Abstract
Objective
To systematically assess the efficacy of oral beta blockage treatment in primary (before established) and secondary (in threshold stages) prevention of severe retinopathy of prematurity (ROP) in premature infants born ≤32 weeks gestational age.
Study design
Following the PRISMA guidelines, published literature was systematically assessed up to April 27, 2018. Trials and observational studies, in which beta blockage was used to prevent severe ROP (defined as stage ≥3, or requiring treatment) were included. Meta-analyses including random effects models were conducted to determine the overall effect of oral beta blockage on prevention of ROP.
Results
Six studies (five clinical trials and one observational study) including 461 infants met inclusion criteria using propranolol. The pooled relative risk (RR) of severe ROP in the primary and secondary prophylaxis groups were 0.65 (95% CI 0.43–0.98, NNT = 7) and 0.48 (95% CI 0.35–0.65, NNT = 6) in RCTs, respectively. The RR of severe ROP in one observational study was 0.21 (95% CI 0.08–0.55) with a NNT of 3. There were low heterogeneity and publication bias. Side effects occurred in 8.4% of participants on propranolol.
Conclusions
Systematic assessment of studies showed that prophylactic oral propranolol appeared to be effective in preventing severe ROP in premature infants ≤32 weeks gestational age. Additional well powered, multinational, randomized control trials reporting on long-term outcomes are needed.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) is one of the major complications of extreme prematurity in modern neonatal intensive care units (NICU) with potential life-long implications such as vision and neurodevelopmental impairments, and blindness [1]. The Canadian Neonatal Network in 2016 reported an ROP stage ≥3 incidence of 7% in infants with birth gestation <32 weeks [2]. While the mainstay of addressing ROP remains prevention and screening, treatment modalities have been refined over the last decades [3]. Cryotherapy has been replaced by diode laser photocoagulation, or anti-vascular endothelial growth factor (anti-VEGF) intraocular injections, at earlier stages of the disease, and vitreoretinal surgery with later stages [4]. While 5% of infants <32 weeks required treatment, 57% of those received anti-VEGF in Canadian NICUs in 2016 [2]. These treatments have potential side effects such as corneal edema, hemorrhage, and cataract formation with the more invasive laser, along with concerns for systemic effects with anti-VEGF injections such as bevacizumab (Avastin). Anti-VEGF medications are under investigation. Though they lack safety data, they are used off-label as intraocular injectable for the indication of ROP [5].
During retinal vascular development there are two distinct phases: the earlier relative hyperoxic vasoobliterative phase, and the hypoxic neovascularization phase, usually occurring around 31–32 weeks of gestation [6, 7]. VEGF is the main modulating cytokine secreted by the maturing avascular retina, with production being regulated by oxygen tension: hypoxia inducing its transcription and hyperoxia inhibiting it [8, 9]. An array of other cytokines such as insulin-like growth factor-1 (IGF-1), hypoxia-inducible factor-1, transcription factors, and potential genetic predisposition are implicated in the final downstream effect of development of oxygen induced ROP [6].
Propranolol is a nonselective beta-blocker with wide indications in pediatric populations such as supraventricular tachycardia, tetralogy of fallot, and more recently for infantile haemangioma [10]. Common, usually transient, side effects include hyperkalemia, hypoglycemia, hypotension, and bradycardia. Beta receptors, more specifically receptor types 2 and 3, are present in the developing eye and upregulated by hypoxia [11]. Activation favors angiogenic factor transcription such as VEGF, and leads to uncontrolled angiogenesis, which can be blocked by beta receptor blockage in animal models [12]. Topical propranolol given in mice successfully treated oxygen-induced ROP [13]. However, other animal studies reported neither oral, subcutaneous, or intraperitoneal propranolol successfully prevent development of ROP [14], while others caution against potential systemic antiadrenergic side effects [15]. Beta blockers, due to their anti-VEGF properties, have been employed as primary and secondary preventing agent for ROP in both animal models, and preterm infants, but there was not enough evidence to support or refute its use for ROP in premature infants at the time of a recent Cochrane review [16]. The Cochrane review included only few studies related with the use of propranolol. Therefore, we planned to carry out an updated systematic review including all clinical trials and observational studies for the meta-analysis. The objective of our study was to evaluate whether, in premature infants born ≤32 weeks gestational age, propranolol treatment in primary (before established) and secondary (in threshold stages) prevention for severe ROP is effective compared with standard care or placebo.
