Abstract
To compare the therapeutic effect of less invasive surfactant administration (LISA) followed by synchronized nasal intermittent positive pressure ventilation (SNIPPV) and traditional intubate-Surfactant-Extubate (InSurE) strategy for the treatment of neonatal respiratory distress syndrome (NRDS). A single-center, non-randomized and single- blinded study Tertiary neonatal intensive care unit 89 infants enrolled were preterm with gestational age < 366/7 weeks and clinically diagnosed with neonatal RDS (NRDS) Interventions: 32 infants were assigned to the LISA + SNIPPV group and 57 infants to the InSurE + nCPAP group. No statistically significant differences were noted in the baseline characteristics of the enrolled infants. A lower proportion of infants developed BPD in the LISA + SNIPPV group compared to the InSurE + CPAP group [10 (31.25%) vs. 21 (36.84%), P > 0.05]; however, there was no statistically significant difference. The number needed to treat (NNT) with LISA + SNIPPV to prevent BPD development is 18. The mortality rate was not significant between our study arms [1 (3.13%) vs 2 (3.51%), P > 0.05]. There were no statistically significant differences in the durations (days) of MV [(12.18 ± 13.89) vs. (11.35 ± 11.61), P > 0.05], oxygen therapy [(35.03 ± 19.13) vs. (39.75 ± 17.91), P > 0.05] and re-intubation rates [(0.19 ± 0.40) vs. (0.21 ± 0.45), P > 0.05] between the two study groups. In terms of complications, the incidence of patent ductus arteriosus (PDA) [24 (75.00%) vs. 27 (47.37%), P < 0.05] was higher and a lower rate of disturbed liver function [1 (3.23%) vs. 19 (33.33%), P < 0.05] were observed in the LISA + SNIPPV group. Acid–base imbalances were reportedly significantly higher in the InSurE group (P < 0.05). No significant differences in other complications were noted. In the interventional group, FiO2 requirements were significantly lower up until the 3rd week of treatment [FiO2 at day 0, (30.75 ± 4.78) vs. (34.66 ± 9.83), P < 0.05; FiO2 at day 21, (25.32 ± 3.74) vs. (29.11 ± 8.17), P < 0.05], as was RSS on days 2 [(0.77 ± 0.38) vs. (1.94 ± 0.75), P < 0.05] and 3 [(0.66 ± 0.33) vs. (1.89 ± 0.82), P < 0.05] after treatment. Additionally, infants in the standard group had a significantly prolonged hospital stay (days) [(45.97 ± 16.93) vs. (54.40 ± 16.26), P < 0.05]. The combination of LISA and SNIPPV for NRDS can potentially lower the rate of BPD, FiO2 demand and shorten the length of hospitalization.
Similar content being viewed by others
Introduction
To minimize neonatal lung injury, pulmonary surfactant administration has also evolved towards less invasive strategies over the years. Although the intubate-surfactant-extubate (InSurE) strategy has gained popularity since its initial description two decades ago and is currently the mainstay of treatment for RDS, it is still associated with short-term positive pressure ventilation. With advances in neonatal care and increasing data to support less invasive techniques of surfactant administration, less invasive surfactant administration (LISA) or minimally-invasive surfactant therapy (MIST) techniques are emerging. These revolutionary techniques have alleviated the reliance on MV and instead use non-invasive ventilatory modes to support spontaneously breathing infants during the procedure. Our study combines the use of LISA and synchronized nasal intermittent positive pressure ventilation (SNIPPV) for more advantageous outcomes in our study population.
With the increasing survival rate of extremely preterm infants due to advanced care in neonatal intensive care units (NICUs) and delivery room interventions for neonatal pulmonary conditions, a parallel increase in the incidence of BPD has been noted1. Hence, investigation to acquire the optimal and least invasive therapeutic plan for RDS is ongoing.
In a randomized controlled trial (RCT) aiming to eliminate the detrimental effects of long-term MV, Verder et al. were the first to analyze the InSurE (intubation-surfactant-extubation) method followed by nasal continuous positive airway pressure (nCPAP) in RDS infants2. They found a reduced need for MV and better oxygenation in the InSurE group, compared to the nCPAP only group. Dani et al. also reported a significant reduction in the duration of oxygen therapy, MV, hospital stay and decreased requirement for repeated surfactant doses in their surfactant-nCPAP compared to the surfactant-MV group3. The advantageous effects of InSurE were once again described in a retrospective, bi-center study in Stockholm, where a 50% reduction in the need for MV was observed after InSurE implementation at one of the centers4. Further reports also found InSurE to be associated with reduced need for subsequent MV, lower incidence of air leak syndromes (pulmonary interstitial emphysema and pneumothorax) and BPD5,6. Nonetheless, the adverse effects of premedication associated with the InSurE procedure cannot be neglected7. InSurE failure has been well-documented and although remifentanil and propofol are promising drugs for premedication, there is currently no consensus to guide their use during the InSurE procedure in neonates7. A further hindrance associated with InSurE is the acquisition of prompt intubation skills, which are of paramount importance. With the widespread use of nCPAP in recent years, it is nowadays harder for the junior pediatric personnel to gain adequate experience with newborn intubation. A study by O’Donnell et al. observed successful intubation only about 60% of the time8.
