Abstract
Objective
To compare the PF-PCO2 equation—partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2) minus partial pressure of carbon dioxide (PCO2)—to three other tools for postnatal prediction of survival in infants with congenital diaphragmatic hernia (CDH).
Study design
A retrospective analysis of 203 infants with CDH from 1 January 2003 to 30 June 2018. Area under the curve (AUC) analysis was performed for survival and secondary outcomes of survival without extracorporeal membrane oxygenation support (ECMO) and death despite ECMO. Predictive scores were calculated to determine cutoff for PF-PCO2 score.
Results
The PF-PCO2 tool had the highest AUC (0.84 for survival, 0.92 for survival without ECMO, and 0.83 for death despite ECMO). PF-PCO2 best predicted survival when >−60 and survival without ECMO when >+80. There was no optimal cutoff score for death despite ECMO.
Conclusion
The PF-PCO2 tool best predicted postnatal survival in infants with CDH.
Similar content being viewed by others
Introduction
Congenital diaphragmatic hernia (CDH) occurs in ~1 in 3000 live births and has a mortality rate between 20–40% [1,2,3,4]. Prenatal predictors of survival, based on ultrasound and magnetic resonance imaging findings [5, 6], can aid in prenatal counselling of families regarding survival and prognosis. Ultimately, the severity of pulmonary hypoplasia and ability to oxygenate and ventilate determines survival in these patients [2, 7,8,9,10].
Several postnatal models for predicting survival of infants with CDH have been developed and tested in both local populations and the Congenital Diaphragmatic Hernia Study Group (CDHSG) registry, which includes infants with CDH from North America, Europe, and Australia. These include tools created specifically for infants with CDH, such as the CDHSG Probability of Survival Equation (CDHSG-PS) [1], Wilford Hall Santa Rosa Prediction Formula (WHSRpf) [8], and Brindle tool [11], as well as predictors of morbidity and mortality in general neonatal and pediatric populations [12, 13]. No postnatal prediction tool is applied consistently across institutions caring for infants with CDH. Furthermore, problems with existing tools include poor generalizability and difficulty in calculating a score at the patient’s bedside.
Of the tools developed specifically for the CDH population, the WHSRpf tool includes measures of oxygenation and ventilation. This equation is the difference between the best partial pressure of arterial oxygen (PaO2) and partial pressure of carbon dioxide (PaCO2) in the first 24 h of life [8]. However, PaO2 alone is a poor measure of hypoxemia. Thus, we modified the WHSRpf equation to include the PaO2/fraction of inspired oxygen (FiO2) (PF) ratio to better account for the degree of hypoxemia in infants with CDH [14, 15]. The final equation we refer to as the PF-PCO2 tool, or the difference between the PF ratio and PCO2, where PaO2 and PaCO2 are the best values (not necessarily from the same blood gas) obtained in the first 24 h of life and FiO2, is the FiO2 recorded at the same time as the PaO2. Chandrasekharan et al. have also described this equation in a brief letter to the editor [16].
Given the importance of a clinical prediction tool in guiding therapy and postnatal counseling, we performed a retrospective review comparing the equation, PF-PCO2, with three other published tools (CDHSG-PS, WHSRpf, and Brindle score) to predict survival in our local population of CDH infants. We also determined the predictive ability of this equation for the secondary outcomes of survival without extracorporeal membrane oxygenation support (ECMO) and death despite ECMO. Our hypothesis was that the PF-PCO2 tool will be most predictive of survival, survival without ECMO, and death despite ECMO.
Methods
Eligible infants were identified retrospectively from an internal database of infants with CDH admitted to the University of Utah or Primary Children’s Hospital Neonatal Intensive Care Units (NICU’s) from 1 January 2003 to 30 June 2018. This database contains prospectively captured data on each infant, including need for ECMO and measures of ventilation and oxygenation, including blood gas (PaO2 and PaCO2) and ventilator support data (FiO2 and mean airway pressure) at various time points. Infants admitted to the University of Utah or Primary Children’s NICU’s pre-repair were eligible for this study. Exclusion criteria were diagnosis of CDH after 24 h of life, gestational age less than 340/7 weeks, birth weight less than 1800 g, and presence of other major anomalies or chromosomal disorders. All infants were repaired at our center. Major cardiac anomalies were defined as all cardiac anomalies except for patent foramen ovale, patent ductus arteriosus, ventricular septal defect, or atrial septal defect.
