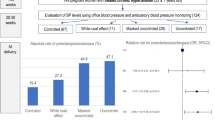
Abstract
De novo - or as a continuum of antenatal hypertension -postpartum hypertension complicates ~2% of pregnancies. Many maternal complications, such as eclampsia and cerebrovascular accidents, occur in the postpartum period. Despite widespread use of antihypertensives during pregnancy and childbirth, there is a paucity of data on preferred medications in the postpartum period. This randomized controlled study enrolled 130 women who were started on antihypertensives. They were randomized to receive oral Labetalol(LAB, maximum 900 mg per day in three doses) or oral Amlodipine(AML, maximum 10 mg per day given in two doses). In the immediate postpartum, all women were closely monitored for neurological symptoms, blood pressure, heart rate, respiratory rate, urine output, and deep tendon reflex. The primary outcome was the time to achieve sustained blood pressure control for 12 h from the initiation of medication; secondary outcomes included side effects of both medications. Mean time to achieve sustained blood pressure control was lower in women who received AML than in those who received LAB-(mean difference 7.2 h; 38 95% CI 1.4, 12.9, p = 0.011). There were fewer severe hypertensive episodes among those with AML than in those who received LAB. However, the proportion of women who continued to require antihypertensives at discharge was higher in the AML group than in the LAB group (55.4% versus 32.3%, p = 0.008). None of the participants developed drug-related side effects. Among women with postpartum persistence or new-onset hypertension, oral AML achieved sustained blood pressure control in a shorter duration, with fewer hypertensive emergencies than oral LAB. (CTRI/2020/02/023236).
Trial Registration details: The study protocol was registered with Clinical Trial Registry of India with CTRI Number CTRI/2020/02/023236 dated 11.02.2020. Protocol can be accessed at https://www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=40435&EncHid=&modid=&compid=%27,%2740435det%27.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The data underlying this paper will be shared on reasonable request to the corresponding author.
References
Payne BA, Hanson C, Sharma S, Magee LA, von Dadelszen P. Epidemiology of the hypertensive disorders of pregnancy, In Magee LA, von Dadelszen P, Stones W, Mathai M, editors The FIGO Textbook of Pregnancy Hypertension. United Kingdom. The Global Library of Women’s Medicine: 2016. 63–74.
Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021;398:341–54.
Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40:213–20.
Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206:470–5.
Keepanasseril A, Monarrez-Espino J, Vadivelu P, Kumar Maurya D. Risk factors of pulmonary edema in women with preeclampsia from south India: a case-control study. J Hum Hypertens. 2020;34:727–34.
McDermott M, Miller EC, Rundek T, Hurn PD, Bushnell CD. Preeclampsia: Association With Posterior Reversible Encephalopathy Syndrome and Stroke. Stroke. 2018;49:524–30.
Hauspurg A, Jeyabalan A. Postpartum preeclampsia or eclampsia: defining its place and management among the hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2022;226:S1211–S1221.
Cairns AE, Pealing L, Duffy JMN, Roberts N, Tucker KL, Leeson P, et al. Postpartum management of hypertensive disorders of pregnancy: a systematic review. BMJ Open. 2017;7:e018696.
National Institute for Health and Care Excellence Hypertension in pregnancy: diagnosis and management. [Internet] [London]: NICE; 2019 [updated 2023 Apr; cited 2023 Apr 10](NICE guideline NG133). Available from https://www.nice.org.uk/guidance/ng133.
Kumar NR, Hirshberg A, Srinivas SK. Best Practices for Managing Postpartum Hypertension. Curr Obstet Gynecol Rep. 2022;11:159–68.
Sharma KJ, Greene N, Kilpatrick SJ. Oral labetalol compared to oral nifedipine for postpartum hypertension: A randomized controlled trial. Hypertens Pregnancy. 2017;36:44–47.
Veena P, Perivela L, Raghavan SS. Furosemide in postpartum management of severe preeclampsia: A randomized controlled trial. Hypertens Pregnancy. 2017;36:84–89.
Ainuddin J, Javed F, Kazi S. Oral labetalol versus oral nifedipine for the management of postpartum hypertension a randomized control trial. Pak J Med Sci. 2019;35:1428–33.
Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31.
ACOG Committee Opinion No. 767 Summary: Emergent Therapy for Acute-Onset, Severe Hypertension During Pregnancy and the Postpartum Period. Obstet Gynecol 2019;133:e174–80.
Watson DL, Bhatia RK, Norman GS, Brindley BA, Sokol RJ. Bromocriptine mesylate for lactation suppression: a risk for postpartum hypertension? Obstet Gynecol. 1989;74:573–6.
Kirschen GW, Hoyt K, Johnson E, Patel S. Eclampsia with RCVS: Postpartum seizure provoked by methergine. Pregnancy Hypertens. 2022;27:131–3.
Powles K, Gandhi S. Postpartum hypertension. CMAJ. 2017;189:E913.
Joshi A, Beyuo T, Oppong SA, Moyer CA, Lawrence ER. Preeclampsia knowledge among postpartum women treated for preeclampsia and eclampsia at Korle Bu Teaching Hospital in Accra, Ghana. BMC Pregnancy Childbirth. 2020;20:625.
Firoz T, Sanghvi H, Merialdi M, von Dadelszen P. Pre-eclampsia in low and middle income countries. Best practice & research. Clin Obstetrics Gynaecol. 2011;25:537–48.
Anderson PO. Treating Hypertension During Breastfeeding. Breastfeed Med. 2018;13:95–6.
Pucci G, Battista F, Schillaci G. Effects of antihypertensive drugs on central blood pressure: new evidence, more challenges. Hypertens Res. 2014;37:10–2.
McGaughey TJ, Fletcher EA, Shah SA. Impact of Antihypertensive Agents on Central Systolic Blood Pressure and Augmentation Index: A Meta-Analysis. Am J Hypertens. 2016;29:448–57.
Misra A. Ethnic-Specific Criteria for Classification of Body Mass Index: A Perspective for Asian Indians and American Diabetes Association Position Statement. Diabetes Technol Ther. 2015;17:667–71.
Cheymol G. Clinical pharmacokinetics of drugs in obesity. An update. Clin Pharmacokinet. 1993;25:103–14.
Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39:512–9.
Mulrenin IR, Garcia JE, Fashe MM, Loop MS, Daubert MA, Urrutia RP, et al. The Impact of Pregnancy on Antihypertensive Drug Metabolism and Pharmacokinetics: Current Status and Future Directions. Expert Opin Drug Metab Toxicol. 2021;17:1261–79.
Fischer JH, Sarto GE, Hardman J, Endres L, Jenkins TM, Kilpatrick SJ, et al. Influence of gestational age and body weight on the pharmacokinetics of labetalol in pregnancy. Clin Pharmacokinet. 2014;53:373–83.
Author information
Authors and Affiliations
Contributions
AK and AG conceived the study. All authors contributed to the design. DN, and JS carried out the data collection and guarantees data integrity. AK performed statistical analyses. AG, JS, and DN reviewed the analysis, and AK and JS wrote the first draft. All authors contributed to revising and finalization of the paper. AK (corresponding author) guarantees all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, A., Nayak, D., Sharma, J. et al. Comparing the efficacy of oral labetalol with oral amlodipine in achieving blood pressure control in women with postpartum hypertension: randomized controlled trial (HIPPO study—Hypertension In Pregnancy & Postpartum Oral-antihypertensive therapy). J Hum Hypertens 37, 1056–1062 (2023). https://doi.org/10.1038/s41371-023-00841-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-023-00841-x