ZA, Maheshwari A, Rao R, Mathur AM. Anemia of prematurity and cerebral near-infrared spectroscopy: should transfusion thresholds in preterm infants be revised? J Perinatol. 2018;38:1022–9.
van Hoften JC, Verhagen EA, Keating P, ter Horst HJ, Bos AF. Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch Dis Child Fetal Neonatal Ed. 2010;95:F352–8.
Alderliesten T, Dix L, Baerts W, Caicedo A, van Huffel S, Naulaers G, et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr Res. 2016;79:55–64.
Wardle SP, Yoxall CW, Weindling AM. Determinants of cerebral fractional oxygen extraction using near infrared spectroscopy in preterm neonates. J Cereb Blood Flow Metab. 2000;20:272–9.
Balegar KK, Stark MJ, Briggs N, Andersen CC. Early cerebral oxygen extraction and the risk of death or sonographic brain injury in very preterm infants. J Pediatr. 2014;164:475–80.e1.
Verhagen EA, Keating P, ter Horst HJ, Martijn A, Bos AF. Cerebral oxygen saturation and extraction in preterm infants with transient periventricular echodensities. Pediatrics. 2009;124:294–301.
Whitehead HV, Vesoulis ZA, Maheshwari A, Rambhia A, Mathur AM. Progressive anemia of prematurity is associated with a critical increase in cerebral oxygen extraction. Early Hum Dev. 2019. https://doi.org/10.1016/j.earlhumdev.2019.104891.
Sandal G, Oguz SS, Erdeve O, Akar M, Uras N, Dilmen U. Assessment of red blood cell transfusion and transfusion duration on cerebral and mesenteric oxygenation using near-infrared spectroscopy in preterm infants with symptomatic anemia. Transfusion. 2014;54:1100–5.
Aktas S, Ergenekon E, Ozcan E, Aksu M, Unal S, Hirfanoglu IM, et al. Effects of blood transfusion on regional tissue oxygenation in preterm newborns are dependent on the degree of anaemia. J Paediatr Child Health. 2019;55:1209–13.
Andropolous DB, Stayer SA, Diaz LK, Ramamoorthy C. Neurological monitoring for congenital heart surgery. Anesth Analg. 2004;99:1365–75.
Hirsch JC, Charpie JR, Ohye RG, Gurney JG. Near-infrared spectroscopy: what we know and what we need to know—a systematic review of the congenital heart disease literature. J Thorac Cardiovasc Surg. 2009;137:154–9. 159e1-12
Bonestroo HJ, Lemmers PM, Baerts W, van Bel F. Effect of antihypotensive treatment on cerebral oxygenation of preterm infants without PDA. Pediatrics. 2011;128:e1502–10.
Tina LG, Frigiola A, Abella R, Artale B, Puleo G, D’Angelo S, et al. Near infrared spectroscopy in healthy preterm and term newborns: correlation with gestational age and standard monitoring parameters. Curr Neurovasc Res. 2009;6:148–54.
Limperopoulos C, Gauvreau KK, O’Leary H, Moore M, Bassan H, Eichenwald EC, et al. Cerebral hemodynamic changes during intensive care of preterm infants. Pediatrics. 2008;122:e1006–13.
MohanKumar K, Namachivayam K, Song T, Jake Cha B, Slate A, Hendrickson JE, et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun. 2019;10:3494.
Fortune PM, Wagstaff M, Petros AJ. Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med. 2001;27:1401–7.
Singh R, Visintainer PF, Frantz ID 3rd, Shah BL, Meyer KM, Favila SA, et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol. 2011;31:176–82.
DeRienzo C, Smith PB, Tanaka D, Bandarenko N, Campbell ML, Herman A, et al. Feeding practices and other risk factors for developing transfusion-associated necrotizing enterocolitis. Early Hum Dev. 2014;90:237–40.
Nowicki PT. Effects of sustained flow reduction on postnatal intestinal circulation. Am J Physiol. 1998;275:G758–768.
Perget L, Mukhopadhyay D, Komidar L, Wiggins-Dohlvik K, Uddin MN, Beeram M. Maternal pre-eclampsia as a risk factor for necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2016;S15:889–97.
Giannone PJ, Luce WA, Nankervis CA, Hoffman TM, Wold LE. Necrotizing enterocolitis in neonates with congenital heart disease. Life Sci. 2008;82:341–7.
Jayanthi S, Seymour P, Puntis JW, Stringer MD. Necrotizing enterocolitis after gastroschisis repair: a preventable complication? J Pediatr Surg. 1998;33:705–7.