CDHSG-PS, WHSRpf, and Brindle scores were calculated for all eligible infants based on previously published formulae (Table 1).(1,8,11) PF-PCO2 was calculated for all eligible infants per the formula—[PaO2/FiO2]-PaCO2—where PaO2 and PaCO2 are the highest and lowest values, respectively, from post-ductal arterial blood gases obtained in the first 24 h of life. The FiO2 is the value recorded at the time of the PaO2. Institutional review board approval and a waiver of informed consent due to retrospective nature of the study were obtained from the University of Utah and Primary Children’s Hospital.
The primary outcome was the ability of each tool to predict survival. Secondary outcomes included: ability of the PF-PCO2 tool to predict survival without ECMO and death despite ECMO; and defining cutoff values that best predict all outcomes for the PF-PCO2 tool. To determine these cutoff points, positive (PPV) and negative predictive values (NPV) were calculated for the PF-PCO2 tool at intervals of 20 from −80 to +80.
The primary outcome and other categorical measures were analyzed by Chi-square or Fisher’s Exact test. Student’s t-test was used for analysis of normally distributed continuous data and Mann–Whitney U test was applied for ordinal data or continuous data that was not normally distributed. Two-sided P-values less than 0.05 were considered statistically significant and no adjustment was made for multiple comparisons. Area under the curve analysis was performed. SPSS (version 24, IBM, Armonk NY) was used for statistical analysis.
Results
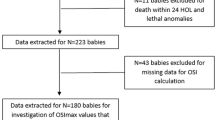
Figure 1 shows the patient flowchart. After exclusion of 70 infants, the final study group of 203 included 165 infants who survived and 38 infants who died. Within each group, missing data precluded WHSRpf and PF-PCO2 score calculation for seven infants. Of the 19 infants diagnosed with CDH after 24 h of life, two (11%) were born at <34 weeks GA and <1800 g and three (16%) had a severe anomaly. The remaining 14 infants diagnosed after 24 h of life underwent primary repair of their defect and survived without ECMO. Missing data precluded PF-PCO2 calculation for these infants. Patient characteristics are shown in Table 2. Infants who survived had a higher birth weight, but this was not statistically significant. They were also less likely to have pulmonary hypertension pre-repair, liver in the thorax, and to undergo ECMO. There was a significant difference in type of repair, with surviving infants more likely to undergo primary repair (p < 0.001). There were no differences in survival (p = 0.119) or ECMO use (p = 0.322) when infants were divided into 5-year epochs corresponding to their birth year (1 = 2003–2007, 2 = 2008–2012, and 3 = 2013–2018). This was also true on regression analysis, where birth epoch did not influence survival (p = 0.057 for epoch 1 versus 3; p = 0.079 for epoch 2 versus 3). For the PF-PCO2 and WHSRpf equations, the median age of the highest PaO2 and lowest PaCO2 values was 7 h (interquartile range 3–14 h) and 6 h (4–14 h), respectively. There was a significant difference in the median predictive score for each tool between groups (Table 2).
As seen in Fig. 2, the PF-PCO2 tool best predicted survival, with an AUC of 0.84 (95% confidence interval (CI) 0.77–0.91), versus 0.80 (95% CI 0.72–0.88) for WHSRpf, 0.77 (95% CI 0.69–0.86) for CDHSG-PS. The AUC’s for the Brindle score are not included in the figure as they are inversely related to the other scores. The AUC for survival by the Brindle tool was the lowest among the predictive scores tested at 0.74 (95% CI 0.65–0.83). For survival without ECMO, PF-PCO2 outperformed the other tools with an AUC of 0.92 (95% CI 0.87–0.96) versus 0.87 (95% CI 0.81–0.93) for WHSRpf, 0.72 (95% CI 0.65–0.80) for CDHSG-PS, and 0.75 (95% CI 0.68–0.82) for the Brindle tool. Finally, for death despite ECMO, PF-PCO2 had the highest AUC at 0.83 (95% CI 0.76–0.91) versus 0.82 (95% CI 0.75–0.89) for WHSRpf, 0.75 (95% CI 0.65–0.85) for CDHSG-PS, and 0.77 (95% CI 0.67–0.88) for the Brindle tool.
