Abstract
Non-dipping blood pressure (BP) pattern is a predictor for cardiovascular (CV) events and mortality. We evaluated dipping status change and its association with incidence of non-fatal CV events in middle-aged subjects. The OPERA study was carried out during the years 1991–1993, with a follow-up study 21.7 years later. In this study, we included 452 participants with 24-h ambulatory BP measurements (ABPM) available in both surveys. The study population was divided into four groups according to the dipping pattern change: dipping–dipping (n = 152/33.6%), dipping–non-dipping (n = 198/43.8%), non-dipping–dipping (n = 20/4.4%), and non-dipping–non-dipping (n = 82/18.1%). Sixty-five participants experienced a CV event (14.4%) during the 21.7 (SD 0.8) years of follow-up. The incidence of events was highest (28%) in the non-dipping–non-dipping group, and lowest (6.6%) in the dipping–dipping group (p < 0.001). In Cox regression analyses the covariates were age, sex, total cholesterol, hypertension and use of antihypertensive medication, systolic office BP and ambulatory mean or nighttime systolic BP, as well as the change in the variables during the follow-up period. After adjustments, the association of the non-dipping–non-dipping pattern with CV events compared with the dipping–dipping pattern remained significant (HR 4.01; 95% CI 1.89–8.67, p < 0.001). In summary, non-dipping–non-dipping pattern was associated with non-fatal CV events in the long term, and the effect was independent of the conventional risk factors including office and ambulatory BP levels.
Similar content being viewed by others
Introduction
Ambulatory blood pressure measurement (ABPM) is a method which usually provides 24-h assessment of blood pressure (BP) readings and its diurnal variation in a domestic setting during normal daily activities. Recent guidelines recommend ABPM for screening of masked hypertension, detecting white coat hypertension, for diagnosing hypertension, and as the most accurate method for assessing cardiovascular (CV) risk and for detecting nocturnal hypertension [1, 2]. BP dipping is a physiological phenomenon in which nighttime BP declines 10–20% compared with daytime BP levels. In a proportion of individuals, however, the normal circadian BP variation is inadequate, and the decrease in BP is therefore less than 10%. This is considered a non-dipping BP pattern, which was first described by O’Brien et al. [3]. The non-dipping phenotype has been shown to be an independent risk factor for CV events in hypertensive patients and in different populations [3,4,5,6,7]. Non-dipping has also been associated with metabolic abnormalities [8], end organ damage [7, 9, 10] and new-onset diabetes [11]. Furthermore, some studies have suggested that nocturnal BP levels, sometimes accompanied by a non-dipping pattern, are better at predicting CV events, CV mortality and total mortality than office BP [12].
Earlier studies on ambulatory BP characteristics have been mostly based on the risk of outcomes associated with only one monitoring. However, only limited information is available regarding the long-term consistency of the non-dipping pattern. In this study we present long-term data on the changes of ABPM characteristics and the association of these changes with CV morbidity. To the best of our knowledge, there are no prior studies with follow-up data that are as long and with baseline examinations that are as detailed as in the present study.
Methods
Study population
This research is part of the OPERA (Oulu Project Elucidating Risk of Atherosclerosis) project, a population-based cohort study designed to evaluate the risk factors and endpoints of atherosclerotic CV disease. The Ethics Committee of the Faculty of Medicine, University of Oulu approved the OPERA study, and it was conducted by the principles of the Declaration of Helsinki. All study subjects gave an informed consent. The details of the study population and the selection criteria have been published earlier [13]. In short, 600 hypertensive men and women aged 40–62 years, were randomly selected from the register of reimbursement for hypertension medication. In addition, an age- and sex-matched cohort of 600 normotensive subjects was randomly sampled from the social register of the area.
Baseline study
Out of the 1200 individuals invited, 1045 (87.1%) participated in the baseline study during the years 1990–1993. The participants attended a clinical examination and answered a questionnaire, and laboratory samples were drawn after fasting [13]. The office BP was measured by a registered study nurse, using an appropriately fitted cuff size and an automatic oscillometric device (Dinamap Procare 100, Criticon, Tampa, Florida, USA) [14]. BP was measured three times in a sitting position, after a rest of a minimum of 5 min, with 1-min intervals between the measurements. The mean of the second and the third measurement was used for the analyses.