Ito Y, Doelle SM, Clark JA, Halpern MD, McCuskey RS, Dvorak B. Intestinal microcirculatory dysfunction during the development of experimental necrotizing enterocolitis. Pediatr Res. 2007;61:180–4.
Cortez J, Gupta M, Amaram A, Pizzino J, Sawhney M, Sood BG. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J Matern Fetal Neonatal Med. 2011;24:574–82.
Mintzer JP, Parvez B, Chelala M, Alpan G, LaGamma EF. Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates. J Neonatal Perinat Med. 2014;7:199–206.
Sood BG, Cortez J, McLaughlin KL, Gupta M, Amaram A, Kolli M, et al. Near infrared spectroscopy as a biomarker for necrotizing enterocolitis following red blood cell transfusion. J Near Infrared Spectrosc. 2014;22:375–88.
Schat TE, van Zoonen AGJF, van der Laan ME, Mebius MJ, Bos AF, Hulzebos CV, et al. Early cerebral and intestinal oxygenation in the risk assessment of necrotizing enterocolitis in preterm infants. Early Hum Dev. 2019;131:75–80.
American College of Obstetricians and Gynecologists. Committee opinion number 814: delayed umbilical cord clamping after birth. American College of Obstetricians and Gynecologists; 2020. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2020/12/delayed-umbilical-cord-clamping-after-birth. Accessed 26 Feb 2021.
Australian and New Zealand Society of Blood Transfusion. Guidelines for transfusion and immunohaematology laboratory practice. 1st ed. Australian and New Zealand Society of Blood Transfusion; 2016. https://anzsbt.org.au/wp-content/uploads/2021/01/FINAL-Guideline_-for_Transfusion_and_Immunohaematology_Laboratory_Practice_Published_20210125.pdf. Accessed 26 Feb 2021.
Resuscitation Council (UK). Resuscitation and support of transition of babies at birth. Resuscitation Council (UK); 2015. https://www.resus.org.uk/library/2015-resuscitation-guidelines/resuscitation-and-support-transition-babies-birth. Accessed 26 Feb 2021.
Mercer JS, Erickson-Owens DA, Deoni SCL, Dean DC 3rd, Collins J, Parker AB, et al. Effects of delayed cord clamping on 4-month ferritin levels, brain myelin content, and neurodevelopment: a randomized controlled trial. J Pediatr. 2018;203:266–72.e2.
Rana N, Ko A, Malqvist M, Subedi K, Andersson O. Effect of delayed cord clamping of term babies on neurodevelopment at 12 months: a randomized controlled trial. Neonatology. 2019;115:36–42.
Levy T, Blickstein I. Timing of cord clamping revisited. J Perinat Med. 2006;34:293–7.
Lin JC, Strauss RG, Kulhavy JC, Johnson KJ, Zimmerman MB, Cress GA, et al. Phlebotomy overdraw in the neonatal intensive care nursery. Pediatrics. 2000;106:E19.
Madan A, Kumar R, Adams MM, Benitz WE, Geaghan SM, Widness JA. Reduction in red blood cell transfusions using a bedside analyzer in extremely low birth weight infants. J Perinatol. 2005;25:21–25.
Widness JA, Madan A, Grindeanu LA, Zimmerman MB, Wong DK, Stevenson DK. Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics. 2005;115:1299–306.
Worthington-White DA, Behnke M, Gross S. Premature infants require additional folate and vitamin B12 to reduce the severity of the anemia of prematurity. Am J Clin Nutr. 1994;60:930–5.
Haiden N, Klebermass K, Cardona F, Schwindt J, Berger A, Kohlhauser-Vollmuth C, et al. A randomized, controlled trial of the effects of adding vitamin B12 and folate to erythropoietin for the treatment of anemia of prematurity. Pediatrics. 2006;118:180–8.
Haiden N, Schwindt J, Cardona F, Berger A, Klebermass K, Wald M, et al. Effects of a combined therapy of erythropoietin, iron, folate, and vitamin B12 on the transfusion requirements of extremely low birth weight infants. Pediatrics. 2006;118:2004–13.
American Academy of Pediatrics, Committee on Nutrition. Iron deficiency. In: Kleinman RE, editor. Pediatric nutrition handbook. Elk Grove Village, IL: American Academy of Pediatrics; 1998. pp. 299–312.