AUC curves for each predictive tool. Brindle curves are not shown due to low AUC and opposite test direction compared to the other scores. ECMO extracorporeal membrane oxygenation, CDHSG-PS Congenital Diaphragmatic Hernia Study Group Probability of Survival Equation, WHSRpf Wilford Hall Santa Rosa Prediction Formula, PF-PCO2 (PaO2/FiO2)-PCO2 equation
The scatterplot diagram in Fig. 3 shows the distribution of PF-PCO2 scores for the outcomes of survival, survival without ECMO, and death despite ECMO. A PF-PCO2 score >−60 was most predictive of survival and >+80 was most predictive of survival without ECMO (Table 3). More subjects with a PF-PCO2 score >−60 survived (97%, p < 0.001) and score >+80 survived without ECMO (93%, p < 0.001). The PF-PCO2 score had poor predictive ability for the outcome of death despite ECMO.
Discussion
This retrospective review showed that, in our single center experience, the PF-PCO2 tool was superior to the CDHSG-PS, Brindle, and WHSRpf tools for early postnatal prediction of survival in infants with CDH. We also found the PF-PCO2 tool to be most predictive of survival without ECMO, but not death despite ECMO. In our patient population, a score >−60 had an 84% PPV and 100% NPV to predict survival and a score >+80 had a 90% PPV and 89% NPV to predict survival without ECMO.
The CDHSG-PS and Brindle scores predicted survival the poorest in our patient population, with an AUC of 0.77 and 0.74, respectively. Javid et al. [17] showed that survival rates for infants with CDH in the Canadian Neonatal Network were better than that predicted by the CDHSG-PS tool. Similar results were obtained by Downard et al. [18] in an analysis of their CDH population, where actual versus predicted survival was 93% versus 68%, respectively. In contrast, Skarsgard et al. [12] and Baird et al. [19] found the CDHSG-PS tool predicted mortality well in their databases of infants with CDH from multiple centers in Canada. Gentili et al. [13] also found this model to be effective at predicting mortality (AUC 0.84) in their local population of infants with CDH. Furthermore, the CDHSG-PS model significantly predicted survival in a local population of CDH infants who underwent ECMO [10]. Regarding the Brindle tool, the tool stratified infants into low, intermediate, and high risk for mortality categories with an AUC of 0.81 in the derivation model, but only 0.77 in the validation model [11]. Finally, Akinkuotu et al. [2] found the Brindle score to independently predict mortality at 6 months, but not risk for ECMO.
In our infants with CDH, the WHSRpf tool predicted survival second best, with an AUC of 0.80. Authors applying this tool to their local populations have shown good prediction of survival or mortality, with Shultz et al. [8] showing an AUC of 0.87 in their local derivation model and Gentili et al. [13] an AUC of 0.84. However, the predictive ability declined when applied to larger databases [8, 17]. In a CDH-ECMO population, Hoffman et al. [20] found the WHSRpf tool predicted survival in CDH infants who underwent ECMO with an AUC of 0.71.
It is unclear why the CDHSG-PS and Brindle tools had lower predictive scores for survival, survival without ECMO, and death despite ECMO in our population. One reason could be that the authors of these tools did not exclude infants with other major cardiac or chromosomal anomalies, both of which would increase the risk of mortality via the Brindle score. As infants with other major anomalies may frequently not be offered (or parents may decline) surgical intervention or ECMO, we excluded this group of patients to isolate the effect of the diaphragmatic hernia on mortality. Additionally, we excluded infants born at a gestational age of less than 340/7 weeks or birth weight less than 1800 g, as ECMO is not typically offered to these patients [21, 22] and low birth weight and prematurity increase mortality [11, 22, 23]. Including these infants would likely reduce the predictive ability of the PF-PCO2 equation, as factors other than pulmonary function could play a role in an infants’ death. Finally, we excluded infants diagnosed after 24 h of life, because these infants typically have a mild defect and favorable prognosis [3]. Among the 19 infants we excluded due to diagnosis after 24 h, survival without ECMO was 100%. Thus, for this group of CDH infants, a tool predicting survival seems unnecessary. As expected, the WHSRpf score had good predictive ability, with an AUC of 0.80 for survival. The PF-PCO2 score likely performed better due to the inclusion of the PF ratio, rather than PaO2 alone, thereby providing a better measure of oxygenation [14, 15]. An advantage of the PF-PCO2 score is the ease with which it can be calculated at the bedside, in contrast to the CDHSG-PS and Brindle tools, which are algorithm based.
