Abstract
Objective
To compare HR pattern of vigorous newborns during the first 180 s with early (≤60 s, ECC) or delayed (>60 s, DCC) cord clamping.
Study design
Observational study including dry-electrode ECG monitoring of 610 vaginally-born singleton term and late-preterm (≥34 weeks) who were vigorous after birth.
Results
198 received ECC while 412 received DCC with median cord clamping at 37 s and 94 s. Median HR remained stable from 30 to 180 s with DCC (172 and 170 bpm respectively) but increased with ECC (169 and 184 bpm). The proportion with bradycardia was higher among ECC than DCC at 30 s and fell faster in the DCC through 60 s. After adjusting for factors affecting timing of cord clamping, ECC had significant risk of bradycardia compared to DCC (aRR 1.51; 95% CI; 1.01–2.26).
Conclusion
Early heart instability and higher risk of bradycardia with ECC as compared to DCC supports the recommended clinical practice of DCC.
Similar content being viewed by others
Introduction
During the first minutes after birth, two key physiological events—aeration of the newborn lungs and umbilical cord clamping—impact the successful transition of neonates from the intra-uterine to extra-uterine environment [1,2,3]. Crying at birth generates large trans-pulmonary pressure differences and increases blood oxygen content through pulmonary aeration. As a result, pulmonary vascular resistance drops, resulting in increased pulmonary blood flow and transition of flow across the ductus arteriosus from the fetal pattern of right-to-left to dominant left-to-right [4]. Umbilical cord clamping at birth plays a critical role in this transition with respect to hemodynamics, as umbilical venous return supplies preload for the left ventricle through the patent foramen ovale during fetal life. Clamping the umbilical cord limits cardiac venous return in the absence of adequate ventilation and pulmonary blood flow [5]. As a result, cardiac output falls precipitously and may remain compromised, resulting in bradycardia. Heart rate (HR) is used as a critical indicator of the need for resuscitation [6]. As per the International Liaison Committee on Resuscitation (ILCOR) guidelines and Neonatal Resuscitation Program a HR below 100 beats per minute (bpm) in an infant who is not breathing well or crying despite stimulation indicates the need for positive-pressure ventilation (PPV) [7].
Generally, a crying infant is assumed to have a heart rate >100, and reflex bradycardia is uncommon with cord clamping in vigorous, crying infants [8]. However, studies which have evaluated normative HRs have provided inconsistent findings regarding rates of bradycardia in these well infants.
Dawson et al. reported HR centile curves in the first 10 minutes after birth among 466 neonates who did not require intervention [9]. Early cord clamping (<1 min) was the standard of care at the time this study was performed. A pulse oximeter placed on infants’ right wrist collected and stored HR data every 2 seconds. At 1 minute, 50% of vigorous neonates had a HR less than 100 bpm and 17% of neonates had a HR less than 60 bpm, raising questions about using HR < 100 bpm as a threshold requiring intervention with PPV. Several studies have shown that pulse oximetry reports lower HRs than electrocardiography (ECG) and exhibits a time lag to detection [10,11,12,13]. In the delivery room, ECG is more effective than pulse oximetry in providing timely and accurate information on HR and is recommended when resuscitation is needed [11].
Bjorland et al. demonstrated that using a wireless dry-electrode ECG monitor with display (NeoBeat, Laerdal Global Health, Stavanger, Norway) the HR centile graphs were re-evaluated in 898 term vigorous, vaginally born neonates with delayed cord clamping (after 1 minute) [14]. A dry-electrode ECG is significantly faster to place and acquires HR more efficiently than pulse oximetry [15]. This study showed that HRs <100 bpm after 1 minute were uncommon in healthy newborns after delayed cord clamping. The differences in HR at 1 minute as reported by Dawson and Bjorland may derive not only from different measurement methods, but also from the timing of cord clamping, reflecting the critical physiologic changes and the potential benefit of ongoing placental transfusion even in a vigorous infants during the first few minutes after birth [9, 14].