ABPM was recorded in 903 participants by a noninvasive fully automatic SpaceLabs 90207 oscillometric unit (SpaceLabs Inc., Redmond, Washington, USA). The measurements were taken every 15 min from 04:00 a.m. to midnight, and every 20 min between midnight and 04:00 a.m. The British Hypertension Society and the US Association for the Advancement of Medical Instrumentation have previously confirmed the accuracy and reproducibility of the BP readings acquired with this device [15]. In each individual, the proper positioning of the cuff was assessed by means of the similarity (difference < 5 mmHg) between four SpaceLabs BP measurements and four auscultatory readings using a Y-connector. Values were automatically excluded from the analysis if systolic BP (SBP) was <70 or >250 mmHg, or if diastolic BP (DBP) was <40 or >150 mmHg, or if the heart rate was <40 or >150 beats per minute. Less than 3% of the BP readings were rejected as artifacts based on these criteria [16]. Further details of the ABPM of the baseline study can be found in a previous publication [17].
Follow-up study
The follow-up study was carried out during the years 2013 and 2014, and 600 subjects took part in it. ABPM was completed in 483 of the original participants (53.5%) of which 30 were excluded for a previous CV event and 1 for missing values, leaving 452 recordings for analyses. During the follow-up period, the ABPM setting was similar compared with the baseline measurement, and the recordings were made with Oscar 2 oscillometric ABP monitor (SunTech Medical) [18]. The mean follow-up time between the recordings was 21.7 ± 0.8 years.
Follow-up of cardiovascular events
Follow-up time of the CV events was defined as the time from the date of the baseline examination to the first non-fatal CV event or until December 31, 2014. The endpoint was the first CV event: a major coronary heart disease (CHD) event or stroke, whichever occurred first. The data on CV events was obtained by the ICD (the International Classification of Diseases) codes from national registries, the Hospital Discharge Register and the Care Register for Health Care of the Finnish Institute for Health and Welfare (THL). Each study subject was identified by a personal identity code. The diagnoses were classified according to the ICD Ninth Revision (ICD-9) before 1996 and the Tenth Revision (ICD-10) thereafter. The ICD-10 code was mandated for use in Finland from January 1, 1996. CHD was based on the following diagnoses: I20, I21, I22 (ICD-10)/410, 4110 (ICD-9) as the main diagnosis, and I21, I22 (ICD-10)/410 (ICD-9) as a first or second side diagnosis or third diagnosis (ICD-9 only), or coronary artery bypass grafting, or coronary angioplasty. Stroke as an endpoint included I61, I63 (not I63.6), I64 (ICD-10) and 431, 4330A, 4331A, 4339A, 4340A, 4341A, 4349A, 436 (ICD-9) as the main diagnosis or as a side diagnosis.
Statistical methods
Data analyses were performed with IBM SPSS Statistics for Windows version 27.0 (IBM Corp. IBM SPSS Statistics for Windows. Armonk, NY: IBM Corp; 2020). A p value < 0.05 was considered as statistically significant. Baseline data are expressed as prevalence, mean ± SD for continuous variables, or median with 25th and 75th quartiles for skewed variables. Continuous variables were tested for difference between the CV event or non-event groups with Student’s t test or with Mann–Whitney’s test, when appropriate. Log transformation was performed for skewed variables for statistical testing. ANOVA Tukey test was used to test difference between the dipping groups in continuous variables, and Pearson’s Chi-square test was used for the categorical variables. Delta variables were formed by subtraction.
The Kaplan–Meier method was used to assess hazard of CV events by dipping status categories and tested by log-rank test. We used Cox regression analysis to estimate the association of the dipping status categories with CV events in multivariate models adjusted by variables with significance in univariate analyses. The covariates (change of dipping status, age, sex, total cholesterol, hypertension, use of antihypertensive medication, office blood pressure, and either 24-h mean SBP or nighttime mean SBP) were selected according to the univariate analyses (shown as Supplementary Tables 1 and 2). Categorical covariates included dipping status (dipping–dipping was the reference), sex (women as a reference), hypertension (no hypertension as a reference) and use of antihypertensive medication (medication as a reference). Hypertension was defined by office BP over 140/90 or use of antihypertensive medication. The other covariates were used as continuous variables. Total cholesterol was selected to the model out of the lipids. If it was replaced by any of the lipids (LDL-cholesterol, HDL-cholesterol, or triglycerides), the significance of the mode, or the dipping status, did not change.