Methods
This systematic review and meta-analysis was based on the guidelines of the PRISMA statement. A predefined protocol was used [17].
Literature search
We performed a comprehensive systemic literature search, assisted by an experienced medical librarian (HLR) using the OVID MEDLINE, OVID EMBASE, CINAHL, and Scopus from the date of inception to April 27, 2018. We also searched the ClinicalTrials.gov database for studies that finished recruitment. One additional study was identified via Pediatric Academic Societies 2018 abstract manual search [18]. Two investigators (AS and SK) independently reviewed all citations and abstracts and selected articles for full-text review. Only articles related to humans and published in English language were included. Combinations of subject headings, keywords and synonyms used included: retinopathy of prematurity, ROP, retrolental fibroplasia, retinal detachment, ROP treatment, outcomes, hemangioma, low birth weight, prematurity, premature, and preterm, infant, newborn, neonate, beta blockers (intravenous, oral, or local administration), propranolol, beta blockage, randomized controlled trial (RCT), human.
Study selection
All clinical trials and observational studies, in which beta blockers were used to prevent or treat ROP in preterm infants were eligible for inclusion [19]. The main a priori exclusion criteria were: infants with congenital cardiac defects or malformation and/or moribund conditions at birth, and beta blocker treatment for any other indication than for ROP. Two reviewers (AS, SK) independently reviewed all abstracts and studies and assessed for inclusion (using title and abstracts). Full papers were retrieved and inclusion criteria applied. Data extraction was done separately by AS and KS using the standardized Neonatal Cochrane Group data abstraction forms. All articles and data abstractions forms were checked and reviewed by AL, he also served as third reviewer in case of disagreement. The protocol for this review was not registered.
Outcomes
The primary outcome was the combination of severe ROP and/or need of treatment for ROP. Severe ROP was defined as stage ≥3 and/or those infants requiring treatment (laser or intraocular injections). Secondary outcomes were progression of ROP, severity (all stages), any treatment required for ROP, and adverse effects noted related to beta blocker treatment. ROP was defined and graded into 1–5 stages and zones I–III according to the International Classification of Retinopathy of Prematurity [20].
Study quality assessment
The methodological quality was independently evaluated by two reviewers (AS, SK) according to the revised Cochrane risk-of-bias tool for RCTs [21]. These instruments assesses quality and risk of bias of clinical trials and include respective 10 and 7 items (Tables 1 and 2). Newcastle–Ottawa Quality Assessment Scale criteria [22] were used to assess the quality of one observational study [23].
The reviewers were not masked to the authors, institution, or journals. In case of need for clarification or more information to conduct analysis, authors of various studies were contacted by email or phone for additional information not available in the publications. One study was identified before publication, information was obtained from personal communication with the author, and the data underwent the same assessment as above [18].
Analysis
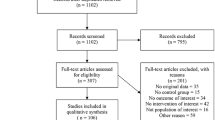
Data were analyzed using a per-eye analysis approach, and combined using Review Manager software (RevMan version 5.3, The Cochrane Collaboration, 2014). Heterogeneity was tested using I2 test and p-value < 0.05 was interpreted as being statistically significant. Outcome data were analyzed and relative risk (RR), absolute risk reduction (ARR) or risk difference, and number needed to treat (NNT) were calculated for dichotomous outcomes. All estimates of treatment effects were reported with 95% confidence intervals (CI). Reporting followed the PRISMA guidelines (Fig. 1) [24]. All forest plots show the number of eyes in the control and in the propranolol groups, rather than number of individual infants.