Our findings suggest that incorporating a better measure of oxygenation and ventilation allows the PF-PCO2 tool to predict survival and survival without ECMO in infants ≥34 weeks gestation with isolated CDH. The severity of pulmonary hypoplasia determines outcome in these patients, including need for ECMO and mortality [2, 7,8,9,10]. Infants with CDH have decreased numbers of alveoli and accompanying vessels compared to normal term infants, although the total ratio is normal [24]. Furthermore, there is a greater percentage of peripheral arteries that are abnormally muscularized or hypertrophied [24], also contributing to pulmonary hypertension. When correlating postmortem findings with blood gas values, Germain et al. [25] found that the severity of pulmonary hypoplasia correlated with the level of hypoxemia in CDH infants. Finally, other investigators have shown that blood gas values predict survival in infants with CDH, including PaCO2 [26] and the highest FiO2 [9] and PaO2 [27, 28]. Regarding ECMO risk, Kays et al. [29] showed that PaCO2 and PaO2 at 1 h of life correlated with an increased risk for ECMO in their group of infants with severe CDH, while Hoffman et al. [30] found that PaCO2 independently predicted survival in CDH infants placed on ECMO. However, PaO2 itself is not the best measure of oxygenation in a patient. Of the various measures of oxygenation or hypoxemia [14, 15], the PF ratio is easy to calculate and has been shown to be significantly different between survivors and non-survivors of CDH [13].
While most studies have looked at survival or mortality alone, we also determined the ability of the PF-PCO2 equation to predict survival without ECMO and death despite ECMO. The equation performed well for predicting survival without ECMO, but not for death despite ECMO. These outcomes are clinically relevant, because infants with CDH who require ECMO have a greater risk of mortality and long-term morbidity, including adverse neurodevelopmental outcomes and chronic lung disease [3, 31]. Thus, knowledge of an infants’ risk for ECMO and death despite ECMO is important not only when considering the risks versus benefits of ECMO, but also when providing informed consent to families regarding this intervention. To date, no model has shown good predictive ability for the isolated outcome of death despite ECMO with CDH.
There are several limitations to this study. This study may have limited generalizability, given that it was a retrospective review conducted at a single center serving a predominantly Caucasian population. Differences in initial care may have contributed to patient outcome, as 42% and 34% of patients in the survived and died groups, respectively, were outborn. Sample size is also relatively small. Additionally, differences in ventilator management can affect PaCO2, PaO2, and FiO2, which may produce different results in another center. Although our center has written guidelines directing the care of infants with CDH, including a gentle ventilation strategy with relative permissive hypercapnia and criteria for ECMO initiation, we do not have data on compliance with this protocol. We also did not assess whether including ventilator parameters, particularly mean airway pressure, would change the predictive ability of the PF-PCO2 equation. Finally, we were unable to validate the PF-PCO2 tool in another population, including the CDHSG registry, as it does not contain data on FiO2. Thus, while we showed good predictive ability of this tool in our population, this may decline when applied to a different population. We plan to test this tool in a much larger independent data set.