We hypothesized that timing of cord clamping (early or delayed) may impact normative HR data in vigorous infants. We hypothesized that early cord clamping would be associated with more bradycardia even in vigorous infants. Both Helping Babies Breathe (HBB) and the Neonatal Resuscitation Program (NRP) recommend that the initial steps of resuscitation can be performed with the umbilical cord intact if the infant is vigorous. As providers in our study were trained in HBB, we defined delayed cord clamping as >60 seconds as recommended with the HBB algorithm. With data from directly observed deliveries including the timing of cord clamping and respiratory status synchronized with continuous HR data obtained by dry electrode, we aimed to assess HR pattern in the first 180 seconds after birth among vigorous infants who received early cord clamping (ECC) as compared to delayed cord clamping (DCC).
Materials/subjects and methods
Design
The study was conducted with a single-center, prospective, observational design.
Setting
The study was conducted at Pokhara Academy of Health Sciences, Pokhara, Nepal from May to December 2020. The hospital had 6500 annual births and is the referral hospital of the province. A nurse-midwife attends all births with on-call support from a general medical doctor, pediatrician and obstetrician when needed.
REFINE, a heart-rate-guided neonatal resuscitation protocol, was implemented by providing training in Helping Babies Breathe (HBB) supplemented by a half-day orientation on the use of NeoBeat at all births [16]. With this curriculum, providers were taught to provide delayed cord clamping if an infant was vigorous, which was defined as maintaining the cord intact for at least 60 seconds in HBB. Within REFINE, periodic meetings were facilitated by the quality improvement team to discuss progress on implementation of heart-rate-guided neonatal resuscitation care using a Plan-Do-Study-Act (PDSA) process [17].
Participants
All women admitted to the labor room with a singleton pregnancy at gestational age 34 weeks or more were eligible for this study. Eligible women who agreed to participate provided written consent for enrollment. Infants born by caesarean section were not included as observation during childbirth in the operation theatre was not feasible. All newborns who were not vigorous at birth or required respiratory support (bag mask ventilation) were excluded from this study. Vigorous newborns were defined as those who cried immediately (within approximately 15 seconds) after birth and required no intervention beyond thorough drying immediately after birth.
Data collection and management
Study research nurses were present in the labor and delivery room to collect data using mobile device with a purpose-built Liveborn application to record real-time observations of newborn care in the first few minutes after birth as previously described [18]. A research nurse attending the delivery noted time of birth, time of cord clamping, time of infant cry, and start and stop of resuscitation interventions such as stimulation, suctioning, and ventilation by annotating the events in the Liveborn application. After delivery the neonate was thoroughly dried by the health worker and a research nurse applied NeoBeat on the upper abdomen. NeoBeat incorporates two dry electrodes into a semi-circular carrier with an integrated digital display that is easily visible to the provider (Supplementary Fig. 1). Continuous HR data was recorded by was recorded by Neobeat, live streamed to the Liveborn application and synchronized with activities recorded in the application. Movement or handling of the baby could temporarily prevent detection or cause interference with HR.
Vigorous newborns were positioned on the mother’s chest after NeoBeat was placed. Drying of newborns was done on mother’s chest. Cord clamping was performed at the health worker’s discretion, although training on HBB recommended cord clamping after 1 minute and the majority of providers had received recent training. Demographics, obstetric history and obstetric complications during labour (Supplementary Table 1) and neonatal characteristics were collected from medical records [19]. The Liveborn application automatically integrated heart rate with any intervention provided after time of birth. A single unique ID was provided for each participant to link the Liveborn app and demographic/medical history data for final data analysis.
Data analysis
For the data analysis, we excluded a participant’s data when there was an indication that the birth time registered in Liveborn application was not correct, i.e. if NeoBeat recording started before “baby born” was pressed in the Liveborn application. Other exclusion criteria included too little (<30 s), too late (>120 s after birth) or poor signal quality data. We categorized the participants into two different groups based on the timing of umbilical cord clamping, defined as early cord clamping (ECC) at 60 seconds or less and delayed cord clamping (DCC) at 61 seconds or more. The obstetric characteristics of ECC and DCC were analyzed including mean maternal age, parity, induction of labor, gestation, and obstetric complications during labor. The neonatal characteristics analyzed included gestational age in weeks, birth weight in grams, gender, Apgar score at 1 and 5 minutes, time of first HR in seconds and immediate drying after birth. Means were compared using independent t-test, medians by Mann-Whitney U test and proportions via Pearson’s Chi-square test.