Results
The PRISMA flow diagram of study selection is shown in Fig. 1. Our initial search without duplicates yielded 302 citations. One more RCT was included based on the recent Pediatric Academics Society Meeting 2018 abstracts. After review of titles and abstracts, 264 citations were excluded. After full text review of the remaining 39 articles, 33 articles were excluded with reason (review article, animal studies) (Fig. 1). The remaining six studies were included in this review. Characteristics of these studies are summarized in Table 3.
Of the six included studies, one study addressed primary prophylaxis [25], and the other five studies addressed secondary prophylaxis [23, 26,27,28]. Of the five studies with secondary prophylaxis, one was observational [23] and the other four were RCTs. One excluded study used 0.1% topical eye drops in 23 newborns with stage 2 ROP at 66.5 ± 25 days of life [29]. This study was discontinued early for lack of efficacy and not included in this meta-analysis as intervention was topical. We present the results of the meta-analysis of the one primary, the four RCT secondary prophylaxis, and the one observational studies.
Characteristics of the included studies
Table 3 summarizes the included study characteristics. In this meta-analysis 461 preterm infants, 225 receiving propranolol, and 236 controls, were included. All studies included premature infants ≤32 weeks’ gestation and used oral propranolol admixed with feeds. No study was found that used a different beta blocker or intravenous administration.
Quality assessment of included studies
On the revised Cochrane risk-of-bias tool for RCTs, the Sanghvi and Bancalari (2018), and Filippi studies had lower risk of bias and were rated high quality, whereas the Korkmaz and Makhoul studies had moderate to higher risk of bias (Tables 1 and 2). Newcastle–Ottawa Quality Assessment Scale criteria were used to assess the quality of one observational study (Table 4) [23]. Based on the assessment of this cohort study with historical control group, the quality of the study was deemed good and had minimal risk of bias. No significant heterogeneity was noted using I2 statistics.
Primary prophylaxis
Meta-analyses of RCTs
The meta-analyses included one RCT including 109 infants of <32 weeks and ≤7 days old who received primary prophylactic oral propranolol or placebo until 37 weeks’ completed or fully vascularized retina [25]. The primary outcome of ROP requiring treatment was significantly reduced by 35.0% (RR: 0.43; 95% CI: 0.43–0.98) in the intervention group (P < 0.04) (Fig. 2). The ARR was 13.8%, from 39.2 to 25.4%, and the NNT was 7 (P = 0.03).
There were decreasing trends favouring propranolol in all ROP-related outcomes, however, none were significant: ROP diagnosis was reduced to 56.8% in the treatment group vs 68.6% in the control group (P = 0.39). There were fewer injections (P = 0.09) and laser treatments (P = 0.37). No side effects were noted, and the visual outcomes at 12 months were comparable (Table 3).
Secondary prophylaxis (treatment) studies
Meta-analysis of RCTs
The meta-analysis of RCTs using propranolol as a secondary agent to prevent severe ROP or treatment included four studies, with a total of 313 eyes in the control group, and 299 eyes in the propranolol group. The RR of severe ROP or treatment in the propranolol group compared with placebo was 0.48 (95% CI; 0.35–0.65) (Fig. 2). In a systematic review and meta-analysis of clinical trials, regarding our primary outcome, progression to severe ROP and/or requirement of treatment, for the secondary prevention studies, the ARR was 16.5%, from 31.9 to 15.4%, and the NNT was 6 (P < 0.00001) (Fig. 2).
In regards to need of any treatment, all four included RCTs reported on this outcome, with a RR of 61.0% (95% CI; 0.27–0.57), ARR of 15.8%, from 26.2 to 10.4%, and NNT of 6. The risk of progression to stage 2 with plus disease was reported by three of the included studies, with a total of 264 eyes (Fig. 3). It shows a risk reduction of 86.0% (RR 0.14; 95% CI: 0.03–0.61), ARR of 9.0%, and NNT of 11. Publication bias was not detected in meta-analysis of clinical trials (Fig. 4).