This study’s strengths include use of a prospectively maintained database with multiple data points, including physiologic measures of respiratory function as well as morbidity and mortality information. Additionally, data were available for all patients to calculate CDHSG-PS and Brindle scores and all but 7 patients to calculate WHSRpf and PF-PCO2 scores. Furthermore, our exclusion criteria attempted to hone in on a subpopulation of CDH infants in whom a predictive tool would be clinically useful. Specifically, CDH infants, who are eligible for ECMO (≥340/7 weeks gestation and >1800 g), do not have a mild defect (diagnosed <24 h of life), and do not have other conditions that could influence their outcome. Although this study was limited to a single center, the University of Utah and Primary Children’s Hospital NICU’s are academic level III and IV units, respectively, and receive neonatal and maternal transfers from across Utah and neighboring states. Finally, despite this study covering a long time period, there were no differences in survival of or ECMO use in patients with CDH.
In summary, the PF-PCO2 tool predicted survival better than the CDHSG-PS, Brindle, and WHSRpf tools in our local population of infants with CDH. This easy to use clinical tool may aid in postnatal therapeutic decisions and counselling of families of CDH infants. Further studies applying this tool to other populations are necessary to validate this equation. Additional studies should also consider analysis of the PF-PCO2 tool at different time points within the first 24 h of life.
References
Lally KP, Jaksic T, Wilson JM, Clark RH, Hardin WD, Ronald BH, et al. Estimating disease severity of congenital diaphragmatic hernia in the first 5 min of life. J Pediatr Surg. 2001;36:141–45.
Akinkuotu AC, Cruz SM, Abbas PI, Lee TC, Welty SE, Olutoye OO, et al. Risk stratification of severity for infants with CDH: prenatal versus postnatal predictors of outcome. J Pediatr Surg. 2016;51:44–48.
Losty PD. Congenital diaphragmatic hernia: where and what is the evidence? Semin Pediatr Surg. 2014;23:278–82.
Murthy K, Pallotto EK, Gien J, Brozanski BS, Porta NFM, Zaniletti I, et al. Predicting death or extended length of stay in infants with congenital diaphragmatic hernia. J Perinatol. 2016;36:654–59.
Cannie M, Jani J, Meersschaert J, Allegaert K, Marchal G, Deprest J, et al. Prenatal prediction of survival in isolated diaphragmatic hernia using observed to expected total fetal lung volume determined by magnetic resonance imaging based on either gestational age or fetal body volume. Ultrasound Obstet Gynecol. 2008;32:633–39.
Bebbington M, Victoria T, Danzer E, Moldenhauer J, Khalek N, Johnson M, et al. Comparison of ultrasound and magnetic resonance imaging parameters in predicting survival in isolated left-sided congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2014;43:670–74.
Numanoglu A, Morrison C, Rode H. Prediction of outcome in congenital diaphragmatic hernia. Pediatr Surg Int. 1998;13:564–68.
Shultz CM, DiGeronimo RJ, Yoder BA. Congenital diaphragmatic hernia: a simplified postnatal predictor of outcome. J Pediatr Surg. 2007;42:510–16.
Lupo E, Castoldi F, Maestri L, Rustico M, Dani C, Lista G. Outcome of congenital diaphragmatic hernia: analysis of implicated factors. Minerva Pediatr. 2013;65:279–85.
Chiu PPL, Sauer C, Mihailovic A, Adatia I, Bohn D, Coates AL, et al. The price of success in the management of congenital diaphragmatic hernia: is improved survival accompanied by an increase in long-term mortality? J Pediatr Surg. 2006;41:888–92.
Brindle ME, Cook EF, Tibboel D, Lally PA, Lally KP. A clinical prediction rule for the severity of congenital diaphragmatic hernias in newborns. Pediatrics . 2014;134:e413–19.
Skarsgard ED, MacNab YC, Qiu Z, Little R, Lee SK. SNAP-II predicts mortality among infants with congenital diaphragmatic hernia. J Perinatol. 2005;25:315–19.
Gentili A, Pasini L, Lannella E, Landuzzi V, Lima M, Bacchi Reggiani ML, et al. Predictive outcome indexes in neonatal congenital diaphragmatic hernia. J Matern Fetal Neonatal Med. 2015;28:1602–07.
Aboab J, Louis B, Jonson B, Brochard L. Relation between PaO2/FiO2 ratio and FiO2: a mathematical description. Intensive Care Med. 2003;32:1494–97.
Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–82.