Hence, in 2007, Kribs et al. first reported the feasibility of a novel, less invasive surfactant administration (LISA) method, whereby direct laryngoscopy and Magill forceps are used to pass a thin catheter (feeding tube) into the trachea for surfactant administration in spontaneously breathing infants maintained on CPAP9. Similarly, in 2011, a different adaptation of LISA was performed by Dargaville et al. in which they used a 16 gauge vascular catheter for tracheal catheterization and the procedure was termed minimally-invasive surfactant therapy (MIST). The latter was also found to be an effective approach for surfactant delivery10. The goal of our research was to further optimize the strategy of surfactant administration via tracheal instillation and strive to find the most suitable post-extubation non-invasive ventilation (NIV) strategy so as to avoid InSurE and CPAP failure. Studies have previously demonstrated the superiority of nasal intermittent positive pressure ventilation (NIPPV) over nCPAP in premature infants in terms of lesser need for intubation and prevention of respiratory failure11,12. NIPPV has also been found to significantly reduce post-extubation failure compared to nCPAP12. As the beneficial roles of synchronized ventilation in premature infants have also been well-documented, synchronized NIPPV (SNIPPV) mode was applied to LISA-treated infants to maximize the benefits for our infants. SNIPPV improves thoraco-abdominal synchrony, decreases inspiratory effort and need for intubation and has a favorable effect on surfactant distribution in the lungs12,13,14,15. SNIPPV has also been found to be superior to NIPPV and nCPAP as it lowers the overall incidences of central apnea, desaturations and bradycardia16. Lower incidences of BPD and air leaks have also been reported. Taken together, we aimed to further assess the application of the promising LISA technique in combination with the more advantageous SNIPPV non-invasive respiratory support in the treatment of RDS in premature and low-birth weight infants.
Methods
Study design
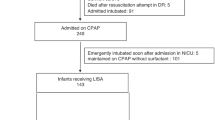
This was a single-center, non-randomized and single-blinded study. Masking of the treatment allocation was not possible but data documentation and analysis were performed in a blinded manner. The study was conducted in the NICU of The First Affiliated Hospital of Nanjing Medical University, Nanjing, China, between August 2018 and December 2019. A total of 89 infants were recruited in our study, 32 infants were assigned to the experimental LISA + SNIPPV group and 57 infants were assigned to the traditional InSurE + nCPAP group (Fig. 1). The decision for treatment allocation was based on a mutual decision involving the neonatologists and the parents. The study was approved by the ethics committee and institutional review board of The First Affiliated Hospital of Nanjing Medical University (2018-SR-161). And we have registered the experiment with ClinicalTrails.gov, the registration number:NCT03989960(Date:18/06/2019). This study was conducted in accordance with the Declaration of Helsinki. The study did not include adults, only premature infants. Written informed consent was obtained from the parents of each infant participating in the study.
Management of premature infants
Delayed cord ligation was not performed in asphyxiated neonates, and the resuscitation steps were performed according to the Chinese resuscitation guidelines (revised in 2016 and 2021)17,18. The radiant warming table was preheated in advance, and the temperature of the radiant warming table was set at 32 ~ 34 °C for term infants, and according to its neutral temperature for preterm infants. All infants were required to have their heads dried and kept warm. Full-term infants were wrapped in preheated towels, dried and placed on the radiant warming table. For resuscitation of preterm infants < 32 weeks of gestational age and/or birth weight < 1500 g, the body and limbs below the head were wrapped in a clean plastic film/bag or covered with plastic wrap and placed on the radiant warming table, and initial resuscitation was continued in the correct position.
Inclusion and exclusion criteria
The inclusion criteria for our study were:
-
(1)
Premature infants with birth weight < 2500 g and gestational age (GA) < 366/7 weeks,
-
(2)
High-risk premature infants with early symptoms of RDS or infants clinically diagnosed as RDS,
-
(3)
Written parental informed consents obtained.
