Abstract
Background
As smoking prevalence has decreased in Canada, particularly during pregnancy and around children, and technological improvements have lowered detection limits, the use of traditional tobacco smoke biomarkers in infant populations requires re-evaluation.
Objective
We evaluated concentrations of urinary nicotine biomarkers, cotinine and trans-3’-hydroxycotinine (3HC), and questionnaire responses. We used machine learning and prediction modeling to understand sources of tobacco smoke exposure for infants from the CHILD Cohort Study.
Methods
Multivariable linear regression models, chosen through a combination of conceptual and data-driven strategies including random forest regression, assessed the ability of questionnaires to predict variation in urinary cotinine and 3HC concentrations of 2017 3-month-old infants.
Results
Although only 2% of mothers reported smoking prior to and throughout their pregnancy, cotinine and 3HC were detected in 76 and 89% of the infants’ urine (n = 2017). Questionnaire-based models explained 31 and 41% of the variance in cotinine and 3HC levels, respectively. Observed concentrations suggest 0.25 and 0.50 ng/mL as cut-points in cotinine and 3HC to characterize SHS exposure. This cut-point suggests that 23.5% of infants had moderate or regular smoke exposure.
Significance
Though most people make efforts to reduce exposure to their infants, parents do not appear to consider the pervasiveness and persistence of secondhand and thirdhand smoke. More than half of the variation in urinary cotinine and 3HC in infants could not be predicted with modeling. The pervasiveness of thirdhand smoke, the potential for dermal and oral routes of nicotine exposure, along with changes in public perceptions of smoking exposure and risk warrant further exploration.
Similar content being viewed by others
Introduction
Tobacco smoke exposure has been studied extensively for its negative health effects and is known to be particularly harmful to children [1]. The health effects of firsthand smoking are now well appreciated and research priorities have shifted to understanding secondhand (SHS) and thirdhand smoking (THS) [2,3,4]. SHS both in utero and in childhood has been repeatedly linked to asthma, as well as sudden infant death syndrome, low birth weight, cancer, dental caries, hearing loss, metabolic syndromes, and a breadth of poor behavioral and cognitive outcomes [3, 5]. SHS occurs as a result of passive exposure to the smoke from the burning end of a cigarette and the smoke breathed out by smokers. THS results from SHS that has been absorbed onto surfaces such as carpeting and upholstery, or settled on dust where it can persist a long period of time and be re-emitted into the air long after a smoking event [6].
Accurately assessing prenatal and early life tobacco smoke exposure is important to understand and reduce childhood asthma and wheeze [7]. Questionnaires are a flexible and relatively inexpensive method of assessing exposure, but biomarkers of tobacco smoke exposure are more accurate, objective, and can be obtained with little burden to the participant using passive urine sample collection. Nicotine is an important component of cigarettes for researchers because it can be reliably detected in humans at concentrations proportional to cigarette smoke exposure [8]. While many metabolites of nicotine can be analyzed, cotinine is the most widely used biomarker of recent tobacco smoke exposure, has an average half-life of 16–19 h in children ages 2 months to 4 years [9, 10], and is heralded as the best biomarker of tobacco smoke exposure [11]. More than 70% of nicotine is metabolically converted into cotinine, which is then metabolically converted into trans-3’-hydroxycotinine (3HC) [10].
When smoking prevalence is high, the use of cotinine to measure tobacco smoke exposure aids in verification of the confirmed exposure. However, over the past two decades the rates of smoking in Canada have been decreasing, particularly during pregnancy and in the presence of children. This is a combined result of policy, increased awareness, product labeling, clinician counseling, and social pressures [11,12,13,14,15,16,17,18]. Smoking rates during pregnancy are trending downwards in Canada—from an estimated at 24% in the 1990s [19], to approximately 17% in 2000 [17], and 11% in 2006 [12]. The Canadian Tobacco Use Monitoring Survey reported that in 2012, 22.8% of Canadian women age 20–24 years and 4.8% of women age 25–44 years reported smoking during their most-recent pregnancy [20]. Excluding the Yukon, Northwest territories, and Nunavut, the overall smoking rate among all Canadians over the age of 15 has dropped as well, from 25% in 1999 to 16% in 2012 [21]. Tests with lower levels of detection for cotinine have been put into practice. However, there is limited knowledge of how these refinements may impact the use of this biomarker for public health in low-smoking settings.
The purpose of this study was to better understand cotinine and 3HC as biomarkers of tobacco smoke exposure in a population with little to no reported tobacco smoke exposure. The research questions were “What is the amount of tobacco smoke exposure in a population of urban Canadian infants?”, “What are the key sources or predictors of tobacco smoke exposure?”, and “What are the research implications and the relevance of nicotine biomarker concentrations to policy makers, researchers and clinicians?”. This allows for a better understanding of how biomarker data could be organized within cohorts, and how low-level nicotine metabolites should be considered and interpreted as biomarkers by environmental health researchers and how policy might reduce exposure to infants.
Materials, participants, and methods
Participants
This study uses secondary data from the CHILD Cohort Study, a four-center (Vancouver, Edmonton, Manitoba, and Toronto) longitudinal, population-based birth-cohort study that enrolled 3455 mother–child pairs between 2008 and 2012. The main focus of CHILD was to identify environmental and genetic determinants of allergic disorders and asthma. The demographic characteristics of the cohort reflect lower smoking rates relative to the general Canadian population, with a very small proportion of mothers in the study reporting that they smoked during pregnancy or during the child’s early life [22]. Data were collected using a combination of questionnaires, in-home visits, and urine samples. Questionnaires and biological samples pertaining to the growing infants’ exposures, lifestyle, and health were collected at various time points, with many questions repeated to allow for longitudinal analyses [23].