Results
Our study population consisted of the OPERA study cohort whose participants underwent ABPM in the baseline study and again 21.7 years later (SD ± 0.7, range 20.4–23.8 years) during the follow-up study (Fig. 1). Twenty-seven persons were excluded from the analyses based on an earlier diagnosis of coronary artery disease, three persons because of a history of previous stroke, and one because of missing nighttime ABPM values.
In the analyses, there were altogether 452 eligible individuals, of which 228 (50.4%) were women and 224 (49.6%) men (Table 1). During the recruitment, the mean age of the participants was 49.7 ± 5.4 years, and the age range was from 40.2 to 62.0 years. Non-fatal CV events (n = 65, in 14.4% of the subjects) were assessed during the follow-up until the first event. As expected, those who experienced a CV event were predominantly men (p < 0.001), older (p = 0.012), had higher 24-h mean systolic (p = 0.013), daytime systolic (p = 0.033), nighttime systolic (p < 0.001) and nighttime diastolic BP (p = 0.001) and more unfavorable lipid profile than those without a CV event. Almost half of the subjects were hypertensive (47.6%), and 45.4% were treated with at least one antihypertensive medication. The prevalence of diabetes was 6.9%. The average body mass index was 27.2 ± 4.3 kg/m2. Of all the subjects, 22.6% were non-dippers. The prevalence of non-dippers was significantly higher in those with a CV event than in those without (40.0% vs. 19.6%, p < 0.001) (Table 1).
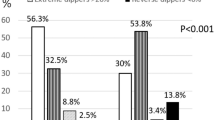
The cohort was divided into four groups according to the dipping status change from the baseline to the follow-up study (Fig. 2). Dipping–dipping pattern was present in 33.6% of the whole cohort, and non-dipping–non-dipping pattern in 18.2%. Non-dipping phenotype was the most consistent: it prevailed in the follow-up in 80.4% of the baseline non-dippers, whereas 43.4% of the baseline dippers remained dippers. A large proportion of the original dippers changed their phenotype to non-dippers (56.6%).
Altogether, 65 non-fatal CV events occurred, and the incidence was the highest (28.0%) in the non-dipping–non-dipping group and the lowest (6.6%) in the dipping–dipping group, p < 0.001 (Table 2). Age, office BPs, 24-h mean BPs and nighttime BPs differed between the groups and were significantly higher in the non-dipping–non-dipping group compared with the dipping–dipping group. The non-dipping–non-dipping group also had lower estimated glomerular filtration rate (eGFR), total cholesterol, LDL cholesterol and triglycerides levels compared with the dipping–dipping group. Additionally, there was a trend of rising prevalence of hypertension (p < 0.001) and antihypertensive medication use (p < 0.001) in the non-dipping–non-dipping group (linear by linear association p < 0.001).
Kaplan–Meier survival curves for CV events by different dipping patterns are presented in Fig. 3.
We analyzed the changes in BP, metabolic factors, and prevalence of hypertension and medication use during the follow-up time (Table 3) by the dipping categories. In the non-dipping–non-dipping group, the incidence of new-onset diabetes was significantly higher than in the dipping–dipping group, and it was reported earlier [11].
A general positive development over time in BP measurements can be observed, with the DBP decreasing more, as expected, with age. There were statistically significant differences in office BP and nighttime BP between the dipping groups. In the non-dipping–non-dipping group, the reduction of office BP was the greatest and in the dipping–dipping group the smallest. In nighttime mean BP, the groups also differed from each another, but in post hoc analyses, there was no statistically significant difference in the change in the nighttime BP between the dipping–dipping and the non-dipping–non-dipping group.
As expected, the prevalence of any BP medication use increased over time in all the dipping groups, especially the proportions of the RAA system agents. The non-dipping–non-dipping group had more often BP medication than the dipping–dipping group (86.6% vs. 63.2%, p < 0.001 between the groups, data shown as Supplementary Table 3).
In the follow-up, a lipid lowering agent was used by 45.4% of the study population, compared to only 2.4% at the baseline. Non-dippers tended to use statins more often than the other groups in the follow-up, but the difference did not reach statistical significance.
In the multivariate Cox model, we included variables, that were statistically significant in univariate analyses (data shown only as Supplementary Tables 1 and 2): dipping change pattern (dipping–dipping group as a reference), age, sex, hypertension, office SBP, 24-h mean SBP or nighttime mean SBP, total cholesterol and use of antihypertensive agent (Table 4). The baseline fasting blood glucose levels, prevalence of diabetes or smoking did not differ between the dipping groups and were therefore left out of the Cox model. The non-dipping–non-dipping pattern was independently associated with CV events when adjusted with the above covariates and the 24-h mean SBP (HR 4.01; 95% CI 1.86–8.67, p < 0.001) (Table 4a). The association between the non-dipping–non-dipping pattern and CV events also remained statistically significant when the adjustment in this model was made with the nighttime mean SBP instead of the 24-h mean SBP (HR 3.19; 95% CI 1.41–7.20, p = 0.005) (Table 4b).