Infants were excluded if they had:
-
(1)
Severe congenital malformations,
-
(2)
Cyanotic congenital heart disease,
-
(3)
Hereditary metabolic diseases,
-
(4)
Emergency intubation requirement after birth,
-
(5)
No parental informed consent.
Surfactant administration
Calf Pulmonary Surfactant (Calsurf) was supplied in ready-to-use, single-use vials containing 70 mg of surfactant phospholipids. Calsurf is licensed through Beijing Double Crane Pharmaceutical CO., Beijing, China. Calsurf is a natural surfactant, prepared from bovine lungs, containing almost exclusively polar lipids, in particular phosphatidylcholine (≥ 55% of the total phospholipids) and 1–2% of surfactant associated apoproteins SP-B and SP-C. Dipalmitoyl-phosphatidylcholine, palmitic acid, and tripalmitin are added to standardize the composition and to mimic the surfactant properties of natural lung surfactant. Pulmonary surfactant dosage used was 70–140 mg/kg.
The LISA + SNIPPV and InSurE + nCPAP technique
All infants were initially the same treatment strategy19,20. Without prior sedation or analgesia, the vocal cords were gently visualized using the laryngoscope and the thin, semi-rigid, narrow-bore tracheal catheter (Double Crane Pharmaceutical CO., Beijing, China) directly inserted under laryngoscopy, was carefully passed 1.5 to 2 cm beyond the cords without using Magill’s forceps. Calsurf was instilled into the tracheal catheter over 10 min by mini-boluses. Tracheal catheterization was only attempted by trained neonatal specialists, neither LISA nor InSurE use sedative drags. Respiratory support following the procedure was SNIPPV mode and breathing synchrony was achieved using an abdominal Graseby capsule placed on the subxiphoid region. Criteria for surfactant doses and management in InSurE + nCPAP group were the same as for the LISA group. Infants with RDS were immediately supported with nCPAP at 6–8 cmH2O and FiO2 was initially set at 0.21–0.4 and adjusted to obtain SpO2 of 90–94% by setting alarm limits. NIPPV group (peak inspiratory pressure 15–25 cm H2O, PEEP 4–6 cmH2O, inspiratory time 0.3–0.5 s, breathing frequency 15–40 breaths/min, FIO2 0.21–0.40). After the child's condition is stabilised, the pressure can be gradually reduced, and CPAP can be considered to be discontinued when the pressure is < 4 ~ 5 cm H2O, FiO2 ≤ 0.25, there is no apnoea and bradycardia, TcSO2 does not fall, and the work of respiration does not increase. Similarly, when the child's condition is stabilised, all the parameters can be gradually reduced, and evacuation can be considered to be completed when the FiO2 is < 0.30, the peak inspiratory pressure (PIP) is < 14 cmH2O, PEEP < 4 cmH2O, respiratory rate < 15 breaths/min, the child has no apnoea, bradycardia, and TcSO2 has not decreased, the withdrawal of NIPPV can be considered21.
Outcomes
Primary outcome
The primary outcome of the study was the rate of BPD22 and FiO2 requirements.
Secondary outcomes
The secondary outcomes analyzed included:
-
(1)
Mortality rate,
-
(2)
Duration of mechanical ventilation,
-
(3)
Duration of oxygen therapy,
-
(4)
Re-intubation rate,
-
(5)
Incidence of complications,
-
(6)
Electrolyte disturbances,
-
(7)
Acid–base disturbances,
-
(8)
Respiratory severity score (RSS)23: defined as FiO2 × (ventilatory support) + (medications), assessing the severity of neonatal lung disease and the risk of subsequent pulmonary morbidity,
-
(9)
Length of hospital stay.
Statistical analysis
Statistical analysis of data was performed using SAS software, version 9.4. Comparison between the two groups was done using the chi-square test or Fisher exact test for categorical variables. Normally distributed continuous variables were compared by student's t-test; abnormally distributed continuous variables were compared by Wilcoxon rank sum test. A P value of < 0.05 was considered statistically significant.
Ethics approval
The study was approved by The First Affiliated Hospital of Nanjing Medical University (number: 2018-SR-161). And We have registered the experiment with ClinicalTrails.gov, the registration number: NCT03989960 (Date:18/06/2019).
Results
Study population
The baseline characteristics of the study population are similar in both groups (Table 1).