Sampling design
Ethics approval was obtained through Simon Fraser University, deeming this project to be a minimal risk study [2018s0608]. Research ethics approval for the overall CHILD study was obtained at each recruitment site and through the Hamilton Integrated Ethics Board (certificate 07-2929).
Urine samples were collected by trained research assistants during the 3-month in-home visits. The procedure involved placing a plastic Tegaderm™ film over the wetting area of the baby’s diaper to prevent urine absorption by the diaper. Cotton pads were placed on top of the film and the baby then wore the diaper for the duration of the home visit. At the end of the visit, the mother removed the diaper, and the research assistant placed the cotton pads into a syringe, aliquoted the sample into six vials, and measured the specific gravity (SG) of the sample using a calibrated refractometer. The samples were stored at –80 degrees Celsius [23]. In the laboratory, β-glucuronidase was used to de-conjugate any glucoronidated cotinine and 3HC molecules. After extraction, the samples were analyzed by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry.
Analyte concentrations were calculated using least-squares linear regression of the peak area ratios of native to internal standards. The limit of detection for both cotinine and 3HC was 0.030 ng/mL [24]. To account for dilution of the urine samples, biomarker concentrations were corrected for SG [22, 23]. The following formula was applied to adjust concentrations to a standardized SG: PC = P × [(SGMED – 1)/(SG – 1)], where P is the uncorrected metabolite concentration, PC is the corrected concentration, SGMED is the median urine specific gravity of the study population (1.004) and SG is the specific gravity measure of the sample. Samples with SG measurements outside the normal human range (3 standard deviations above the median) were excluded from the analysis. Since the metabolite concentrations were approximately log-normally distributed, the concentrations were log-transformed (base 2) prior to correction for SG (i.e., dilution). Concentrations below the level of detection (LOD) were imputed with a concentration between 0 and the LOD in a way that matched the log-normal distribution of the truncated concentration [25]. Concentrations were determined for each of the two metabolites in ng/mL.
Tobacco smoke exposure can be firsthand, secondhand, thirdhand, or any combination of these. While it cannot be determined based on a measure of biomarker concentration alone whether the participant was exposure to recent THS, or SHS some time ago, questionnaires offer a valuable means of characterizing exposure. The exposure variables were taken from three sources: a questionnaire completed by the mother during pregnancy, a parent-completed household exposures questionnaire at 3 months, and a research assistant-completed questionnaire on household exposures completed at the 3-month home visit. Many questions originally asked about the mother’s environment during pregnancy were asked again during the child’s 3-month questionnaire. Potential predictors of exposure were derived from questionnaires that captured smoking-related exposure, housing characteristics, and demographics that have been linked in the literature to tobacco smoke exposure, be it secondhand, or thirdhand [6, 8, 26,27,28,29,30].
Statistical methods
Geometric means and 95% confidence intervals of both cotinine and 3HC concentrations were calculated and reported by predictor variable. For normally distributed variables, t-tests or one-way analysis of variance (ANOVA) tests were used to assess whether the difference in means between levels or groups of a variable were statistically significant. When an ANOVA test was significant, Tukey Honest Significant Differences tests were run to assess multiple pairwise comparisons between multiple levels of a predictor. ANOVA and t-tests assume normality in the distribution of the means being compared. For non-normally distributed variables (determined using a Shapiro–Wilk test), a Wilcoxon test was used in place of a t-test, and a Kruskal–Wallis test in place of an ANOVA analysis. Spearman correlation tests were used when comparing the biomarker concentrations to a continuous predictor variable.
A random forest regression (RFR) of all potential predictor variables (specified a priori) against each metabolite concentration produced variable importance scores for each predictor. RFR is a machine learning technique that allows a system to automatically learn from the data and produce results with minimal subjectivity, offering an objective perspective on the variable selection process. The RFR was set to run models on 1000 trees, a relatively large number for RFR, to help ensure that the average model fit best reflected the data and avoids overfitting. These variable importance scores reflected the overall influence of the predictor in a prediction model, by showing how much the mean squared error (MSE) of a model would increase as a result of the predictor being excluded from the model. The MSE is a measure of closeness of a fitted line to actual data points, with values between zero and infinity. Having a well-fit model with variables of high importance will result in a lower MSE.
Using variable importance scores derived from RFR, we created multivariable linear regression (MLR) models to predict urinary concentrations as the outcome. A MLR model was then selected based on questionnaire variables identified as “important,“ and an a priori assessment to best explained the cotinine concentrations of the sample with detectable metabolite data. The models were built using manual selection, starting with the predictor with the highest variable importance score. The predictor with the next highest score was then added to the model. The added predictor was included if it had appropriate directionality, increased the model’s coefficient of determination (R2), and had a p < 0.15, as opposed to the more-restrictive p < 0.05. This was repeated for many predictor variables (see Appendix) until it was clear that adding more predictors did not further improve model performance. The coefficient of determination and measures of model fit were then used to determine how well questionnaire-based models explain variation in urinary cotinine concentrations in infants. This process was repeated for 3HC, as some variables may be more important for one metabolite than another. Plots of predicted versus observed biomarker concentrations were created for each final model to assess model fit. Regression coefficients and their 95% confidence intervals were reported for each predictor in the final MLR models against the log-transformed cotinine or 3HC concentration. These coefficients were then inverse-log-transformed to reflect the multiplicative change in urinary concentration (i.e., 1.10 means a 10% increase in concentration). The coefficients and back-transformations from unadjusted regression against the log-transformed urinary concentrations were also calculated for each predictor.
