Abstract
Objective
To compare short term respiratory outcomes in preterm infants treated with bovine lipid extract surfactant or poractant alfa.
Study design
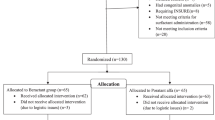
Prospective comparative effectiveness cohort study of infants <32 weeks’ gestational age requiring surfactant in thirteen centers. Each center provided bovine lipid extract surfactant for a set period of time in the year 2019 and then changed to poractant alfa for the remainder of the year. The primary outcome was total duration of respiratory support.
Result
968 infants were included. 494 received bovine lipid extract surfactant and 474 received poractant alfa. No difference was observed in the total duration of respiratory support (mechanical ventilation or non-invasive) (median 38 vs 40.5 days), need to re-dose surfactant, bronchopulmonary dysplasia, survival to discharge, or length of admission.
Conclusion
In this pragmatic study, we did not identify any difference in short term outcomes between the groups based on the type of surfactant received.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The data sets generated and analyzed during the current study are with the corresponding author but current data transfer approval agreements do not allow for data to be available to others.
References
Ardell S, Pfister RH, Soll R. Animal derived surfactant extract versus protein free synthetic surfactant for the prevention and treatment of respiratory distress syndrome. Cochrane Database Syst Rev. 2015;8:CD000144. https://doi.org/10.1002/14651858.CD000144.pub3.
Lam BCC, Yiu KN, Kar YW. Randomized trial comparing two natural surfactants (Survanta vs. bLES) for treatment of neonatal respiratory distress syndrome. Pediatr Pulmonol. 2005;39:64–9.
Sandri F, Plavka R, Ancora G, Simeoni U, Stranak Z, Martinelli S, et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics. 2010;125:e1402–9.
Gharehbaghi MM, Sakha SHP, Ghojazadeh M, Firoozi F. Complications among premature neonates treated with beractant and poractant alfa. Indian J Pediatr. 2010;77:751–4.
Baroutis G, Kaleyias J, Liarou T, Papathoma E, Hatzistamatiou Z, Costalos C. Comparison of three treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Eur J Pediatr. 2003;162:476–80.
Singh N, Hawley KL, Viswanathan K. Efficacy of porcine versus bovine surfactants for preterm newborns with respiratory distress syndrome: systematic review and meta-analysis. Pediatrics. 2011;128:e1588–95.
Lemyre B, Fusch C, Schmölzer GM, Bouali NR, Reddy D, Barrowman N, et al. Poractant alfa versus bovine lipid extract surfactant for infants 24+0 to 31+6 weeks gestational age: A randomized controlled trial. PLoS One. 2017;12:e0175922.
Sarokolai ZK, Niknafs P, Azizzadeh F, Bijari BB, Mousavi H. BLES versus curosurf for treatment of respiratory distress in preterm neonates and their adverse effects. Iran J Pediatr. 2018;28:e11734.
Coshal H, Mukerji A, Lemyre B, Ng EH, Alvaro R, Ethier G, et al. Characteristics and outcomes of preterm neonates according to number of doses of surfactant received. J Perinatol. 2021;41:39–46.
Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: Prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;84:527–32.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34.
Shah PS, Seidlitz W, Chan P, Yeh S, Musrap N, Lee SK. Internal audit of the canadian neonatal network data collection system. Am J Perinatol. 2017;34:1241–9.
Rigo V, Lefebvre C, Broux I. Surfactant instillation in spontaneously breathing preterm infants: a systematic review and meta-analysis. Eur J Pediatr. 2016;175:1933–42.
Lau CSM, Chamberlain RS, Sun S. Less invasive surfactant administration reduces the need for mechanical ventilation in preterm infants. Glob Pediatr Heal. 2017;4:2333794X17696683.
Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: A systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017;102:F17–23.
Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasiveventilation strategies withmortality and bronchopulmonarydysplasiaamong preterm infants: A systematic review and meta-analysis. In: JAMA—Journal of the American Medical Association. 2016;316:611–24.
Dargaville PA, Aiyappan A, Cornelius A, Williams C, De Paoli AG. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed. 2011;96:F243-8.
Paul S, Rao S, Kohan R, McMichael J, French N, Zhang G, et al. Poractant alfa versus beractant for respiratory distress syndrome in preterm infants: A retrospective cohort study. J Paediatr Child Health. 2013;49:839–44.
Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013;164:955–60.
Sekar K, Fuentes D, Krukas-Hampel MR, Ernst F. Health economics and outcomes of surfactant treatments for respiratory distress syndrome among preterm infants in US level III/IV neonatal intensive care units. J Pediatr Pharm Ther. 2019;24:117–27.
Sardesai S, Biniwale M, Wertheimer F, Garingo A, Ramanathan R. Evolution of surfactant therapy for respiratory distress syndrome: past, present, and future. Pediatr Res. 2017;81:240–8.
Acknowledgements
Authors would like to acknowledge the contribution of Dr. Petros Pechlivanoglou for his statistical input.
Funding
Although no specific funding was received for this study, organizational support for the Canadian Neonatal Network was provided by the Maternal-infant Care Research Centre (MiCare) at Mount Sinai Hospital in Toronto, Ontario, Canada. MiCare is supported by a Canadian Institutes of Health Research (CIHR) Team Grant (CTP 87518), the Ontario Ministry of Health and Long-Term Care, and the participating hospitals. PSS holds a CIHR Applied Research Chair in Reproductive and Child Health Services and Policy Research (APR-126340). Additionally, Metapharm Specialty Pharmaceuticals provided poractant alfa at the same cost per mL as bovine lipid extract surfactant to participating sites, for the duration of the study, but had no involvement in design, conduct of analyses, interpretation and reporting of the results. The funding bodies played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
BL, TL, MO, and PSS conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. JB, SD, MD, DL, LM, AM, GMS, BS, and JW participated in study design and data interpretation, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lemyre, B., Lacaze-Masmonteil, T., Shah, P.S. et al. Poractant alfa versus bovine lipid extract surfactant: prospective comparative effectiveness study. J Perinatol 42, 468–475 (2022). https://doi.org/10.1038/s41372-022-01346-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01346-0