Finally, the delta values of nighttime SBP or 24-h mean SBP, as well as delta office SBP, total cholesterol, new onset diabetes, new onset hypertension, and the change in BP medication were added to multivariate models. The statistical significance of the association of the dipping pattern with CV events remained (for nighttime SBP delta: HR 3.40; 95% CI 1.47–7.88, p = 0.004 and for 24-h mean SBP: HR 3.13; 95% CI 1.38–7.11, p = 0.006).
Discussion
This population-based study consisted of 452 originally middle-aged Finnish hypertensive and normotensive females and males who underwent ABPM at the baseline and at the end of the follow-up time, 21.7 years later. The prevalence of non-dipping varies among studies, from 20% in white young adult women [19] to 86% in CKD patients [20]. In this study, at the baseline, 22.6% were non-dippers. Non-dipping–non-dipping pattern of nighttime BP, which was detected in 80.4% of the original non-dippers, was associated with non-fatal CV events during the follow-up even when adjusting with demographics, the classic CV risk factors, and either 24-h mean SBP or nighttime SBP.
In the present study, the association of the non-dipping status and non-fatal CV events was not explained by the unfavorable development of the conventional risk factor levels or the differences in them between the groups. In our previous study, we showed that the non-dipping–non-dipping group developed new-onset diabetes more often than the dipping–dipping group [11]. However, in the present study, all the multivariate analyses were controlled for diabetes as well as other risk factors. The trend toward lower risk factor levels of several variables during the follow-up period was more enhanced in the non-dipping–non-dipping group: BP declined more in non-dippers than dippers, but only the difference in the office BP reached statistical significance. Also, lipid levels improved in general, and the change was more prominent in non-dippers than in dippers. Furthermore, the use of antihypertensive and lipid lowering agents was more prevalent in the non-dipping–non-dipping group than in the dipping–dipping group. Nevertheless, the CV risk was greater in those with non-dipping–non-dipping. In a cohort of hypertensive middle-aged patients, with ABPM seven years apart, the unmedicated baseline ABPM predicted future CV events better than the latter recording in a 10-year follow-up [21] .
Our study has strengths, but also limitations. One strength is that the nighttime BP was measured three times per hour. If BP is recorded only once or twice an hour, the value of hourly mean BP is rapidly diminished [22]. Other strengths are the comprehensive data of the population and hospital records, which are available and collectable by a personal identity code provided to every citizen, as well as high participation percentages in both our surveys.
There are also limitations: one is that the reproducibility of BP phenotypes identified by both office BP and ABP has been questioned. According to a recent review, in hypertensive patients on antihypertensive medication, only a limited number of individuals maintained the same dipping pattern over a few years of follow-up, unrelated to the type of antihypertensive medication used [23]. On the contrary, there is also data of higher agreement between ABPM recordings 2.5 years apart and a coherence of up to 76% [24]. Nighttime BP has been associated with poor CV outcome both in population studies and in hypertensive persons [25], and overall reproducibility is considered the best in the majority of subjects with the non-dipping pattern of nocturnal BP [26].
The day-to-day variation of dipping status has been suspected of being to some extent a result of sleep quality [27]. In this study we did not collect data regarding the quality of sleep or the prevalence of sleep apnea. Instead of diary records, fixed-clock intervals were used in defining daytime and nighttime, and the transitional times in the morning and in the evening were not excluded from the analyses. In a 17-year-long study, however, the researchers stated that while diary records are preferable, standard fixed-time intervals are also suitable in population-based studies [28]. Furthermore, we do not have data on timing of the antihypertensive medication which was common among our study population.
To conclude, the non-dipping–non-dipping pattern of BP was independently associated with non-fatal CV events in a randomly selected normotensive and hypertensive middle-aged population in a two-decade follow-up study.
Summary
What is known about the topic
-
Non-dipping blood pressure pattern is associated with cardiovascular events, end-organ damage, and metabolic abnormalities.
-
Nocturnal BP levels may be better to predict CV events and mortality than office BP.