A ten-fold cross validation (CV) was also applied to the final 3HC and cotinine prediction models to evaluate prediction error for both models:
-
1.
The dataset was randomly divided into ten sub-groups, or “folds,” with approximately the same number of observations in each group.
-
2.
The predictive model was parameterized based on data from nine of the ten groups.
-
3.
The estimated coefficients were used to predict log-transformed urinary cotinine (or 3HC) concentrations for observations in the excluded group.
-
4.
Steps 1–3 were repeated to obtain predictions for all ten groups and, therefore, all observations.
-
5.
Log-transformed urinary concentration predictions and measurements on the untransformed scale were compared and model performance was evaluated based on R2.
Biomarkers can also be used to predict or verify the exposure level of participants. Density plots of the urinary metabolite concentrations by important questionnaire questions were used to examine the separation of the participants by questionnaire response [31]. We then compared density proportions of these responses within cut-point bounds to gain consensus about what average concentration is found in those who likely have no household exposure, those who have some moderate exposure, and those who have concentrations consistent with confirmed household SHS exposure. In some cases, these levels were continuous (e.g., week of gestation that mother quit smoking), while others were factors (e.g., location). Recommended cut-points for urinary cotinine concentrations from previous studies were assessed [32].
Analysis was undertaken using R version 3.5.1 (2018-07-02).
Results
Of 2017 infants with complete data (Supplementary Fig. 1), 76% had detectable cotinine and 89% had detectable 3HC concentrations. Participants from Manitoba (Winnipeg, Morden, and Winkler) made up the largest proportion of our sample (31%), followed by Vancouver (27%), Edmonton (22%), and Toronto (20%). More than half of our participants had a household income over $100,000/year (55%), lived in a single-family home (56%), and had a mother over age 30 (60%). A minority of the sample (34%) lived in a rented home, and 31% had at least one parent with a history of asthma. Less than 3% reported actively smoking during their pregnancy, while 21% reported being recently exposed to smoke during pregnancy. Nearly 18% of mothers were smokers but had quit prior to their pregnancy. Of the 6% of our sample who did not quit prior to their pregnancy, 64% reported quitting during the pregnancy, leaving just 2% who continued to smoke throughout their pregnancy. Most (62%) of mothers were exclusively breastfeeding their child, 26% were partially breastfeeding, and 12% reported not breastfeeding their child at 3–4 months of age. Only 12% reported that smoking had occurred at the home since the child’s birth, with the majority of household smoking occurring outdoors. Table 1 contains more information of the demographic makeup and self-reported smoke exposure of our sample along with how the urinary concentrations differ based on these characterizations. Metabolite concentrations for additional characteristics can be found in the Supplementary information (Supplementary Table 1a–c), along with their corresponding importance scores (Supplementary Fig. 2a, b and Supplementary Table 2).
Urinary concentrations
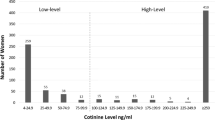
After correcting for urine dilution and imputing those below detection (Supplementary Fig. 3a, b and Supplementary Table 3), the geometric mean cotinine concentration was 0.12 ng/mL (95% CI: 0.11–0.13), and the geometric mean 3HC concentration was 0.22 ng/mL (95% CI: 0.21–0.24) (Table 2). Without correction for dilution, the geometric mean concentrations would be slightly higher at 0.13 ng/mL (95% CI: 0.12–0.14) for cotinine and 0.25 ng/mL (95% CI: 0.21–0.24) for 3HC. The arithmetic mean (and median) concentrations for cotinine were 1.87 ng/mL (median 0.08 ng/mL), and 6.67 ng/mL (median 0.16 ng/mL) for 3HC (Table 2). Based on geometric mean concentrations and density plots (Fig. 1), those who reported no known exposure to tobacco smoke had a geometric mean urinary cotinine concentration of 0.09–0.12 ng/mL, while those who reported some exposure had a more variable range, and those with reported SHS exposure had an average cotinine concentration of at least 0.25 ng/mL depending on the characterizing question.
The distribution of the log-transformed urinary cotinine (left) and 3HC (right) concentrations by two questions: “How many cigarettes (on average) are smoked at the home daily in the child’s early life”, and “How many smokers lived at the home during pregnancy?”. Vertical lines reflect cut-points of assumed exposure to very little to no SHS or THS (left), moderate SHS (middle), and regular SHS exposure (right). The dashed lines reflect 0.25 and 30 ng/mL, while dotted lines indicate 0.50 and 60 ng/mL. Density curves were not created for categories with two or fewer participants.