We analyzed the continuous HR data for the ECC and DCC groups from 10 until 180 seconds after birth using centile curves at 3rd, 10th, 25th, 50th, 75th, 90th and 97th. The centile curves were smoothed using piecewise cubic spline fitting. Median HRs at 12 serial time points (10, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165 and 180 s) were compared using Mann Whitney U test with post hoc Bonferroni correction of p-values to adjust for multiple repeated measures (p = 0.05/12 = p ≤ 0.004 for significance).
Proportion of bradycardic infants with 95% CI in the ECC and DCC groups was graphically presented using generalized additive models (GAM). The relative risk of ever being bradycardic (HR < 100 bpm) after cord clamping was determined by cord clamping group after birth using generalized linear model regression with binomial distribution and log link function. Gestational age in weeks, birthweight in grams, presence of bradycardia at time of cord clamp, and obstetric complications during labor were included as covariates. Results are presented as the crude relative risk (cRR) and adjusted relative risk (aRR), with 95% confidence intervals (CI). Analyses were performed using R version 44.1.2 (Vienna, Austria) and Matlab R2021a (Mathworks Inc, Natick, MA).
Results
Study population
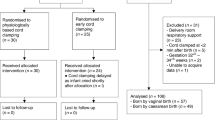
During the study period, NeoBeat was applied on 1172 neonates. Of them, 269 were excluded due to incorrect birth time or duplicate case ID, 23 were excluded due to insufficient HR data captured (<30 s), 60 were excluded due late application of NeoBeat to the upper abdomen (>120 s after birth), 42 were excluded due to poor signal quality, and 38 cases were excluded due to not having a cord clamp time registered in Liveborn. Among the remaining 740 neonates, 610 were vigorous neonates and included in the final analysis. Of the neonates included, 198 had cord clamping at 60 seconds or less, and 412 had cord clamping after 60 seconds (Fig. 1).
Patient characteristics
Comparison of obstetric and neonatal characteristics of the ECC and DCC cohorts identified that the cohorts were largely similar, with the exception that the ECC group had a higher rate of obstructed labor (18.7% vs 10.7%; p = 0.01). Comparable maternal age, parity, birthweight, gestational age, rates of induction, gender distribution and Apgar scores were observed. (Table 1).
The proportion of neonates dried was comparable in the ECC and DCC groups (99.0% vs 98.5%, p = 0.49). The median times (quartiles) to cord clamping were 37 (25, 48) seconds among ECC and 94 (75, 121) seconds among the DCC (p < 0.0001). There was no significant difference in median time (quartiles) to HR in ECC as compared to DCC (17.0 [12.0, 23.0] vs 17.0 [13.0, 23.5], p = 0.68). The proportion of infants with bradycardia any time before cord clamping was comparable between the groups (9.1% vs 9.5%, p = 0.88). However, in 2% of infants in the ECC group, the first HR was obtained after cord clamping, while HR was obtained before clamping for all infants in the DCC group. The median HR [bpm] at the time of cord clamping was similar between the groups (169.0 [145.2, 183.8] vs 168.0 [153.0, 182.0], p = 0.63) (Table 2).
Heart rate centiles
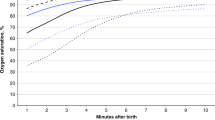
The 3rd, 10th, 25th, 50th, 75th, 90th and 97th centile smoothed HRs in ECC and DCC groups are displayed in Fig. 2A, B (numerical values are listed in Supplementary Tables 2 and 3). The 3rd centile HR in the ECC cohort remained below 100 bpm to 60 seconds and 3rd centile HR in the DCC cohort remained below 100 bpm to 45 seconds. There were no significant differences in median HRs from 10 to 180 seconds after birth between ECC and DCC groups at the prespecified time intervals. However, median HRs [bpm] remained stable from 30 to 180 seconds with DCC [172 (152, 183) at 30 s and 170 (156, 184) at 180 s] whereas gradual increase was noted with ECC [169 (143, 183) at 30 s and 184 (173, 198) at 180 s] (Supplementary Table 4).