Maier RF, Obladen M, Kattner E, Natzschka J, Messer J, Regazzoni BM, et al. High-versus low-dose erythropoietin in extremely low birth weight infants. The European Multicenter rhEPO Study Group. J Pediatr. 1998;132:866–70.
Vamvakas EC, Strauss RG. Meta-analysis of controlled clinical trials studying the efficacy of rHuEPO in reducing blood transfusions in the anemia of prematurity. Transfusion. 2001;41:406–15.
Aher SM, Ohlsson A. Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Datbase. Syst Rev. 2006;3:CD004868.
Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;3:CD004863.
Ohlsson A, Aher SM. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst Rev. 2017;11:CD004863.
Juul SE, Comstock BA, Wadhawan R, Mayock DE, Courtney SE, Robinson T. for the PENUT Trial Consortium et al. A randomized trial of erythropoietin for neuroprotection in preterm infants. N Engl J Med. 2020;382:233–43.
Juul SE, Vu PT, Comstock BA, Wadhawan R, Mayock DE, for the Preterm Erythropoietin Neuroprotection Trial Consortium, et al. Effect of high-dose erythropoietin on blood transfusions in extremely low gestational age neonates: post hoc analysis of a randomized clinical trial. JAMA Pediatr. 2020; 174:933–43.
Calhoun DA, Christensen RD, Edstrom CS, Juul SE, Ohls RK, Schibler KR, et al. Consistent approaches to procedures and practices in neonatal hematology. Clin Perinatol. 2000;27:733–53.
Fredrickson LK, Bell EF, Cress GA, Johnson KJ, Zimmerman MB, Mahoney LT, et al. Acute physiological effects of packed red blood cell transfusion in preterm infants with different degrees of anaemia. Arch Dis Child Fetal Neonatal Ed. 2011;96:F249–53.
Ghirardello S, Dusi E, Cortinovis I, Villa S, Fumagalli M, Agosti M, et al. Effects of red blood cell transfusions on the risk of developing complications or death: an observational study of a cohort of very low birth weight infants. Am J Perinatol. 2017;34:88–95.
Sloan, SR. Blood products used in the newborn. In: Cloherty JP, editor. Manual of neonatal care. Philadelphia: Lippincott Williams & Wilkins; 2012. pp. 221–5.
Kirpalani H, Whyte RK, Anderson C, Asztalos EV, Heddle N, Blajchman MA, et al. The premature infants in need of transfusion (PINT) study: a randomized controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–7.
Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusions in preterm infants. Pediatrics. 2005;115:1685–91.
Blank JP, Sheagren TG, Vajaria J, Mangurten HH, Benawra RS, Puppala BL. The role of RBC transfusion in the premature infant. Am J Dis Child. 1984;138:831–3.
Keyes WG, Donohue PK, Spivak JL, Jones MD Jr, Oski FA. Assessing the need for transfusion of premature infants and the role of hematocrit, clinical signs, and erythropoietin level. Pediatrics. 1989;84:412–7.
Brooks SE, Marcus DM, Gillis D, Pirie E, Johnson MH, Bhatia J. The effect of blood transfusion protocol on retinopathy of prematurity: a prospective, randomized study. Pediatrics 1999;104:514–8. https://doi.org/10.1542/peds.104.3.514.
Franz AR, Engel C, Bassler D, Rudiger M, Thome UH. for the ETTNO Investigators, et al. Effects of liberal vs restrictive transfusion thresholds on survival and neurocognitive outcomes in extremely low-birth-weight infants: the ETTNO randomized clinical trial. JAMA. 2020;324:560–70.
Lopriore E. Updates in red blood cell and platelet transfusions in preterm neonates. Am J Perinatol. 2019;36:S37–S40.
British Committee for Standards in Haematology: British Committee for Standards in Haematology Clinical Guideline. Transfusion for fetuses, neonates, and older children. London: BCSH; 2016.
Miller Y, Bachowski G, Benjamin R. Practice guidelines for blood transfusion, 2nd ed. Washington: American Red Cross; 2007. pp. 5–17.
Ohls RK. The use of erythropoietin in neonates. Clin Perinatol. 2000;27:681–96.
Bednarek FJ, Weisberger S, Richardson DK, Frantz ID 3rd, Shah B, Rubin LP. Variations in blood transfusions among newborn intensive care units. SNAP II Study Group. J Pediatr. 1998;133:601–7.