Concentration cut-points
Applying cut-points of presumed exposure to our samples, based on threshold identified by Benowitz et al. for a population of adolescents with low-smoking exposure [32], we suspect that 1.6% (n = 33) of our sample were actively exposed or exposed to recent heavy SHS (≥30 ng/mL), 21.9% (n = 441) were exposed to moderate SHS (0.25–30 ng/mL), and 76.5% (n = 1543) were exposed to low second or THS to none at all (<0.25 ng/mL). The same concentration thresholds did not apply well to 3HC concentrations, so cotinine thresholds of 0.25 and 30 ng/mL were doubled. When the data are visualized in density curves, the cut-point line would need to be shifted for the 3HC concentrations to match the location on the curve for the cut-point on the cotinine concentration density curves, based upon key questionnaire responses (Fig. 1). Our proposed cut-point values are based on corrected concentrations, and uncorrected concentrations would require a slightly higher cut-point to similarly differentiate infants with suspected tobacco smoke exposure. For uncorrected cotinine, this would mean using 0.31 and 37.6 ng/mL instead of 0.25 and 3 ng/mL. For uncorrected 3HC, cut-points would be approximately 0.63 and 75 ng/mL instead of 0.50 and 60.0 ng/mL.
Prediction models
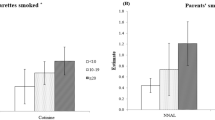
The final model selected for cotinine (Fig. 2) used fewer predictors than the final 3HC model (Fig. 3). The selected MLR models predicted 31% of the log-transformed cotinine concentration using 13 predictors and 41% of the variation in the log-transformed 3HC concentration using 19 predictors (Fig. 4). 3HC may require more predictors because its higher concentrations are more sensitive to a breadth of exposure sources. Ten-fold CV found that the models performed slightly poorer, at 30% and 37%, respectively. The most important predictors were whether or not the mother smoked and/or quit prior to the pregnancy, the number of cigarettes smoked at the home during the pregnancy, and whether someone had smoked at the home since the child’s birth, though all predictors added value to the model. Adjusted model coefficients show that those who had not quit prior to their pregnancy had an infant with twice the urinary cotinine concentration compared with an infant of a mother who reportedly never smoked, and mothers actively smoking during their pregnancy had an infant with more than five times the urinary cotinine concentration of infants of non-smoking mothers. A more detailed description of the predictor variables as well as the regression model coefficients and their multiplicative change equivalents can be found in the Supplementary information (Supplementary Tables 4 and 5a, b and Supplementary Fig. 4a, b).
Standard regression coefficients (point) and their 95% confidence intervals (line) are displayed for each variable in a model predicting log-transformed urinary cotinine concentration. Variables related to secondhand smoke are shown in red, variables not related to smoking in blue, and variables related to household characteristics in gray. Note that the ingestion of breast milk is an indirect source of nicotine from secondhand or thirdhand smoke, or dietary nicotine sources. Intervals with a point estimate of the regression coefficients that are displayed as a circle are based on bivariate analysis between each predictor and urinary cotinine, while estimates displayed with a triangle reflect estimates from a multivariable model.
Standard regression coefficients (point) and their 95% confidence intervals (line) are displayed for each variable in a model predicting log-transformed urinary trans-3’-hydroxycotinine concentration. Variables related to secondhand smoke are shown in red, variables not related to smoking in blue, and variables related to household characteristics in gray. Note that the ingestion of breast milk is an indirect source of nicotine from secondhand or thirdhand smoke, or dietary nicotine sources. Intervals with a point estimate of the regression coefficients that are displayed as a circle are based on bivariate analysis between each predictor and urinary trans-3’-hydroxycotinine concentration while estimates displayed with a triangle reflect estimates from a multivariable model.
Discussion
Urinary nicotine metabolite concentrations
Nicotine exposure was nearly ubiquitous in our study population, with nearly 90% of the urine samples having some detectable level of nicotine metabolite(s). This is similar to a Korean cohort study in which 88% of infants from non-smoking homes had infants with detectable concentrations of cotinine [33] and a study of American adolescents that found that nearly all participants were exposed to tobacco smoke but that the majority of exposure was from light SHS and THS exposure sources [32]. Compared to similar studies [32, 34,35,36], the urinary concentrations of nicotine metabolites in our study sample were reflective of those with low SHS and/or THS exposure.
3HC had a larger interquartile range (0.38 vs. 0.19 ng/mL) than cotinine, indicating that it varies more in the population and may be a more sensitive biomarker of nicotine exposure. On average, the 3HC concentrations were at least twice that of the cotinine concentrations. Some have proposed that this is due to the difference in the metabolism of nicotine and cotinine between infants and adults. Neonates and children under 1 year of age have lower nicotine metabolism rates, with a nicotine half-life three to four times longer than adults, but the metabolism of cotinine into 3HC in neonates is similar to that of older children and adults [37, 38]. Part of this difference may be due to initially low hepatic blood flow in the transition away from umbilical flow [39], and the lower metabolic enzyme activity for nicotine in early life [40].
Prediction models
Our study found that prediction models explained less than half of the variation in urinary biomarker concentrations. Overall, approximately half of the predictors included in our models pertained directly to reported SHS exposure (Figs 2 and 3). The predictive multiple linear regression models explained 31.4% of log-transformed cotinine, and 40.9% of 3HC concentrations in our cohort. This prediction is consistent but slightly lower than was reported by other studies of urinary cotinine (33–45%) in children with at least one household smoker that differs from the CHILD cohort [41, 42]. Overall, questionnaire-based models similarly predict biomarker levels across a range of exposure patterns. Parental reports are helpful in characterizing smoke exposure, but less helpful when completed by non-smoking mothers [36]. Others have had more success in predicting serum cotinine (R2 of 61%) when including indoor air nicotine levels, duration of exposure and ventilation measures [43]. Plotted measured vs. predicted concentrations show some fanning of the observations, particularly at higher concentrations suggesting that the residuals have non-constant variance and that confidence intervals and significance tests should be interpreted with caution (Fig. 4). While our models show how much we could reasonably predict of our biomarker concentrations, they may not be the most parsimonious models. The first two predictors added to the model explained 23% of the log-transformed cotinine concentration, and subsequently added predictors adding between 0.5 and 2% to the R2 while meeting our inclusion criteria. Some may argue that a model with just these predictors would be a sufficient balance between predictability and the number of predictors required.