Primary outcome
FiO2 requirements were significantly lower in infants who underwent the LISA followed by SNIPPV up to the 3rd week after treatment [FiO2 at day 0, (30.75 ± 4.78) vs. (34.66 ± 9.83), P < 0.05; FiO2 at day 21, (25.32 ± 3.74) vs. (29.11 ± 8.17), P < 0.05] (Fig. 2). The rate of BPD was lower in the experimental group compared to the standard treatment group, without statistically significant [10 (31.25%) vs. 21 (36.84%), P > 0.05] (Table 2) (Fig. 3). The number needed to treat (NNT) with the combination of LISA + SNIPPV to prevent BPD development is 18.
Secondary outcomes
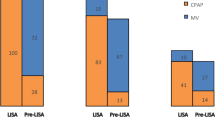
Mortality rates were not statistically different between the two study groups (Table 2). The duration of MV, oxygen therapy and re-intubation rates were statistically similar between the two study arms (Table 2) (Fig. 4). LISA-treated infants has a significantly lower RSS on days 2 [(0.77 ± 0.38) vs. (1.94 ± 0.75), P < 0.05] and 3 [(0.66 ± 0.33) vs. (1.89 ± 0.82), P < 0.05] after birth (Fig. 5). Analyzing complications incidences in the two groups revealed no statistically significant differences in complications. However, the LISA-treated group had a significantly higher rate of patent ductus arteriosus (PDA) and a higher rate of PDA closure (P < 0.05) and a significantly lower proportion of infants with disturbed liver function [1 (3.23%) vs. 19 (33.33%), P < 0.05] (Table 2). No intestinal perforation occurred in our study population. Furthermore, a significantly lower proportion of infants in the LISA group had acid–base disturbances and electrolyte disturbances (Table 2). The length of hospitalization was significantly lower for the LISA-treated infants compared to InSurE-treated ones [(45.97 ± 16.93) vs. (54.40 ± 16.26), P < 0.05] (Table 2) (Fig. 6).
The blue line indicates infants receiving LISA + SNIPPV treatment and the red line indicates those receiving InSurE + nCPAP. The FiO2 requirement was significantly different immediately after treatment for both groups (At Day 0, mean FiO2 (%) for LISA + SNIPPV group was 30.75 ± 4.78 vs. 34.66 ± 9.83 for the standard group). A significantly lower FiO2 demand was sustained up to the 2rd (14 days) and 3rd week (21 days) after treatment (P < 0.05). *Indicates significant differences with a P < 0.05.
Discussion
In this era of high-quality perinatal and neonatal care, moving away from the traditional use of MV to administer surfactant and searching for the least invasive method of surfactant administration is crucial. While having numerous benefits compared to conventional MV, the InSurE technique still requires the use of an endotracheal tube and the damaging effects of short-term MV on the immature lungs have been demonstrated by animal models studies24. Transitioning from the use of an endotracheal tube to a nasogastric feeding tube or vascular catheter has revolutionized the strategy of surfactant administration and the LISA (nasogastric feeding tube) and MIST (vascular catheter) techniques have evolved to further minimize lung injury and prevent BPD9,10. The large Avoidance of Mechanical Ventilation (AMV) trial, investigating infants of 26 to 28 weeks’ GA, deducted that the LISA method lessens the need for MV25. Further investigation of LISA led to the Nonintubated Surfactant Application trial, which was conducted over three years and involved 13 level III NICU in Germany. It studied the application of LISA to preterm infants of 23 to 26 weeks’ GA with RDS, which was associated with reduced rates of intubation and a significantly lower incidence of major complications (pneumothorax and severe intraventricular hemorrhage); hence, emphasizing the sizeable impact on the infants’ quality of life26.
Apart from the method of surfactant administration, the post-extubation NIV support is another important concern to resolve. We combined LISA with SNIPPV as one of the goals of our study was to avoid CPAP failure. Despite the acclaimed positive effects of nCPAP such as reducing the need for and duration of MV and oxygen therapy, the use of nCPAP may not be sufficient in patients with severe surfactant deficiency26,27,28. Failure of CPAP support is an incontestable weakness of the CPAP technique, which occurs in more than 50% of extremely premature infants29. CPAP failure can be predicted in infants supported by CPAP in the initial hours after birth by an FiO2 exceeding 0.30. Some studies have also reported predictive factors of CPAP failure, including gestational age, male gender, birth weight, Apgar score at 1 or 5 min and oxygenation parameters29,30,31.
Evidence from a previous study comparing nCPAP to NIPPV during the MIST procedure found NIPPV to be more favorable for preterm infants with RDS32. Compared to nCPAP, NIPPV reduces the risk of extubation failure and the need for re-intubation, but has no effect on chronic lung disease and mortality33. As described above, the benefits of NIPPV over nCPAP have been well-acknowledged in literature. Synchronization further amplifies the positive effects of NIPPV and as reported by Huang et al. it improves gas exchange and reduces respiratory effort after extubation34. Moreover, SNIPPV compared to CPAP after the INSURE procedure was found to decrease the need for MV15.