Howarth C, Banerjee J, Aladangady N. Red blood cell transfusion in preterm infants: current evidence and controversies. Neonatology. 2018;114:7–16.
dos Santos AM, Guinsburg R, de Almeida MF, Procianoy RS, Marba ST, Ferri WA. for the Brazilian Network on Neonatal Research, et al. Factors associated with red blood cell transfusions in very-low-birth-weight preterm infants in Brazilian neonatal units. BMC Pediatr. 2015;15:113
Fergusson D, Hebert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–51.
Cooke RW, Clark D, Hickey-Dwyer M, Weindling AM. The apparent role of blood transfusions in the development of retinopathy of prematurity. Eur J Pediatr. 1993;152:833–6.
Cooke RW, Drury JA, Yoxall CW, James C. Blood transfusion and chronic lung disease in preterm infants. Eur J Pediatr. 1997;156:47–50.
Paul DA, Mackley A, Novitsky A, Zhao Y, Brooks A, Locke RG. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics. 2011;127:635–41.
Christensen RD, Lambert DK, Henry E, Wiedmeier SE, Snow GL, Baer VL, et al. Is ‘transfusion-associated necrotizing enterocolitis’ an authentic pathogenic entity? Transfusion. 2010;50:1106–12.
Baer VL, Lambert DK, Henry E, Snow GL, Butler A, Christensen RD. Among very-lowbirth-weight neonates is red blood cell transfusion an independent risk factor for subsequently developing a severe intraventricular hemorrhage? Transfusion. 2011;51:1170–8.
McCoy TE, Conrad AL, Richman LC, Lindgren SD, Nopoulos PC, Bell EF. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol. 2011;17:347–67.
Nopoulos PC, Conrad AL, Bell EF, Strauss RG, Widness JA, Magnotta VA, et al. Long-term outcome of brain structure in premature infants: effects of liberal vs. restricted red blood cell transfusions. Arch Pediatr Adolesc Med. 2011;165:443–50.
Whyte RK, Kirpalani H, Asztalos EV, Andersen C, Blajchman M, Heddle N. for the PINTOS Study Group, et al. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123:207–13.
Ibrahim M, Ho SK, Yeo CL. Restrictive versus liberal red blood cell transfusion thresholds in very low birth weight infants: a systematic review and meta-analysis. J Paediatr Child Health. 2014;50:122–30.
Whyte R, Kirpalani H. Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants. Cochrane Syst Rev. 2011;11:CD000512.
Kirpalani H, Bell E, Hintz SR, Tan S, Schmidt B, Chaudhary AS, et al. Higher or lower hemoglobin transfusion thresholds for preterm infants. N Engl J Med. 2020;383:2639–51.
Kamper-Jorgensen M, Ahlgren M, Rostgaard K, Melbye M, Edgren G, Nyren O, et al. Survival after blood transfusion. Transfusion. 2008;48:2577–84.
Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in critical care investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17.
Kneyber MC, Hersi MI, Twisk JW, Markhorst DG, Plotz FB. Red blood cell transfusion in critically ill children is independently associated with increased mortality. Intensive Care Med. 2007;33:1414–22.
Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–52.
Somani A, Steiner ME, Hebbel RP. The dynamic regulation of microcirculatory conduit function: features relevant to transfusion medicine. Transfus Apher Sci. 2010;43:61–68.
dos Santos AM, Guinsburg R, de Almeida MF, Procianoy RS, Leone CR, Marba ST. for the Brazilian Network on Neonatal Research, et al. Red blood cell transfusions are independently associated with intra-hospital mortality in very low birth weight preterm infants. J Pediatr. 2011;159:371–6, e1-3.
Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI). Br J Haematol. 2007;136:788–99.
Rashid N, Al-Sufayan F, Seshia MM, Baier RJ. Post transfusion lung injury in the neonatal population. J Perinatol. 2013;33:292–6.
Grev JE, Stanclova M, Ellsworth MA, Colby CE. Does red blood cell transfusion-related acute lung injury occur in premature infants? A retrospective cohort analysis. Am J Perinatol. 2017;34:14–18.
Keir A, Pal S, Trivella M, Lieberman L, Callum J, Shehata N, et al. Adverse effects of red blood cell transfusions in neonates: a systematic review and meta-analysis. Transfusion. 2016;56:2773–80.
Hall N, Ong EG, Ade-Ajayi N, Fasoli L, Ververidis M, Kiely EM, et al. T cryptantigen activation is associated with advanced necrotizing enterocolitis. J Pediatr Surg. 2002;37:791–3.