Our models are not more predictive of the variation in cotinine concentrations for a few reasons. The half-life of cotinine leads to higher variability for those with low or inconsistent exposure [44]. Another reason may be that our questionnaires are subject to reporting bias and do not adequately detect all aspects of smoke exposure, namely THS exposure. Finally, low but detectable levels of cotinine and 3HC may result from nicotine exposure not related to tobacco smoke, such as diet [45].
While cotinine concentrations remain a reliable way of verifying questionnaire reported tobacco smoking exposure in populations with some level of SHS, they alone may not adequately reflect true exposure in populations with little direct exposure. Prediction models created to explain small-for-gestation size in a Chinese cohort of pregnant women found that self-reported smoking better predicted small-for-gestational-age than cotinine measures [46]. As researchers report lower levels of detection, nicotine exposure in many populations becomes nearly ubiquitous and may begin to lose its predictive power as a proxy of health risks linked to tobacco smoke exposure.
The model to predict 3HC contained more variables, with some of these additional variables related to carpeting, home ownership, and the season at the time of sample collection. More influence due to suspected thirdhand tobacco smoke reservoirs than was found in the cotinine model may exist because 3HC may be a more sensitive marker for chronic, low-level exposure sources. Important predictors to both models include tobacco smoke exposure to the mother during pregnancy and the occurrence of household smoking since the child’s birth. Dwelling type, paternal education, income, and breastfeeding status were used in predicting both urinary cotinine and 3HC. Breastfeeding was likely a surrogate for other characteristics related to tobacco exposure, explaining why those who were breastfed the least had the highest concentrations of urinary cotinine and 3HC before adjusting for smoking factors. Multi-unit or lower-income housing is also more likely to experience THS from previous inhabitants, and SHS from neighboring units [47].
In a prediction model where collinearity and correlated predictors were present, the coefficients became less reliably interpretable. Sparse data bias likely plays a role, a problem where coefficients for levels of a variable with relatively few participants are easily skewed. For these reasons, assessing the geometric mean concentrations by our predictors becomes more helpful than consideration of the adjusted model coefficients.
Incorporation of machine learning
Random forest analysis identified exposure items relating to breastfeeding, thirdhand sources of tobacco smoke (e.g., carpeting, area rugs), and household characteristics (e.g., single-family detached vs. shared buildings) as being particularly important in predicting the nicotine metabolite concentrations. The advantages of using machine learning methods in environmental epidemiology were recently described as an intelligent way to assess a magnitude of potential exposures and pathways [48].
Secondhand smoke
Prenatal smoking exposure and related behaviors were important predictors of early life cotinine and 3HC concentrations. Approximately 92% of mothers reported that they never smoked when they became pregnant. Half of the remaining sample of mothers quit during pregnancy. However, one-fifth of mothers reported some recent exposure to tobacco smoke during their pregnancy, a proportion closer to the 24% of mothers who had smoked at some point before or during their pregnancy. Only 7.4% of the participants reported some tobacco smoke to their child at 3–4 months, and 4.4% of the sample reported that the baby had been exposed to tobacco smoke in the past week. By comparison, the 2009 Canadian Maternity Experiences Survey reported that 22% of women smoking daily or occasionally before pregnancy and this proportion reduced to 10.5% during the last 3 months of pregnancy [20]. Of the 183 parents that reported cigarettes were smoked at the home daily in the child’s early life, 62% reported that consumption averaged between 1 and 5 cigarettes per day.
In households where smoking was reported, it was predominantly reported to occur outdoors. This behavior limits the extent and proximity of smoking to the child, and so reflects that parents have some understanding of the dangers of smoking indoors or around an infant. While 12% of our sample reported that someone had smoked at the home since the child’s birth, 11% of the sample reported that smoking occurred outside of the house, and 1.6% reported that smoking occurs near a window or in the garage. Only 0.5% reported smoking occurred inside the home. Although the proportion of smokers in the home was similar before and after birth, the location of where the smoking occurred changed slightly suggesting that avoidance behavior may be greater in the presence of a child than a pregnant woman. While the motivations behind and effects of primarily outdoor smoking are encouraging, smoking outdoors was still associated with approximately three times the urinary cotinine concentrations in infants compared to infants who lived in a home with no household smoking. This suggests the pervasiveness of SHS that overrides efforts to minimize exposure by smoking outdoors.
Socioeconomic factors
Household income, education level, and maternal age were inversely related to the child’s urinary concentrations. Younger mothers tend to have less formal education, and are less successful in quitting smoking [19]. The dwelling type of the home was important in both prediction models. Those living in apartments or multi-family homes had higher urinary concentration of cotinine and 3HC. SHS is a prominent issue for those in multi-family and multi-unit housing [49]. Children living in rented homes (23%) had higher concentrations of urinary cotinine and 3HC than those who owned their home. Income, education, and housing are all interrelated factors that influence the likelihood of a child being exposed to tobacco smoke in their early life. The inclusion of these variables in prediction models are expected to add value because they capture smoke exposure not already captured by the questions directly related to SHS exposure. We hypothesize that younger, less educated and lower-income mothers may be more likely to have visiting friends and family who smoke or visit public areas where smoking occurs, which may not be reflected in SHS-related responses to our questionnaires.