Risk of bradycardia following cord clamping
Compared to the DCC group with ECC, the crude risk of being bradycardic did not defer (cRR 0.91; 95% CI; 0.61–1.36; p < 0.64). After adjustment for factors which influenced timing of cord clamping including gestational age, birthweight, presence of bradycardia at time of cord clamp, and obstructed labor, ECC group had a significantly greater risk of bradycardia after cord clamping compared to the DCC group (aRR 1.51; 95% CI; 1.01–2.26; p = 0.047) (Table 3). In sensitivity analysis without obstructed labour in both groups, in the fully adjusted model at the time of cord clamping, RR for ever being bradycardic after cord clamping in the ECC group was 1.57 higher relative to the DCC group (aRR 1.57; 95% CI; 1.05–2.35; p = 0.027) (Supplementary Table 5 and Supplementary Fig. 2). In additional sensitivity analysis without all obstetric complication in both groups, in the fully adjusted model at the time of cord clamping, adjusted Odds ratio for ever being bradycardic after cord clamping in the ECC group was 1.83 higher relative to the DCC group not statistically significant (aOR 1.83; 95% CI; 0.95–3.53; p = 0.07) (Supplementary Table 6 and Supplementary Fig. 3).
The proportion of infants who were bradycardic was similar 10 s after birth in the ECC and DCC groups (8.8% vs 12.3%, p = 0.59). Bradycardia was more common in the ECC group at both 30 and 45 s (8.0% vs 3.1%, p = 0.01; 6.3% vs 2.8%, p = 0.04) then remained similar in groups until 120 seconds. The proportion of babies with bradycardia fell faster in the DCC as compared to ECC group through 60 s (Fig. 3 and Supplementary Table 7).
Discussion
Our data demonstrates comparable median HRs in vigorous infants who receive ECC and DCC, with more stable HR over the first few minutes in infants who received DCC. While these data are from non-randomized observations, they suggest higher rates of bradycardia in the first few minutes in infants who received ECC adjusting for risk factors for use of ECC. ECC may therein place infants at risk for further deterioration with resuscitative interventions such as airway suctioning. Rapid decline in bradycardia among the cohort of vigorous infants who received DCC further suggests that transplacental transfusion may provide added benefit by improving cardiovenous return and support cardiac output even with adequate lung aeration.
Our study provides an update to data provided by Dawson et al. [9] including a HR of less than 100 bpm at 180 seconds among 7% of vigorous neonates who received early cord clamping. Our data suggests that even with ECC this degree of bradycardia is rare beyond the first minute of life and does not replicate the gradual rise in heart rates observed in the Dawson cohort which was obtained with less accurate and less efficient pulse oximeter [10]. Our results align more closely with Bjorland et al. [14], who also used dry electrode ECG and observed less than 3% of their DCC study population having HR less than 100 at 30 seconds and more rapid stabilization of heart rate over the first few minutes.
Delayed cord clamping, defined as that occurring beyond 1 min after birth, is the recommended standard of care by the World Health Organization worldwide with growing recognition of its benefits on cardiovascular transition and newborn outcomes [20,21,22]. Prior attempts to prospectively define HR have yielded different results and were done in spontaneously breathing infants not infants who cried after birth. Our previously published randomized controlled study among the spontaneously breathing infants using pulse oximetry identified lower median HRs with DCC as compared to ECC in healthy late preterm and term neonates [23]. In contrast Padilla-Sanchez et al. used pulse oximetry but documented higher HRs with use of DCC [24]. These studies differed in inclusion of near term versus term infants as well as did not include only crying infants, which may have impacted disparate findings.
NeoBeat has been shown to have accuracy comparable to that of a conventional 3-lead ECG monitor in newborns and can be placed within seconds of birth [25]. Our data utilizing this more robust technology more accurately characterizes early heart rate patterns and identified higher adjusted rates of bradycardia with ECC.
Methodological consideration
There are several limitations to this study. The main limitation is potential selection bias of neonates included in ECC and DCC cohort allocation of into ECC and DCC was not randomized. While our study population was defined as vigorous infants, some neonates who received early cord clamping might have had more subtle clinical indicators leading to an anticipated need for resuscitation. However, the presence of obstetric factors and other demographics which might impact timing of cord clamping were controlled for, we identified an increased risk of bradycardia with ECC. While randomization would be optimal to minimize the selection bias, it provides ethical challenges as DCC is recommended by the WHO for all infants who are vigorous at birth [26, 27]. Despite being trained to delay at least 1-minute, notable variation in timing of cord clamping existed in our dataset with about 1/3 of the infants receiving ECC.
Clinical research staff were used to observe and document the events that occurred following birth, including the time of birth, presence of cry and timing of cord clamping. There is potential for observer bias in classification (vigorous) or miss documentation of events. Data entered manually during a busy resuscitation may have inaccuracies as multiple events need to be documented in a short time period.
