Abstract
Objective
Exogenous surfactant therapy is vital in managing respiratory distress syndrome (RDS) in preterm infants, with less invasive surfactant administration (LISA) gaining popularity. This study aimed to assess the efficacy and short-term outcomes of LISA using beractant and poractant alfa.
Study design
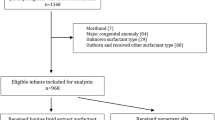
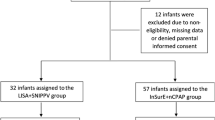
In a randomized controlled trial, we enrolled preterm infants (28–33+6 weeks) with RDS requiring surfactant. LISA was employed, with beractant at 100 mg/kg or poractant-alfa at 200 mg/kg. Primary outcome was the need for intubation within 72 hours.
Results
Among 120 infants, 3.3% in both groups required intubation within 72 hours (p value 1.00, 95% CI 0.14–6.86). No significant differences in secondary outcomes were noted. However, beractant was significantly more economical than poractant-alfa, with a significantly lower surfactant cost and total care cost for infant hospital stays.
Conclusion
Beractant and poractant-alfa exhibit similar efficacy in LISA for preterm infants with RDS. Economic considerations, especially in LMICs, favour beractant.
Clinical trial registation
(CTRI/2023/03/050375).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
References
Sweet DG, Carnielli VP, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology. 2023;120:3–23. https://doi.org/10.1159/000528914.
Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017;102:F17–F23. https://doi.org/10.1136/archdischild-2015-310299.
Kakkilaya V, Gautham KS. Should less invasive surfactant administration (LISA) become routine practice in US neonatal units? Pediatr Res. 2023;93:1188–98. https://doi.org/10.1038/s41390-022-02265-8.
Taylor G, Jackson W, Hornik CP, Koss A, Mantena S, Homsley K, et al. Surfactant administration in preterm infants: drug development opportunities. J Pediatr. 2019;208:163–8.
Survanta [package insert] North Chicago, IL: AbbVie, Inc; 2023. https://www.rxabbvie.com/pdf/survanta_pi.pdf Accessed December 17, 2023.
Curosurf [package insert] Parma, Italy: Chiesi Farmaceutici SpA; 2023. https://resources.chiesiusa.com/Curosurf/CUROSURF_PI.pdf). Accessed December 17, 2023.
Polin RA, Carlo WA, Committee on Fetus and Newborn; American Academy of Pediatrics. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133:156–63. https://doi.org/10.1542/peds.2013-3443.
Singh N, Halliday HL, Stevens TP, Suresh G, Soll R, Rojas-Reyes MX. Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. 2015;2015:CD010249. https://doi.org/10.1002/14651858.CD010249.pub2.
Tridente A, De Martino L, De Luca D. Porcine vs bovine surfactant therapy for preterm neonates with RDS: systematic review with biological plausibility and pragmatic meta-analysis of respiratory outcomes. Respir Res. 2019;20:28 https://doi.org/10.1186/s12931-019-0979-0.
Sánchez Luna M, Bacher P, Unnebrink K, Martinez-Tristani M, Ramos Navarro C. Beractant and poractant alfa in premature neonates with respiratory distress syndrome: a systematic review of real-world evidence studies and randomized controlled trials. J Perinatol. 2020;40:1121–34. https://doi.org/10.1038/s41372-020-0603-7.
Dargaville PA, Kamlin COF, Orsini F, Wang X, De Paoli AG, Kanmaz Kutman HG, et al. Effect of minimally invasive surfactant therapy vs sham treatment on death or bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome: The OPTIMIST-a randomized clinical trial. JAMA. 2021;326:2478–87. https://doi.org/10.1001/jama.2021.21892.
Kribs A, Roll C, Göpel W, Wieg C, Groneck P, Laux R, et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 2015;169:723–30. https://doi.org/10.1001/jamapediatrics.2015.0504.
Silverman WA, Andersen DH. A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics. 1956;17:1–10.
Gupta BK, Saha AK, Mukherjee S, Saha B. Minimally invasive surfactant therapy versus InSurE in preterm neonates of 28 to 34 weeks with respiratory distress syndrome on non-invasive positive pressure ventilation-a randomized controlled trial. Eur J Pediatr. 2020;179:1287–93. https://doi.org/10.1007/s00431-020-03682-9.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. https://doi.org/10.1016/s0022-3476(78)80282-0.
Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, et al. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology. 2021;128:e51–e68. https://doi.org/10.1016/j.ophtha.2021.05.031.
Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17:213–88. https://doi.org/10.1016/0045-9380(87)90031-4.