Chandrasekharan P, Konduri G, Basir M, Klein J, Lakshminrusimha S. Risk stratification for congenital diaphragmatic hernia—is it all oxygenation but not ventilation? [Letter]. J Perinatol. 2018;38:608–09.
Javid PJ, Jaksic T, Skarsgard ED, Lee S., the Canadian Neonatal Network. Survival rate in congenital diaphragmatic hernia: the experience of the Canadian neonatal network. J Pediatr Surg. 2004;39:657–60.
Downard CD, Jaksic T, Garza JJ, Dzakovic A, Nemes L, Jennings RW, et al. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg. 2003;38:729–32.
Baird R, MacNab YC, Skarsgard ED. Mortality prediction in congenital diaphragmatic hernia. J Pediatr Surg. 2008;43:783–87.
Hoffman SB, Massaro AN, Gingalewski C, Short BL. Survival in congenital diaphragmatic hernia: use of predictive equations in the ECMO population. Neonatology . 2011;99:258–65.
Gray B, Rintoul N. Guidelines for neonatal respiratory failure. In: ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support. Extracorporeal life support organization. Ann Arbor. 2017. https://www.elso.org/Resources/Guidelines.aspx. Accessed Aug 30, 2018.
Chandrasekharan PK, Rawat M, Madappa R, Rothstein DH, Lakshminrusimha S. Congenital diaphragmatic hernia—a review. Matern Health Neonatol Perinatol. 2017; 3:6. https://doi.org/10.1186/s40748-017-0045-1.
Shanmugam H, Brunelli L, Botto LD, Krikov S, Feldkamp ML. Epidemiology and prognosis of congenital diaphragmatic hernia: a population-based cohort study in Utah. Birth Defects Res. 2017;09:1451–59.
Bohn D, Tamura M, Perrin D, Barker G, Rabinovitch M. Ventilatory predictors of pulmonary hypoplasia in congenital diaphragmatic hernia, confirmed by morphologic assessment. J Pediatr. 1987;111:423–31.
Germain JF, Farnoux C, Pinquier D, Cortez A, Hartmann JF, Sibony O, et al. Can blood gas values predict pulmonary hypoplasia in antenatally diagnosed congenital diaphragmatic hernia? J Pediatr Surg. 1996;31:1634–39.
Salas AA, Bhat R, Dabrowska K, Leadford A, Anderson S, Harmon CM, et al. The value of PaCO2 in relation to outcome in congenital diaphragmatic hernia. Am J Perinatol. 2014;31:939–46.
Kays DW, Islam S, Perkins JM, Larson SD, Taylor JA, Talbert JL. Outcomes in the physiologically most severe congenital diaphragmatic (CDH) patients: whom should we treat? J Pediatr Surg. 2015;50:893–97.
Wilson JM, Lund DP, Lillehei CW, Vacanti JP. Congenital diaphragmatic hernia: predictors of severity in the ECMO era. J Pediatr Surg. 1991;26:1028–34.
Kays DW, Talbert JL, Islam S, Larson SD, Taylor JA, Perkins J. Improved survival in left liver-up congenital diaphragmatic hernia by early repair before extracorporeal membrane oxygenation: optimization of patient selection by multivariate risk modeling. J Am Coll Surg. 2016;222:459–70.
Hoffman SB, Massaro AN, Gingalewski C, Short BL. Predictors of survival in congenital diaphragmatic hernia patients requiring extracorporeal membrane oxygenation: CNMC 15-year experience. J Perinatol. 2010;30:546–52.
Leeuwen L, Fitzgerald DA. Congenital diaphragmatic hernia. J Paediatr Child Health. 2014;50:667–73.
Author information
Authors and Affiliations
Contributions
Dr. MS performed the data analysis and prepared the manuscript. Dr. SF was involved in study design and manuscript review. Dr. BY developed and maintained the database and was involved in study design and manuscript review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sekhon, M.K., Fenton, S.J. & Yoder, B.A. Comparison of early postnatal prediction models for survival in congenital diaphragmatic hernia. J Perinatol 39, 654–660 (2019). https://doi.org/10.1038/s41372-019-0335-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0335-8