Gephart SM. Transfusion-associated necrotizing enterocolitis: evidence and uncertainty. Adv Neonatal Care. 2012;12:232–6.
Rai SE, Sidhu AK, Krishnan RJ. Transfusion-associated necrotizing enterocolitis re-evaluated: a systematic review and meta-analysis. J Perinat Med. 2018;46:665–76.
Christensen RD, Lambert DK, Henry E, Wiedmeier SE, Snow GL, Baer VL, et al. Is “transfusion-associated necrotizing enterocolitis” an authentic pathogenic entity? Transfusion. 2010;50:1106–12.
Keir AK, Wilkinson D. Question 1: do feeding practices during transfusion influence the risk of developing necrotising enterocolitis in preterm infants? Archiv Dis Child. 2013;98:386–8.
Baer VL, Lambert DK, Henry E, Snow GL, Christensen RD. Red blood cell transfusion of preterm neonates with a grade 1 intraventricular hemorrhage is associated with extension to a grade 3 or 4 hemorrhage. Transfusion. 2011;51:1933–9.
Christensen RD, Baer VL, Lambert DK, Ilstrup SJ, Eggert LD, Henry E. Association, among very-low-birthweight neonates, between red blood cell transfusions in the week after birth and severe intraventricular hemorrhage. Transfusion. 2014;54:104–8.
Brooks SE, Marcus DM, Gillis D, Pirie E, Johnson MH, Bhatia J. The effect of blood transfusion protocol on retinopathy of prematurity: a prospective, randomized study. Pediatrics. 1999;104:514–8.
Stark MJ, Keir AK, Andersen CC. Does non-transferrin bound iron contribute to transfusion related immune-modulation in preterms? Arch Dis Child Fetal Neonatal Ed. 2013;98:F424–429.
Stutchfield CJ, Jain A, Odd D, Williams C, Markham R. Foetal haemoglobin, blood transfusion, and retinopathy of prematurity in very preterm infants: a pilot prospective cohort study. Eye. 2017;31:1451–5.
Collard KJ. Transfusion related morbidity in premature babies: possible mechanisms and implications for practice. World J Clin Pediatr. 2014;3:19–29.
Dani C, Reali MF, Bertini G, Martelli E, Pezzati M, Rubaltelli FF. The role of blood transfusions and iron intake on retinopathy of prematurity. Early Hum Dev. 2001;62:57–63.
Hesse L, Eberl W, Schlaud M, Poets CF. Blood transfusion, iron load, and retinopathy of prematurity. Eur J Pediatr. 1997;156:465–70.
Lust C, Vesoulis Z, Jackups R Jr, Liao S, Rao R, Mathur AM. Early red cell transfusion is associated with development of severe retinopathy of prematurity. J Perinatol. 2019;39:393–400.
National Blood Authority (NBA). Patient blood management guidelines: module 6—neonatal and paediatrics; Canberra, Australia: NBA; 2016. p202.
Whyte RK, Jefferies AL. Canadian Paediatric Society, Fetus and Newborn Committee Red blood cell transfusion in newborn infants. Paediatr Child Health. 2014;19:213–7.
Funding
NIH Grants HL124078 and HL133022 (AM).
Author information
Authors and Affiliations
Contributions
CCC performed the literature review and wrote the first draft of the manuscript. RR performed and reviewed the literature and critically edited the review for content. AM coinitiated the concept for the review, performed literature review, and critically reviewed and edited the manuscript for content. AMM initiated the concept for the review, performed literature review, and critically reviewed and edited the manuscript for content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cibulskis, C.C., Maheshwari, A., Rao, R. et al. Anemia of prematurity: how low is too low?. J Perinatol 41, 1244–1257 (2021). https://doi.org/10.1038/s41372-021-00992-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-00992-0
This article is cited by
-
Abdominal Near Infrared Spectroscopy can be reliably used to measure splanchnic oxygenation changes in preterm infants
Journal of Perinatology (2023)
-
Red blood cell transfusions post diagnosis of necrotizing enterocolitis and the deterioration of necrotizing enterocolitis in full-term and near-term infants: a propensity score adjustment retrospective cohort study
BMC Pediatrics (2022)
-
Hematological changes in neonatal mice with phlebotomy-induced anemia
Pediatric Research (2022)
-
Is a higher hemoglobin transfusion threshold better for extremely low birthweight infants?
Journal of Perinatology (2022)
-
Ideal blood inoculant volume for neonatal sepsis evaluation: an alternative approach
Pediatric Research (2021)