Meta-analysis of observational study
We present the results of the meta-analysis of one observational study which compared 20 infants, or 40 eyes, with ROP stage 2 or 3, without plus disease, prospectively treated with propranolol, to 27 (equals 54 eyes) historic controls [23]. The RR for severe ROP was reported as 0.21 (95% CI; 0.08–0.55), with a NNT of 3. Meta-analysis of one observational study [23] concludes an ARR of severe ROP by 38.1% with reduction of severe ROP from 48.1 to 10% in the intervention group (P = 0.00001).
Safety
Three of the six included studies did not report any safety concern related to propranolol [23, 25, 27]. Monitoring included mostly vital signs, glucose levels, Filippi [26] measured propranolol and VEGF levels in safe ranges. Bancalari 2018 [18] reported one case of hypoglycemia. Filippi [26] reported nine, and Korkmaz [28] at least nine adverse events which were related to apnea, bradycardia, hypotension, or “instability”. These 19 reported adverse events constituted 8.4% of the included 225 neonates who received propranolol.
Discussion
In our meta-analysis of five clinical trials and one observational study, we found a significant reduction of severe ROP and ROP progression in preterm infants born ≤32 weeks’ gestational age with primary and secondary prophylactic use of propranolol, compared with placebo. All individual outcomes that indicate worrisome features of ROP, such as progression of disease to stage II or beyond, plus disease, and treatment need for either laser or anti-VEGF injections were significantly reduced with minimal adverse effects. There was no evidence of publication bias in our meta-analysis, and heterogeneity was low.
Clinically, the results of this review are important because propranolol therapy may provide a safe and alternative prophylactic option for primary and secondary prevention of ROP in premature infants. Beta-blockage might ameliorate the second stage of ROP, the increased angiogenesis, via downregulation of the beta2-adrenoreceptor stimulation which in turn upregulates VEGF (Fig. 5). Propranolol as nonselective beta-adreno-receptor blocker has been shown to downregulate both VEGF and IGF-1 expression in a mouse model [11]. Side effects in the included studies were few such as transient hypoglycemia which may be managed with slow increase of treatment dosage, similar to established indications such as cardiac disease and infantile hemangiomas. More serious side effects were rare in included infants in this meta-analysis, and seemed to be mainly restricted to difficulties in surmounting an adequate sympathomimetic response to triggers such as sepsis or anesthesia induction [26]. As in other indications for beta-blockage, clinical prudence would dictate extra caution if a neonate on treatment were to develop a deterioration requiring an intact hemodynamic response system. Topical applications of propranolol seems promising as systemic adverse events might be ameliorated, however, efficacy could not be proven so far, potentially due to too low dosage used [29]. The main advantage of using propranolol to treat ROP is that it can be used orally and does not require intraocular injections of anti-VEGF such as bevacizumab (Avastin). Also, it avoids the use of sedation or anesthetic agents causing hemodynamic instability
Our updated systematic review employed a very recent literature review, even including an unpublished RCT [18], and our results provide data based on the largest number of included infants in the beta-blocker group compared with previously published meta-analysis [16].
Beta-blockage as avenue of treatment in ROP therefore shows promising results so far. However, it needs further trials to ascertain efficacy, safety, and focus also on documenting long-term improvement in visual outcomes with propranolol. Furthermore, ROP screening weekly or biweekly in premature infants is cumbersome. The topical drugs used for ROP screenings and the handling are associated with frequent episodes of desaturation and bradycardia [30]. The compounded risk of neurodevelopmental impairments due to these desaturations and bradycardia are unknown. A different and effective option for ROP prophylaxis may not only reduce the burden of the disease but also lessen the required frequency for screening. This intervention can be easily used in the absence of ophthalmologist services in remote level 2 NICUs when infants are transferred from level 3 NICUs.