Thirdhand smoke
THS is a relatively novel concept, with the term first coined in the late 2000s [50]. THS occurs when SHS interacts with the physical environment and is heavily adsorbed onto surfaces and accumulates in dust [49]. This contamination of surfaces and fabrics from SHS persists to be later released into the air. THS, or residual tobacco smoke pollutants, can be re-emitted as a gas or can react with environmental oxidants or other pollutants to create secondary exposures [6, 49]. Predictors related to household reservoirs of THS, such as carpeting and furnishings, were important in modeling 3HC, though not cotinine. Carpeted flooring and area rugs in the home have been shown to harbor tobacco combustion products [51]. THS exposure can remain elevated for 6 months after smoking cessation, depending on a number of reservoirs such as fabrics, carpets, and dust in the home [52, 53]. Upholstery, carpets, and other fabrics absorb the smoke more readily than other surfaces and can off-gas their contaminants over longer periods of time [52, 54, 55]. Therefore, even if a mother quit smoking prior to her pregnancy, the home may have residual exposure well into her pregnancy.
Characterizing and capturing THS remains a challenge to assessing tobacco smoke exposure. Questionnaires may not accurately capture the complex chemistry of combustion, furnishings, ventilation and human behavior. Thirdhand exposure raises significant challenges for policy makers given the lack of human studies that consider this exposure [6, 49]. Only in the past few years have researchers begun to tease out the effects and pervasiveness of THS [54, 56,57,58]. As a relatively new phenomena in public health, the public lacks awareness and understanding of THS, which may be an important component of tobacco control [59].
There appears to be a lack of understanding of the far-reaching effects of SHS and THS. Some researchers have proposed that nearly 85% of tobacco smoke is invisible [55]. While it may not be odorous or visible, these light exposures still carry risks [60], thereby posing a challenge in knowledge translation and implementation of controls. Although 12% of the sample reported that someone had smoked at the home, only 7.4% reported that any child had any exposure to smoking in early life, illustrating a gap in understanding. Only a quarter of those who reported a household smoker also reported that their baby had some level of smoking exposure. These inconsistencies may be due to social desirability bias, or the lack of awareness of the pervasiveness of SHS and THS exposure. For example, it likely is not widely understood that smoke particles settle onto dust or fabric surfaces, and that day to day activities like crawling can later facilitate THS exposure to an infant.
Quantifiably characterizing exposure
Density plots (Fig. 1) show that cut-points in urinary concentrations meant to characterize exposure are not perfect reflections of true exposure. Similar to the findings of Dostal et al. [31], there was notable overlap in the distribution of the infants’ urinary concentrations by predictors meant to characterizing them as exposed or not. This makes the recommendation of cut-points for this population more difficult. We cautiously agree with the continued use of 0.25 ng/mL of corrected cotinine concentration as a cut-point to differentiate those from some confirmed moderate SHS to those exposed to more intermittent or THS sources. For 3HC, we recommend doubling the concentration used as cut-point in the distribution of cotinine. The use of the metabolites to characterize tobacco smoke exposure in infants of a population with relatively low exposure is challenged by the natural variability that comes with intermittent exposure, the half-life of these metabolites, and the potential for nicotine to be sourced from diet [32, 61] as well as tobacco smoke.
Limitations
We acknowledge that the participants in our study may not reflect the vulnerable population most at-risk for tobacco smoke exposure. While the low prevalence of tobacco smoke exposure in this cohort should be celebrated, this cohort may underestimate true exposure experienced by the Canadian population. Cohort studies like the CHILD Study are also generally more affluent, educated, and predisposed to the diseases under study (asthma and allergies) when compared to the general population. Mothers who smoke during pregnancy are more likely to be low socioeconomic status, non-immigrants, single, have a chronic disease, be without a family doctor, and parenting without having attended prenatal classes [12, 62]. As a result, our recommendations from this study can only be applied to populations similar to our cohort and may not be suitable for individuals from demographics linked to higher cigarette use. At the time of data collection of questionnaire responses used by this study, e-cigarette use was not yet popularized, and marijuana use was still illegal in Canada. We also did not have measure of the maternal use of nicotine-supplementing smoking cessation products, which may contribute to an infant’s nicotine intake through breastfeeding but not be reflective of inhaled smoke. We recognize that we have been unable to assess for more novel means of tobacco smoke exposure and encourage that future analysis make use of data on marijuana and e-cigarette use when characterizing exposure. As well, we were unable to consider variability in CYP2A6 enzyme function in participants, which could affect nicotine metabolism and clearance.