Acknowledging these limitations, we would highlight that this pragmatic study provides high-fidelity data on HR pattern immediately following birth in vigorous neonates and impact of timing of cord clamping. While early cord clamping did not impact the median HR in vigorous infants, it resulted in greater HR instability at birth and a higher risk of bradycardia. Further prospective evaluations are indicated in addition to thoughtful review of thresholds for intervention during resuscitation, as our data supports that a HR less than 100 bpm may not represent pathology among vigorous infants. Algorithms for care of the newborn may need to be updated to consider the impact of a more stable transition with the standardization of DCC.
Disclaimer
The external funding sources had no role in study design, data collection, data analysis, data interpretation, writing of the report or in the decision to submit the paper for publication.
Data availability
We have provided as Supplementary File.
References
Tingay DG, Farrell O, Thomson J, Perkins EJ, Pereira-Fantini PM, Waldmann AD, et al. Imaging the Respiratory Transition at Birth: Unraveling the Complexities of the First Breaths of Life. Am J Respir Crit Care Med. 2021;204:82–91.
Hooper SB, Te Pas AB, Kitchen MJ. Respiratory transition in the newborn: a three-phase process. Arch Dis Child Fetal Neonatal Ed. 2016;101:F266–271.
Brouwer E, Te Pas AB, Polglase GR, McGillick EV, Bohringer S, Crossley KJ, et al. Effect of spontaneous breathing on umbilical venous blood flow and placental transfusion during delayed cord clamping in preterm lambs. Arch Dis Child Fetal Neonatal Ed. 2020;105:26–32.
van Vonderen JJ, Roest AA, Walther FJ, Blom NA, van Lith JM, Hooper SB, et al. The influence of crying on the ductus arteriosus shunt and left ventricular output at birth. Neonatology. 2015;107:108–12.
Hooper SB, Polglase GR, te Pas AB. A physiological approach to the timing of umbilical cord clamping at birth. Arch Dis Child Fetal Neonatal Ed. 2015;100:F355–360.
Hooper SB, Roberts C, Dekker J, Te Pas AB. Issues in cardiopulmonary transition at birth. Semin Fetal Neonatal Med. 2019;24:101033.
Aziz K, Lee CHC, Escobedo MB, Hoover AV, Kamath-Rayne BD, Kapadia VS, et al. Part 5: Neonatal Resuscitation 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2021;147:e2020038505E.
Townsend SF, Rudolph CD, Rudolph AM. Changes in ovine hepatic circulation and oxygen consumption at birth. Pediatr Res. 1989;25:300–4.
Dawson JA, Kamlin CO, Wong C, te Pas AB, Vento M, Cole TJ, et al. Changes in heart rate in the first minutes after birth. Arch Dis Child Fetal Neonatal Ed. 2010;95:F177–181.
van Vonderen JJ, Hooper SB, Kroese JK, Roest AA, Narayen IC, van Zwet EW, et al. Pulse oximetry measures a lower heart rate at birth compared with electrocardiography. J Pediatr. 2015;166:49–53.
Gulati R, Zayek M, Eyal F. Presetting ECG electrodes for earlier heart rate detection in the delivery room. Resuscitation. 2018;128:83–87.
Iglesias B, Rodri Guez MAJ, Aleo E, Criado E, Marti Nez-Orgado J, Arruza L. 3-lead electrocardiogram is more reliable than pulse oximetry to detect bradycardia during stabilisation at birth of very preterm infants. Arch Dis Child Fetal Neonatal Ed. 2018;103:F233–F237.
Katheria A, Rich W, Finer N. Electrocardiogram provides a continuous heart rate faster than oximetry during neonatal resuscitation. Pediatrics. 2012;130:e1177–1181.
Bjorland PA, Ersdal HL, Eilevstjonn J, Oymar K, Davis PG, Rettedal SI. Changes in heart rate from 5 s to 5 min after birth in vaginally delivered term newborns with delayed cord clamping. Arch Dis Child Fetal Neonatal Ed. 2021;106:311–5.
Bush JB, Cooley V, Perlman J, Chang C. NeoBeat offers rapid newborn heart rate assessment. Arch Dis Child Fetal Neonatal Ed. 2021;106:550–552.