With the aim of reinforcing the currently existing evidence surrounding the LISA technique, our study evaluated the combination of the novel LISA technique and SNIPPV compared to InSurE and nCPAP. To improve the odds of initial successful catheterization and reduce the operation duration and incidence of adverse effects (bradycardia and desaturations) during LISA, we chose to use a semi-rigid, narrow-bore tracheal catheter that could be directly inserted under laryngoscopy, without using Magill’s forceps. As shown in Table 1, our two study groups had no differences in their baseline characteristics and all infants were initially management19. The therapeutic plan only differed based on the surfactant administration method and the post-extubation mode of non-invasive respiratory support. In our study center, tracheal catheterization was only attempted by trained neonatal specialists and owing to the simplicity of the LISA technique, the trachea was successfully catheterized at the first attempt for all infants in the LISA group. In relation to airway manipulation with the laryngoscope blade, occasional bradycardic and desaturation events were witnessed, but were self-limited and resolved within a few seconds without resorting to the use of positive pressure ventilation. Surfactant administration via the LISA catheter was followed by an instantaneous physiologic effect, demonstrated by a rapid reduction in FiO2 requirement.
Analysis of the primary outcome of our study revealed a lower rate of BPD development in the experimental group, but it did not differ significantly from the traditional InSurE group. This might be due to our relatively small sample size, which requires expansion in the near future to fully assess the impact of LISA + SNIPPV in terms of BPD development. Secondary outcomes analysis found no statistically significant differences in the duration of MV and oxygen therapy and re-intubation rates for LISA + SNIPPV-treated and for InSurE + nCPAP-treated infants. These results should be interpreted with caution due to our small study sample. Moreover, as illustrated in Fig. 6, the significantly lower FiO2 values for the LISA-treated infants compared to the InSurE-treated ones over the first 3 weeks after treatment shows that lower concentrations of oxygen is required by infants following the LISA method. This finding is favorable for premature infants to reduce the risks of hyperoxia and oxidative stress and may be the reason for the tendency of lower BPD rate in the LISA group10. Moreover, the RSS differed significantly on days 2 and 3 between the two groups; the lower RSS in the experimental group is also related to the lower FiO2 requirements in the LISA group, and it indicates less lung damage and a lower risk of subsequent pulmonary morbidity23. As noted in a previous study, the higher incidence of PDA observed in our LISA-treated infants was possibly due to the left-to-right shunt created by the cascade effect following a decrease in pulmonary vascular resistance following surfactant administration32. However, potential improvement in lung compliance and lowered pulmonary artery pressure with surfactant therapy did not increase the poor prognosis associated with hemodynamically-significant PDA and a higher rate of PDA closure subsequent to oral ibuprofen treatment was witnessed in our experimental group. Although some previous studies have reported a higher incidence of gastrointestinal complications associated with NIPPV, we did not observe similar trends33. NIPPV did not increase the incidence of gastrointestinal complications in the LISA + SNIPPV group and a possible rationale is the role of synchronization, which increases comfort and reduces flatulence35. Similarly, we found normal growth in both groups of preterm infants, neither of which reached the diagnosis of extrauterine growth retardation, which could also be due to our small sample size. Laboratory indexes analysis revealed significantly lower incidences of acid–base disturbances in the LISA-treated group. The latter was also reported by Moretti et al. whereby respiratory acidosis was not a factor for extubation failure in infants treated with SNIPPV36. They concluded that SNIPPV could better stimulate the respiratory drive. The incidence of elevated transaminases was lower in the intervention group and in terms of electrolyte disturbances, there were no statistically significant differences between the two study groups. Consequently, the LISA procedure can be considered safe and well-tolerated. Furthermore, LISA-treated infants also had the added benefit of considerably fewer hospitalization days compared to the standard treatment group. Hence, the experimental group demonstrated improved, short-term clinical outcome, which may significantly improve the prognosis of RDS infants.