Conclusion and future directions
While our smoking rates were low and parents appeared to be motivated to avoid exposing the child to smoke, nicotine metabolites were nearly ubiquitous in infants’ urine. Our results suggest that tobacco smoke questionnaire models may not accurately explain the majority of variation in cotinine or 3HC concentrations within a population with relatively little smoking exposure. Questions that best explained the variation in nicotine metabolite concentrations included whether the mother quit smoking prior to pregnancy, the number of cigarettes smoked daily at the home during pregnancy, and the presence of household smoking since the child’s birth. Tobacco smoke exposure models could use a combination of questionnaire and biomarker data to more accurately assess risk or consider additional exposure assessment tools like air sampling, dust and surface wipe sample collection. Future models should also consider to what extent smoking is occurring at work, hospitality venues, parks, preschools and other places where families with young children gather. As the Canadian population overall is reducing the rates of smoking, particularly during pregnancy and when around children—both as a result of policy and social pressures—our understanding of cotinine as a measure of smoking needs to change. As smoking rates drop, low-level concentrations may be influenced by thirdhand, dietary, and therapeutic sources of nicotine. Researchers need to be aware of the context of their sample population and be purposeful in the selection of the appropriate exposure measures.
With growing public health concern over e-cigarettes and marijuana smoking, this study highlights a lack of awareness of light SHS and THS as an additional frontier of tobacco smoke research and action. Physicians and care providers are encouraged to have conversations with their patients and their families about SHS and THS exposure and possible remedies. This study encourages researchers to look for better means of exposure measurement in populations with relatively low or intermittent tobacco smoking. Future work should focus on collaboration between qualitative and quantitative analysis to better understand the motivations behind smoking cessation or reducing exposure to children, and how the implications of SHS are understood by the public. The pervasiveness of THS and any nicotine ingested through dust or breast milk not necessarily from smoke exposure should be investigated in populations with low reported exposure.
References
Chilmonczyk BA, Salmun LM, Megathlin KN, Neveux LM, Palomaki GE, Knight GJ, et al. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. N Engl J Med. 1993;328:1665–9.
Bird Y, Staines-Orozco H. Pulmonary effects of active smoking and secondhand smoke exposure among adolescent students in Juárez, Mexico. Int J Chronic Obstr Pulm Dis. 2016;11:1459–67.
Zhou S, Rosenthal DG, Sherman S, Zelikoff J, Gordon T, Weitzman M. Physical, behavioral, and cognitive effects of prenatal tobacco and postnatal secondhand smoke exposure. Curr Probl Pediatr Adolesc Health Care. 2014;44:219–41.
Lee M, Ha M, Hong Y-C, Park H, Kim Y, Kim E-J, et al. Exposure to prenatal secondhand smoke and early neurodevelopment: Mothers and Children’s Environmental Health (MOCEH) study. Environ Health. 2019;18:22.
Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicological Sci. 2010;116:364–74.
Jacob P, Benowitz NL, Destaillats H, Gundel L, Hang B, Martins-Green M, et al. Thirdhand smoke: new evidence, challenges, and future directions. Chem Res Toxicol. 2017;30:270–94.
Silvestri M, Franchi S, Pistorio A, Petecchia L, Rusconi F. Smoke exposure, wheezing, and asthma development: a systematic review and meta-analysis in unselected birth cohorts. Pediatr Pulmonol. 2015;50:353–62.
Hukkanen J. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115.
Benowitz NL, Hukkanen J, Jacob P III. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60.
Dempsey DA, Sambol NC, Jacob P, Hoffmann E, Tyndale RF, Fuentes-Afflick E, et al. CYP2A6 genotype but not age determines cotinine half-life in infants and children. Clin Pharmacol Therapeutics. 2013;94:400–6.
Centers for Disease Control and Prevention. Biomonitoring Summaries – Cotinine. 2016. https://www.cdc.gov/biomonitoring/cotinine_biomonitoringsummary.html Accessed Dec. 6, 2018.
Al-Sahab B, Saqib M, Hauser G, Tamim H. Prevalence of smoking during pregnancy and associated risk factors among Canadian women: a national survey. BMC Pregnancy Childbirth. 2010;10:24.
Cawkwell PB, Lee L, Shearston J, Sherman SE, Weitzman M. The difference a decade makes: smoking cessation counseling and screening at pediatric visits. Nicotine Tob Res. 2016;18:2100.
Asbridge M. Public place restrictions on smoking in Canada: assessing the role of the state, media, science and public health advocacy. Soc Sci Med. 2004;58:13–24.
Noar SM, Hall MG, Francis DB, Ribisl KM, Pepper JK, Brewer NT. Pictorial cigarette pack warnings: a meta-analysis of experimental studies. Tob Control. 2016;25:341–54.
Hammond D, Fong GT, Mcdonald PW. Impact of the graphic Canadian warning labels on adult smoking behaviour. Tob Control. 2003;12:391–5.
Millar WJ, Hill G. Pregnancy and smoking. Health Rep. 2004;15:53–6.
Sánchez-Rodríguez JE, Bartolomé M, Cañas AI, Huetos O, Navarro C, Rodríguez AC, et al. Anti-smoking legislation and its effects on urinary cotinine and cadmium levels. Environ Res. 2015;136:227–33.
Connor SK, McIntyre L. The sociodemographic predictors of smoking cessation among pregnant women in Canada. Canadian. J Public Health. 1999;90:352–5.
Government of Canada. Canadian Tobacco Use Monitoring Survey (CTUMS) 2012: supplementary tables. 2013. https://www.canada.ca/en/health-canada/services/publications/healthy-living/canadian-tobacco-use-monitoring-survey-2012-supplementary-tables.html#t7 Accessed Mar. 4, 2021.
Government of Canada. Canadian Tobacco Use Monitoring Survey (CTUMS) 2012. 2013. https://www.canada.ca/en/health-canada/services/publications/healthy-living/canadian-tobacco-use-monitoring-survey-ctums-2012.html Accessed Mar. 4, 2021.