Strengths and limitations
This is the first updated meta-analysis that tries to determine whether beta-blockers might be useful in the prevention or treatment of ROP in at-risk premature infants. The strengths of this meta-analysis, compared with the recently published Cochrane review, include a larger sample size, the inclusion of all RCTs (published and unpublished), and observational studies that give an entire view of this intervention and all clinically important outcomes. We found only one RCT that reported on primary prevention of ROP with lack of power. Therefore, evidence is still lacking whether primary use of propranolol will be beneficial. Because of the low prevalence of ROP in NICUs, we suggest that a multicenter RCT with standardization of treatment for ROP, oxygen saturation limits, and pulse oximetry alarm limits yield adequate power and generalizability of findings. We suggest careful attention to developmental outcomes.
The included studies were reported from various countries [18, 23, 25,26,27,28]. Clinical practices at each of the centers of the included studies might be different, especially oxygen saturation alarm and set limits, thereby questioning the external validity. It is well known that ROP rates vary substantially between countries [31]. Therefore, our results might not be applicable to centers with different baseline risks for ROP and different treatment strategies. We believe that there was heterogeneity in the quality of the studies in terms of risk of bias.
We also are uncertain about the dose response effect of propranolol in preterm infants with the different gestational ages. As well, long-term side effects on neurodevelopmental outcomes with long-term beta blockage at this early age in these fragile infants are lacking.
Conclusion
Prophylactic oral propranolol appeared to be effective in preventing progression to severe ROP and need for rescue treatment with laser or anti-VEGF injections in premature infants ≤32 weeks, without significant short-term side effects. These results warrant a large, multicenter RCT to investigate the treatment efficacy of propranolol in ROP, as well as short term and long term outcomes and safety of its use.
Change history
29 October 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–57.
Canadian Neonatal N. Annual Report. 2016. http://www.canadianneonatalnetworkorg/Portal/LinkClickaspx?fileticket=PJSDwNECsMI%3d&tabid=39. Accessed 15 Jan 2019.
Mutlu F, Sarici SU. Treatment of retinopathy of prematurity: a review of conventional and promising new therapeutic options. Int J Ophthalmol. 2013;6:228–36.
Early Treatment for ROP Cooperative, Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Opthalmol. 2003;121:1684–94.
Mintz-Hittner H, Kennedy KA, Chuang AZ. BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Eng J Med. 2011;364:603–15.
Selvam S, Kumar T, Fruttiger M. Retinal vasculature development in health and disease. Prog Retin Eye Res. 2018;63:1–19.
Smith L. Pathogenesis of retinopathy of prematurity. Growth Horm IGF Res. 2004;14(Suppl A):S140–4.
Pierce E, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Opthalmol. 1996;114:1219–28.
Young T, Anthony DC, Pierce E, Foley E, Smith LE. Histopathology and vascular endothelial growth factor in untreated and diode laser-treated retinopathy of prematurity. J Aapos. 1997;1:105–10.
Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Eng J Med. 2015;372:735–46.
Ristori C, Filippi L, Dal Monte M, Martini D, Cammalleri M, Fortunato P, et al. Role of the adrenergic system in a mouse model of oxygen-induced retinopathy: antiangiogenic effects of beta-adrenoreceptor blockade. Invest Ophthalmol Vis Sci. 2011;52:155–70.
Casini G, Dal Monte M, Fornaciari I, Filippi L, Bagnoli P. The β-adrenergic system as a possible new target for pharmacologic treatment of neovascular retinal diseases. Prog Retin Eye Res. 2014;42:103–29.
Dal Monte M, Casini G, la Marca G, Isacchi B, Filippi L, Bagnoli P. Eye drop propranolol administration promotes the recovery of oxygen-induced retinopathy in mice. Exp Eye Res. 2013;111:27–35.