Previous larger studies have reported a lower incidence of BPD development in infants treated with LISA37,38,39,40. In 2010, the first multicentre report for LISA was published by Kribs et al. which showed significantly decreasing need for MV in the first 72 h, incidence of BPD (10.9% vs. 17.5%, P = 0.004) and mortality rates in the LISA group compared to the standard care group37. Additionally, higher prevalence of surfactant, theophylline, caffeine and doxapram use and less frequent use of analgesics, catecholamines and steroids were observed in the LISA group. In their randomized study, Kanmaz et al. compared the Take Care (LISA) and InSurE methods in preterm infants < 32 weeks38. They not only found a reduced need for and duration of MV, but also a significantly lower rate of BPD, for infants treated with the less invasive technique of surfactant administration. A study in Poland also found similar results for BPD with the use of LISA39. Additionally, a large cohort study found LISA to be associated with a significant reduction in invasive ventilation (12% versus 18%, P = 0.001), reduced postnatal steroid use (2.5% versus 7%, P < 0.001), BPD (12% versus 18%, P = 0.001) and BPD or death (14% versus 21%, P < 0.001)40. Furthermore, Wu et al. analyzed the results from selected trials using LISA and validated the beneficial aspects of the thin-catheter strategy in terms of decreased requirement of MV and, consequently, a reduction in BPD incidence41.
On the other hand, similar to our findings, the effect of LISA was comparable to standard care in some studies42,43,44. A recently published RCT by Gupta et al. compared the MIST and InSurE techniques for the treatment of RDS in preterm infants of < 34 weeks GA, with NIPPV used as primary respiratory support44. They found no differences between the two study groups in terms of need for invasive MV in the first 72 h of life, hsPDA, IVH, BPD or the composite outcome of BPD and death. However they also reported a longer hospital stay for InSurE-treated infants. The reasons for dissimilar proposed conclusions are multi-factorial. The studied sample size, criteria for patient selection, patient demographics and study designs differ among the studies and have an impact on the final inferences. For widespread establishment of the LISA technique in clinical settings, larger randomized trials are warranted to decipher the optimal combination of the type of catheter and NIV support to be used and to address the issue of premedication.
The surfactant preparation used in our study was Calsurf as it has been carefully evaluated in clinical trials since 1997. It has proven to be an excellent candidate for acute and temporary replacement of pulmonary surfactant in infants with RDS45. Calsurf treatment has been reported to reduce the incidence of neonatal mortality in babies with RDS. Meta-analysis demonstrated that modified bovine minced lung surfactant extract was inferior to the porcine minced lung surfactant extract in terms of the risk of mortality prior to hospital discharge, death or oxygen requirement at 36 weeks’ PMA, repeated surfactant doses, etc46. However, no difference in outcome was noted between bovine lung lavage surfactant extract versus porcine minced lung surfactant extract or porcine lung lavage surfactant. A comparative effectiveness study of three surfactant preparations (calfactant, beractant and poractant) demonstrated similar effectiveness in the prevention of air leak syndromes, death, and BPD or death in premature infants47. Compared with Curosurf, Calsurf reduced in-hospital costs but had similar effects on treatment of RDS in full-term and preterm neonates48.
Limitations
Firstly, it was a single-center study and our sample size could be further expanded to obtain results more representative of the population. Secondly, allocation of participants to treatment interventions was not randomized and although blinding was abided by for data analysis, operators could not be blinded to the surfactant administration strategies. Thirdly, it is important to acquire further data by long-term follow-up of discharged patients to gain better insight of RDS infants.
Conclusions
Our research focused on the innovative LISA and SNIPPV, a superior mode of NIV support compared to CPAP. Our study showed a trend of lower rate of BPD in infants treated with LISA followed by SNIPPV. Moreover, preterm infants treated by the LISA + SNIPPV would undeniably positively benefit from the lower RSS, shorter hospitalization and lower FiO2 demands, better prognosis and an enhanced quality of life with a reduced risk of adverse outcomes related to hyperoxia and oxidative stress which are established high-risk factors of BPD. However, Validation of our results warrants a larger sample-sized and multi-centered clinical study for further research.
Data availability
The data can be available upon request from the corresponding author, Dr. Xiao-qing Chen.
Change history
04 March 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-55787-y
References
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 314, 1039–1051 (2015).
Verder, H. et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. danish-swedish multicenter study group. N. Engl. J. Med. 331, 1051–1055 (1994).
Dani, C. et al. Early extubation and nasal continuous positive airway pressure after surfactant treatment for respiratory distress syndrome among preterm infants <30 weeks’ gestation. Pediatrics 113, e560-563 (2004).
Bohlin, K., Gudmundsdottir, T., Katz-Salamon, M., Jonsson, B. & Blennow, M. Implementation of surfactant treatment during continuous positive airway pressure. J. Perinatol. 27, 422–427 (2007).
Stevens, T. P., Harrington, E. W., Blennow, M. & Soll, R. F. Early surfactant administration with brief ventilation selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD003063.pub2 (2007).