Sehgal A, McNamara PJ. Does echocardiography facilitate determination of hemodynamic significance attributable to the ductus arteriosus? Eur J Pediatr. 2009;168:907–14. https://doi.org/10.1007/s00431-009-0983-3.
Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. an evidence-based approach. Am J Respir Crit Care Med. 2019;200:751–9. https://doi.org/10.1164/rccm.201812-2348OC.
Akar S, Topcuoglu S, Dincer E, Ozalkaya E, Karatekin G, Gokmen Yildirim T. Comparison of efficacy of beractant and poractant treatment performed with minimal invasive technique. Iran J Neonatol. 2021. https://doi.org/10.22038/ijn.2020.48388.1838.
Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet. 2011;378:1627–34. https://doi.org/10.1016/S0140-6736(11)60986-0.
Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M, et al. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013;163:955–60.e1. https://doi.org/10.1016/j.jpeds.2013.04.053.
Paul S, Rao S, Kohan R, McMichael J, French N, Zhang G, et al. Poractant alfa versus beractant for respiratory distress syndrome in preterm infants: a retrospective cohort study. J Paediatr Child Health. 2013;49:839–44. https://doi.org/10.1111/jpc.12300.
Fujii AM, Patel SM, Allen R, Doros G, Guo CY, Testa S. Poractant alfa and beractant treatment of very premature infants with respiratory distress syndrome. J Perinatol. 2010;30:665–70. https://doi.org/10.1038/jp.2010.20.
Dizdar EA, Sari FN, Aydemir C, Oguz SS, Erdeve O, Uras N, et al. A randomized, controlled trial of poractant alfa versus beractant in the treatment of preterm infants with respiratory distress syndrome. Am J Perinatol. 2012;29:95–100. https://doi.org/10.1055/s-0031-1295648.
Ramanathan R, Rasmussen MR, Gerstmann DR, Finer N, Sekar K, North American Study Group. A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol. 2004;21:109–19. https://doi.org/10.1055/s-2004-823779.
Lu KW, Pérez-Gil J, Taeusch H. Kinematic viscosity of therapeutic pulmonary surfactants with added polymers. Biochim Biophys Acta. 2009;1788:632–7. https://doi.org/10.1016/j.bbamem.2009.01.005.
Vento M, Bohlin K, Herting E, Roehr CC, Dargaville PA. Surfactant administration via thin catheter: a practical guide. Neonatology. 2019;116:211–26. https://doi.org/10.1159/000502610.
Terek D, Gonulal D, Koroglu OA, Yalaz M, Akisu M, Kultursay N. Effects of two different exogenous surfactant preparations on serial peripheral perfusion index and tissue carbon monoxide measurements in preterm infants with severe respiratory distress syndrome. Pediatr Neonatol. 2015;56:248–55. https://doi.org/10.1016/j.pedneo.2014.11.004.
Brown S, Hurren J, Sartori H. Poractant alfa versus beractant for neonatal respiratory distress syndrome: a retrospective cost analysis. J Pediatr Pharmacol Ther. 2018;23:367–71. https://doi.org/10.5863/1551-6776-23.5.367.
Sekar K, Fuentes D, Krukas-Hampel MR, Ernst FR. Health economics and outcomes of surfactant treatments for respiratory distress syndrome among preterm infants in US Level III/IV neonatal intensive care units. J Pediatr Pharmacol Ther. 2019;24:117–27. https://doi.org/10.5863/1551-6776-24.2.117.
Acknowledgements
We would like to thank Dr Rajib Losan Bora for generation of random sequence and assigning participants to intervention.
Author information
Authors and Affiliations
Contributions
AZ conceived and designed the study, formulated the protocol, managed patient care, and prepared the initial draft. HSk contributed to protocol development, coordinated and supervised data collection, and reviewed and revised the manuscript at all stages. BS assisted in protocol development, coordinated data collection, and provided oversight, while also contributing to the review and revision of the manuscript. AH played a key role in protocol development, critically reviewed the manuscript to enhance its content, and performed the statistical analysis of the data. All authors have given their approval for the final manuscript as submitted and have committed to being accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Informed consent was obtained from the legal guardians of all included participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zamal, A., Sk, M.H., Saha, B. et al. Comparison of efficacy between beractant and poractant alfa in respiratory distress syndrome among preterm infants (28–33+6 weeks gestational age) using the less invasive surfactant administration (LISA) technique: A randomized controlled trial. J Perinatol (2024). https://doi.org/10.1038/s41372-024-01962-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-024-01962-y