Chen J, Joyal JS, Hatton CJ, Juan AM, Pei DT, Hurst CG, et al. Propranolol inhibition of β-adrenergic receptor does not suppress pathologic neovascularizaion in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2012;53;2968–77.
Hård A, Hellström A. On the use of antiangiogenetic medications for retinopathy of prematurity. Acta Paediatr. 2011;100:1063–5.
Kaempfen S, Neumann RP, Jost K, Schulzke SM. Beta-blockers for prevention and treatment of retinopathy of prematurity in preterm infants. Cochrane Cochrane Database Syst Rev. 2018;3:CD011893.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34.
Bancalari A, Schade R, Lazcano C, Munoz T, Sepulveda G. Treatment of retinopathy of prematurity with propranolol: a randomized control trial. PAS-oral presentation. 2018.
Burns P, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:305–10.
International Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. ROB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:14898;1–8
Wells GA, Shea B, O'Connell D, Peterson J, Welche V, Losos M, et al. The Newcastle-Ottawa (NOC) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 15 Jan 2019.
Bancalari A, Schade R, Muñoz T, Lazcano C, Parada R, Pena R. Oral propranolol in early stages of retinopathy of prematurity. J Perinat Med. 2016;44:499–503.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41.
Sanghvi K, Kabra NS, Padhi P, Singh U, Dash SK, Avasthi BS. Prophylactic propranolol for prevention of ROP and visual outcome at 1 year (PreROP trial). Arch Dis Child Fetal Neonatal Ed. 2017;102:F389–94.
Filippi L, Cavallaro G, Bagnoli P, Dal Monte M, Fiorini P, Donzelli G, et al. Oral propranolol for retinopathy of prematurity: risks, safety concerns, and perspectives. J Pediatr. 2013;163:1570–7.
Makhoul I, Peleg O, Miller B, Bar-Oz B, Kochavi O, Mechoulam H, et al. Oral propranolol versus placebo for retinopathy of prematurity: a pilot, randomised, double-blind prospective study. Arch Dis Child. 2013;98:565–7.
Korkmaz L, Baştuğ O, Ozdemir A, Korkut S, Karaca C, Akin MA, et al. The efficacy of propranolol in retinopathy of prematurity and its correlation with the platelet mass index. Curr Eye Res. 2017;42:88–97.
Filippi L, Cavallaro G, Bagnoli P, Dal Monte M, Fiorini P, Berti E, et al. Propranolol 0.1% eye micro-drops in newborns with retinopathy of prematurity: a pilot clinical trial. Pediatr Res. 2017;81:307–14.
Bremond-Gignac D, Jacqz-Aigrain E, Abdoul H, Daruich A, Beresniak A, Baud O, et al. on behalf of the CLAIR FO study group. Ophthalmic insert versus eye drops for Mydriasis in neonates: a randomized clinical trial. Neonatology. 2018;115:142–8.
Darlow B, Lui K, Kusuda S, Reichman B, Håkansson S, Bassler D, et al. International Network for Evaluating Outcomes of Neonates. International variations and trends in the treatment for retinopathy of prematurity. Br J Ophthalmol. 2017;101:1399–404.
Acknowledgements
We would like to thank Dr Aldo Bancalari, Dr Filippi, Dr Sanghvi, and Dr Makhoul for providing extra data through email and phone conversations on their respective studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These finding were presented in an oral presentation at the Pediatric Academic Society Meeting 2018 in Toronto, and as poster presentation at the District VIII American Academy of Pediatrics Meeting 2018 in Utah.
Rights and permissions
About this article
Cite this article
Stritzke, A., Kabra, N., Kaur, S. et al. Oral propranolol in prevention of severe retinopathy of prematurity: a systematic review and meta-analysis. J Perinatol 39, 1584–1594 (2019). https://doi.org/10.1038/s41372-019-0503-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0503-x
This article is cited by
-
Comparative study on optic disc features of premature infants and full‐term newborns
BMC Ophthalmology (2021)