Rojas, M. A. et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: A randomized, controlled trial. Pediatrics 123, 137–142 (2009).
de Kort, E. H. M., Reiss, I. K. M. & Simons, S. H. P. Sedation of newborn infants for the INSURE procedure, are we sure?. Biomed. Res. Int. 2013, 892974 (2013).
O’Donnell, C. P. F., Kamlin, C. O. F., Davis, P. G. & Morley, C. J. Endotracheal intubation attempts during neonatal resuscitation: Success rates, duration, and adverse effects. Pediatrics 117, e16-21 (2006).
Kribs, A., Pillekamp, F., Hünseler, C., Vierzig, A. & Roth, B. Early administration of surfactant in spontaneous breathing with nCPAP: Feasibility and outcome in extremely premature infants (postmenstrual age </=27 weeks). Paediatr. Anaesth. 17, 364–369 (2007).
Dargaville, P. A., Aiyappan, A., Cornelius, A., Williams, C. & De Paoli, A. G. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch. Dis. Child. Fetal Neonatal Ed. 96, F243-248 (2011).
Silveira, C. S. T., Leonardi, K. M., Melo, A. P. C. F., Zaia, J. E. & Brunherotti, M. A. A. Response of preterm infants to 2 noninvasive ventilatory support systems: Nasal cpap and nasal intermittent positive-pressure ventilation. Respir. Care 60, 1772–1776 (2015).
Lemyre, B., Laughon, M., Bose, C. & Davis, P. G. Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants. Cochrane Database Syst. Rev. 12, CD005384 (2016).
Owen, L. S. & Manley, B. J. Nasal intermittent positive pressure ventilation in preterm infants: Equipment, evidence, and synchronization. Semin. Fetal Neonatal Med. 21, 146–153 (2016).
Ramos-Navarro, C., Sanchez-Luna, M., Sanz-López, E., Maderuelo-Rodriguez, E. & Zamora-Flores, E. Effectiveness of synchronized noninvasive ventilation to prevent intubation in preterm infants. AJP Rep. 6, e264-271 (2016).
Gizzi, C. et al. Flow-synchronized nasal intermittent positive pressure ventilation for infants <32 weeks’ gestation with respiratory distress syndrome. Crit. Care Res. Pract. 2012, 301818 (2012).
Gizzi, C. et al. Is synchronised NIPPV more effective than NIPPV and NCPAP in treating apnoea of prematurity (AOP)? A randomised cross-over trial. Arch. Dis. Child. Fetal Neonatal Ed. 100, F17-23 (2015).
Force, C. N. R. P. T. China neonatal resuscitation guideline (revised in 2016, Beijing). Chin. J. Perinat. Med. 19, 481–486 (2016).
Subspecialty Group of Neonatology, the Society of Pediatrics, Chinese Medical Association & Editorial Board, Chinese Journal of Pediatrics. Clinical guideline recommended for neonatal resuscitation in preterm infants with gestational age <32 weeks in China (2022). Zhonghua Er Ke Za Zhi 61, 6–15 (2023).
Sweet, D. G. et al. European consensus guidelines on the management of respiratory distress syndrome - 2016 update. Neonatology 111, 107–125 (2017).
National Guideline Alliance (UK). Evidence Reviews for Respiratory Support: Specialist Neonatal Respiratory Care for Babies Born Preterm: Evidence Review B (National Institute for Health and Care Excellence (NICE), 2019).
Subspecialty Group of Neonatology, The Society of Pediatrics,, Chinese Medical Association. Recommendations for non-invasive ventilation support in preterm infants. Zhonghua Er Ke Za Zhi 56, 643–647 (2018).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Madan, A. et al. A pulmonary score for assessing the severity of neonatal chronic lung disease. Pediatrics 115, e450-457 (2005).
Björklund, L. J. et al. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr. Res. 42, 348–355 (1997).
Göpel, W. et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): An open-label, randomised, controlled trial. Lancet 378, 1627–1634 (2011).
Kribs, A. et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: A randomized clinical trial. JAMA Pediatr. 169, 723–730 (2015).
SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network et al. Early CPAP versus surfactant in extremely preterm infants. N. Engl. J. Med. 362, 1970–1979 (2010).
Morley, C. J. et al. Nasal CPAP or intubation at birth for very preterm infants. N. Engl. J. Med. 358, 700–708 (2008).
De Jaegere, A. P., van der Lee, J. H., Canté, C. & van Kaam, A. H. Early prediction of nasal continuous positive airway pressure failure in preterm infants less than 30 weeks gestation. Acta Paediatr. 101, 374–379 (2012).