What this study adds
-
Non-dipping blood pressure pattern was the most consistent during the 21-year follow-up. Eighty-two (80.2%) out of the 102 baseline non-dippers remained non-dippers, whereas 43.4% (n = 152) of the baseline dippers remained dippers.
-
Non-dipping–non-dipping pattern in the long term was associated with increased risk of non-fatal CV events compared to dipping–dipping pattern.
Data availability
Data cannot be shared publicly because of privacy policy. Data are available from the Oulu University Institutional Data Access for researchers who meet the criteria for access to confidential data. Contact information for the Oulu University Institutional Data Access committee: olavi.ukkola@oulu.fi.
References
Stergiou GS, Palatini P, Parati G, Januszewicz A, Lurbe E, Persu A, et al. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39:1293–302. https://doi.org/10.1097/HJH.0000000000002843.
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2018;138:e426–83. https://doi.org/10.1161/CIR.0000000000000597.
O’Brien E, Sheridan J, O’Malley K. Dippers and non-dippers. Lancet. 1988;2:397. https://doi.org/10.1016/s0140-6736(88)92867-x.
Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–9. https://doi.org/10.1097/00004872-200211000-00017.
Salles GF, Reboldi G, Fagard RH, Cardoso CRL, Pierdomenico SD, Verdecchia P, et al. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the ambulatory blood pressure collaboration in patients with hypertension (ABC-H) meta-analysis. Hypertension. 2016;67:693–700. https://doi.org/10.1161/HYPERTENSIONAHA.115.06981.
Lo L, Hung SWS, Chan SSW, Mak CL, Chan PF, Chao DVK. Prognostic value of nocturnal blood pressure dipping on cardiovascular outcomes in Chinese patients with hypertension in primary care. J Clin Hypertens. 2021;23:1291–9. https://doi.org/10.1111/jch.14304.
Hjortkjær H, Persson F, Theilade S, Winther SA, Tofte N, Ahluwalia TS, et al. Non-dipping and higher nocturnal blood pressure are associated with risk of mortality and development of kidney disease in type 1 diabetes. J Diabetes Complications. 2022;36:108270. https://doi.org/10.1016/j.jdiacomp.2022.108270.
Ukkola O, Vasunta RL, Kesäniemi YA. Non-dipping pattern in ambulatory blood pressure monitoring is associated with metabolic abnormalities in a random sample of middle-aged subjects. Hypertens Res. 2009;32:1022–7. https://doi.org/10.1038/hr.2009.137.
Kalaycioʇlu E, Gökdeniz T, Aykan AÇ, Gül I, Uʇur M, Gürsoy OM, et al. The influence of dipper/nondipper blood pressure patterns on global left ventricular systolic function in hypertensive diabetic patients: a speckle tracking study. Blood Press Monit. 2014;19:263–70. https://doi.org/10.1097/MBP.0000000000000055.
Minutolo R, Agarwal R, Borrelli S, Chiodini P, Bellizzi V, Nappi F, et al. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med. 2011;171:1090–8. https://doi.org/10.1001/archinternmed.2011.230.
Lempiäinen PA, Vasunta RL, Bloigu R, Kesäniemi YA, Ukkola OH. Non-dipping blood pressure pattern and new-onset diabetes in a 21-year follow-up. Blood Press. 2019;28:300–8. https://doi.org/10.1080/08037051.2019.1615369.
Kikuya M, Ohkubo T, Asayama K, Metoki H, Obara T, Saito S, et al. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension. 2005;45:240–5. https://doi.org/10.1161/01.HYP.0000152079.04553.2c.
Rantala AO, Kauma H, Lilja M, Savolainen MJ, Reunanen A, Kesaniemi YA. Prevalence of the metabolic syndrome in drug-treated hypertensive patients and control subjects. J Intern Med. 1999;245:163–74. https://doi.org/10.1046/j.1365-2796.1999.00429.x.
Reinders A, Reggiori F, Shennan AH. Validation of the DINAMAP ProCare blood pressure device according to the international protocol in an adult population. Blood Press Monit. 2006;11:293–6. https://doi.org/10.1097/01.mbp.0000217998.96967.fb.
O´Brien E, Atkins N, Mee F, O´Malley K. Comparative accuracy of six ambulatory devices according to blood pressure levels. J Hypertens. 1993;11:673–5. https://doi.org/10.1097/00004872-199306000-00012.
Ylitalo A. Cardiovascular autonomic regulation in systemic hypertension. Doctoral dissertation, University of Oulu. Acta Universitatis Ouluensis, D, Medica. 1999.