Gurung R, Gurung A, Basnet O, Eilevstjonn J, Myklebust H, Girnary S, et al. REFINE (Rapid Feedback for quality Improvement in Neonatal rEsuscitation): an observational study of neonatal resuscitation training and practice in a tertiary hospital in Nepal. BMC Pregnancy Childbirth. 2020;20:756.
KC A, Ewald U, Basnet O, Gurung A, Pyakuryal SN, Jha BK, et al. Effect of a scaled-up neonatal resuscitation quality improvement package on intrapartum-related mortality in Nepal: A stepped-wedge cluster randomized controlled trial. PLoS Med. 2019;16:e1002900.
Laerdal Global Health. LIVEBORN Application. Stavanger, Norway; 2018.
Gurung R, Jha AK, Pyakurel S, Gurung A, Litorp H, Wrammert J, et al. Scaling Up Safer Birth Bundle Through Quality Improvement in Nepal (SUSTAIN)-a stepped wedge cluster randomized controlled trial in public hospitals. Implement Sci. 2019;14:65.
Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol. 2013;591:2113–26.
Polglase GR, Dawson JA, Kluckow M, Gill AW, Davis PG, Te Pas AB, et al. Ventilation onset prior to umbilical cord clamping (physiological-based cord clamping) improves systemic and cerebral oxygenation in preterm lambs. PLoS One. 2015;10:e0117504.
Ersdal HL, Linde J, Mduma E, Auestad B, Perlman J. Neonatal outcome following cord clamping after onset of spontaneous respiration. Pediatrics. 2014;134:265–72.
KC A, Singhal N, Gautam J, Rana N, Andersson O. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 min of birth - randomized clinical trial. Matern Health Neonatol Perinatol. 2019;5:7.
Padilla-Sanchez C, Baixauli-Alacreu S, Canada-Martinez AJ, Solaz-Garcia A, Alemany-Anchel MJ, Vento M. Delayed vs Immediate Cord Clamping Changes Oxygen Saturation and Heart Rate Patterns in the First Minutes after Birth. J Pediatr. 2020;227:149–56.e141.
Pike H, Eilevstjonn J, Bjorland P, Linde J, Ersdal H, Rettedal S. Heart rate detection properties of dry-electrode ECG compared to conventional 3-lead gel-electrode ECG in newborns. BMC Res Notes. 2021;14:166.
World Health Organization. Guideline: Delayed umbilical cord clamping for improved maternal and infant health and nutrition outcomes. Geneva: WHO; 2014.
KC A, Bergstrom A, Chaulagain D, Brunell O, Ewald U, Gurung A, et al. Scaling up quality improvement intervention for perinatal care in Nepal (NePeriQIP); study protocol of a cluster randomised trial. BMJ Glob Health. 2017;2:e000497.
Acknowledgements
We would like to thank research nurses, pediatricians and nurses in labor and delivery unit at Pokhara Academy of Health Sciences. We would like to thank Omkar Basnet, Pratiksha Bhattarai and Dr. Dela Singh for the clinical advice. Laerdal Foundation for Acute Medicine, Norway (2019–40499) and Grand Challenges Canada (1910–30925).
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
KCA and SYJK designed the study. KCA and RC implemented, supervised and carried out the study and the data collection on site. KCA, JE and SHH extracted and analyzed the heart rate data. SN and SB reviewed data analysis. KCA and SYJK drafted the initial manuscript. SN and SB revised and edited the manuscript. TW assisted with data and statistical analysis and editing of the manuscript. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
SYJK, SHH, JE and HM are employed at Laerdal Medical. All other authors had no other financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years or no other relationships or activities that could appear to have influenced the submitted work.
Ethics approval and consent to participate
The study was approved by the Pokhara Academy of Health Sciences, Institutional Ethics Committee. Written parental/guardian was consent obtained.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41372_2022_1593_MOESM1_ESM.docx
Supplementary figure and tables. Increased risk of bradycardia in vigorous infants receiving early as compared to delayed cord clamping at birth
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
KC, A., Kong, S.Y.J., Haaland, S.H. et al. Increased risk of bradycardia in vigorous infants receiving early as compared to delayed cord clamping at birth. J Perinatol 43, 709–715 (2023). https://doi.org/10.1038/s41372-022-01593-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01593-1