Ammari, A. et al. Variables associated with the early failure of nasal CPAP in very low birth weight infants. J. Pediatr. 147, 341–347 (2005).
Fuchs, H., Lindner, W., Leiprecht, A., Mendler, M. R. & Hummler, H. D. Predictors of early nasal CPAP failure and effects of various intubation criteria on the rate of mechanical ventilation in preterm infants of <29 weeks gestational age. Arch. Dis. Child. Fetal Neonatal Ed. 96, F343-347 (2011).
Oncel, M. Y. et al. Nasal continuous positive airway pressure versus nasal intermittent positive-pressure ventilation within the minimally invasive surfactant therapy approach in preterm infants: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 101, F323-328 (2016).
Lemyre, B., Davis, P. G., De Paoli, A. G. & Kirpalani, H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst. Rev https://doi.org/10.1002/14651858.CD003212.pub2 (2014).
Huang, L. et al. Effects of synchronization during noninvasive intermittent mandatory ventilation in preterm infants with respiratory distress syndrome immediately after extubation. Neonatology 108, 108–114 (2015).
De Paoli, A. G., Davis, P. G. & Lemyre, B. Nasal continuous positive airway pressure versus nasal intermittent positive pressure ventilation for preterm neonates: A systematic review and meta-analysis. Acta Paediatr. 92, 70–75 (2003).
Moretti, C. et al. Synchronized nasal intermittent positive pressure ventilation of the newborn: Technical issues and clinical results. Neonatology 109, 359–365 (2016).
Kribs, A. et al. Surfactant without intubation in preterm infants with respiratory distress: First multi-center data. Klin. Padiatr. 222, 13–17 (2010).
Kanmaz, H. G., Erdeve, O., Canpolat, F. E., Mutlu, B. & Dilmen, U. Surfactant administration via thin catheter during spontaneous breathing: Randomized controlled trial. Pediatrics 131, e502-509 (2013).
Krajewski, P. et al. Surfactant administration without intubation in preterm infants with respiratory distress syndrome–our experiences. J. Matern. Fetal Neonatal Med. 28, 1161–1164 (2015).
Göpel, W. et al. Less invasive surfactant administration is associated with improved pulmonary outcomes in spontaneously breathing preterm infants. Acta Paediatr. 104, 241–246 (2015).
Wu, W., Shi, Y., Li, F., Wen, Z. & Liu, H. Surfactant administration via a thin endotracheal catheter during spontaneous breathing in preterm infants. Pediatr. Pulmonol. 52, 844–854 (2017).
Klebermass-Schrehof, K. et al. Less invasive surfactant administration in extremely preterm infants: Impact on mortality and morbidity. Neonatology 103, 252–258 (2013).
Bao, Y., Zhang, G., Wu, M., Ma, L. & Zhu, J. A pilot study of less invasive surfactant administration in very preterm infants in a chinese tertiary center. BMC Pediatr. 15, 21 (2015).
Gupta, B. K., Saha, A. K., Mukherjee, S. & Saha, B. Minimally invasive surfactant therapy versus inSurE in preterm neonates of 28 to 34 weeks with respiratory distress syndrome on non-invasive positive pressure ventilation-a randomized controlled trial. Eur. J. Pediatr. 179, 1287–1293 (2020).
Dong, S. H. et al. A multi-center randomized controlled clinical trial of surfactant (Calsurf) therapy for neonatal respiratory distress syndrome. Chin. Pediatr. Emerg. Med. 16, 120–124 (2009).
Singh, N. et al. Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst. Rev. 2015, CD010249 (2015).
Trembath, A. et al. Comparative effectiveness of surfactant preparations in premature infants. J. Pediatr. 163, 955-960.e1 (2013).
Shi, Y. et al. Department domestic pulmonary surfactant-calsurf on the treatment of respiratory distress syndrome in full-term neonates. Mod. Med. Chin. 13, 9–12 (2011).
Funding
This work was supported by National Natural Science Foundation of China, 81871195. Meanwhile, this study was supported by Medical key project of Jiangsu Provincial Health Commission, K2023007.
Author information
Authors and Affiliations
Contributions
D.L.P. contributes to the draft of manuscript, Y.H.Z. and Y.F.G. revised the manuscript, H.Y.L. collected the data, X.Q.C. designed the study, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Permall, D.L., Zhang, Y., Li, H. et al. A clinical study evaluating the combination of LISA and SNIPPV for the treatment of respiratory distress syndrome in preterm infants. Sci Rep 14, 1429 (2024). https://doi.org/10.1038/s41598-023-50303-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50303-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.