Perkiömäki JS, Nortamo S, Ylitalo A, Kesäniemi A, Ukkola O, Huikuri H. Ambulatory blood pressure characteristics and long-term risk for atrial fibrillation. Am J Hypertens. 2017;30:264–70. https://doi.org/10.1093/ajh/hpw149.
Goodwin J, Bilous M, Winship S, Finn P, Jones SC. Validation of the Oscar 2 oscillometric 24-h ambulatory blood pressure monitor according to the British Hypertension Society protocol. Blood Press Monit. 2007;12:113–7. https://doi.org/10.1097/MBP.0b013e3280acab1b.
Booth JN, Anstey DE, Bello NA, Jaeger BC, Pugliese DN, Thomas SJ, et al. Race and sex differences in asleep blood pressure: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Clin Hypertens. 2019;21:184–92. https://doi.org/10.1111/jch.13474.
Ida T, Kusaba T, Kado H, Taniguchi T, Hatta T, Matoba S, et al. Ambulatory blood pressure monitoring-based analysis of long-term outcomes for kidney disease progression. Sci Rep. 2019;9:19296. https://doi.org/10.1038/s41598-019-55732-4.
Gosse P, Doublet J, Gaudissard J, Boulestreau R, Cremer A. Long-term evolution of ambulatory blood pressure and cardiovascular events in hypertensive patients. J Hum Hypertens. 2022;36:517–23. https://doi.org/10.1038/s41371-021-00538-z.
di Rienzo M, Grassi G, Pedotti A, Mancia G. Continuous vs intermittent blood pressure measurements in estimating 24-hour average blood pressure. Hypertension. 1983;5:264–9. https://doi.org/10.1161/01.hyp.5.2.264.
Mancia G, Facchetti R, Vanoli J, Dolfini V, Grassi G. Reproducibility of blood pressure phenotypes identified by office and ambulatory blood pressure in treated hypertensive patients. Data from the PHYLLIS study. Hypertens Res. 2022;45:1599–608. https://doi.org/10.1038/s41440-022-00982-5.
McGowan NJ, Gough K, Padfield PL. Nocturnal dipping is reproducible in the long term. Blood Press Monit. 2009;14:185–9. https://doi.org/10.1097/MBP.0b013e32832ff4e1.
O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–68. https://doi.org/10.1097/HJH.0b013e328363e964.
O’Brien E, Kario K, Staessen JA, de la Sierra A, Ohkubo T. Patterns of ambulatory blood pressure: clinical relevance and application. J Clin Hypertens. 2018;20:1112–5. https://doi.org/10.1111/jch.13277.
Hinderliter AL, Routledge FS, Blumenthal JA, Koch G, Hussey MA, Wohlgemuth WK, et al. Reproducibility of blood pressure dipping: relation to day-to-day variability in sleep quality. J Am Soc Hypertens. 2013;7:432–9. https://doi.org/10.1016/j.jash.2013.06.001.
Satoh M, Asayama K, Kikuya M, Inoue R, Tsubota-Utsugi M, Obara T, et al. Nocturnal blood pressure decline based on different time intervals and long-term cardiovascular risk: the Ohasama Study. Clin Exp Hypertens. 2018;40:1–7. https://doi.org/10.1080/10641963.2016.1259324.
Acknowledgements
We want to thank all the research nurses for their contribution throughout the baseline and follow-up studies.
Funding
This study was supported by grants from The Finnish Medical Foundation and The Finnish Kidney Foundation. Open Access funding provided by University of Oulu (including Oulu University Hospital).
Author information
Authors and Affiliations
Contributions
PL contributed to methodology, analysis, visualization and writing the original drafts. OU was the main supervisor, and strongly contributed to methodology and analyses. He reviewed, edited, and partly co-wrote the original draft. HH and AY collected and validated the original baseline data, they also reviewed the paper. YAK was the original supervisor of this study and offered the resources to the baseline study, and reviewed this paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
OPERA study was approved by the Ethics Committee of the Faculty of Medicine, University of Oulu, and it was conducted by the principles of the Declaration of Helsinki. All study subjects gave an informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lempiäinen, P.A., Ylitalo, A., Huikuri, H. et al. Non-dipping blood pressure pattern is associated with cardiovascular events in a 21-year follow-up study. J Hum Hypertens (2024). https://doi.org/10.1038/s41371-024-00909-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41371-024-00909-2