McLean K. Comparison of questionnaire responses with biomarkers of environmental tobacco smoke exposure in a Canadian birth cohort at three months of age. Simon Fraser University; 2013.
Takaro TK, Scott JA, Allen RW, Anand SS, Becker AB, Befus AD, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort study: assessment of environmental exposures. J Exposure Sci Environ Epidemiol. 2015;25:580–92.
Moraes TJ, Lefebvre DL, Chooniedass R, Becker AB, Brook JR, Denburg J, et al. The Canadian Healthy Infant Longitudinal Development Birth Cohort Study: biological samples and biobanking. Paediatr Perinat Epidemiol. 2015;29:84–92.
Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ health Perspect. 2004;112:1691–6.
El-Mohandes AAE, Kiely M, Blake SM, Gantz MG, El-Khorazaty MN. An intervention to reduce environmental tobacco smoke exposure improves pregnancy outcomes. Pediatrics. 2010;125:721–8.
Benowitz NL, Dains KM, Dempsey D, Herrera B, Yu L, Jacob P. Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine Tob Res. 2009;11:954–60.
Dahlström A, Ebersjö C, Lundell B. Nicotine exposure in breastfed infants. Acta Paediatrica. 2004;93:810–6.
Chen A, Krebs NM, Zhu J, Sun D, Stennett A, Muscat JE. Sex/gender differences in cotinine levels among daily smokers in the Pennsylvania Adult Smoking Study. J Womens Health. 2016;26:1222–30.
.Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23:252–61.
Dostál M, Milcová A, Binková B, Kotěšovec F, Nožička J, Topinka J, et al. Environmental tobacco smoke exposure in children in two districts of the Czech Republic. Int J Hyg Environ Health. 2008;211:318–25.
Benowitz NL, Jain S, Dempsey DA, Nardone N, St Helen G, Jacob P. Urine cotinine screening detect nearly ubiquitous tobacco smoke exposure in urban adolescents. Nicotine Tob Res. 2017;19:1048–54.
Kim J, Lee K. Characterization of urinary cotinine in non-smoking residents in smoke-free homes in the Korean National Environmental Health Survey (KoNEHS). BMC Public Health. 2016;16:538.
Becker AB, Manfreda J, Ferguson AC, Dimich-Ward H, Watson WTA, Chan-Yeung M. Breast-feeding and environmental tobacco smoke exposure. Arch Pediatrics Adolesc Med. 1999;153:689–91.
Olivieri M, Bodini A, Peroni DG, Costella S, Pacifici R, Piacentini GL, et al. Passive smoking in asthmatic children: effect of a “smoke-free house” measured by urinary cotinine levels. Allergy asthma Proc. 2006;27:350–3.
Matt GE, Wahlgren DR, Hovell MF, Zakarian JM, Bernert JT, Meltzer SB, et al. Measuring environmental tobacco smoke exposure in infants and young children through urine cotinine and memory-based parental reports: empirical findings and discussion. Tob Control. 1999;8:282–9.
Dempsey D, Jacob P, Benowitz N. Nicotine metabolism and elimination kinetics in newborns. Clin Pharmacol Therapeutics. 2000;67:458–65.
Collier AM, Goldstein GM, Shrewsbury RP, Davis SM, Koch GG, Zhang C-A, et al. Cotinine elimination and its use as a biomarker in young children involuntarily exposed to environmental tobacco smoke. Indoor Built Environ. 1994;3:353–9.
Gow PJ, Ghabrial H, Smallwood RA, Morgan DJ, Ching MS. Neonatal hepatic drug elimination. Pharmacol Toxicol. 2001;88:3–15.
Tateishi T, Nakura H, Asoh M, Watanabe M, Tanaka M, Kumai T, et al. A comparison of hepatic cytochrome P450 protein expression between infancy and postinfancy. Life Sci. 1997;61:2567–74.
Wong GC, Berman BA, Hoang T, Bernaards C, Jones C, Bernert JT, et al. Children’s exposure to environmental tobacco smoke in the home: comparison of urine cotinine and parental reports. Arch Environ Health Int J. 2002;57:584–90.
Matt GE, Hovell MF, Zakarian JM, Bernert JT, Pirkle JL, Hammond SK. Measuring secondhand smoke exposure in babies: the reliability and validity of mother reports in a sample of low-income families. Health Psychol. 2000;19:232–41.
Kalkbrenner AE, Hornung RW, Bernert JT, Hammond SK, Braun JM, Lanphear BP. Determinants of serum cotinine and hair cotinine as biomarkers of childhood secondhand smoke exposure. J Exposure Sci Environ Epidemiol. 2010;20:615–24.
Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, et al. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob Res. 2011;13:202–8.
Siegmund B, Leitner E, Pfannhauser W. Determination of the nicotine content of various edible nightshades (Solanaceae) and their products and estimation of the associated dietary nicotine intake. J Agric food Chem. 1999;47:3113–20.
Xie C, Wen X, Niu Z, Ding P, Liu T, He Y, et al. Comparison of secondhand smoke exposure measures during pregnancy in the development of a clinical prediction model for small-for-gestational-age among non-smoking Chinese pregnant women. Tob Control. 2015;24:e179–87.
Matt GE, Quintana PJE, Hoh E, Zakarian JM, Dodder NG, Record RA, et al. Persistent tobacco smoke residue in multiunit housing: legacy of permissive indoor smoking policies and challenges in the implementation of smoking bans. Preventive Med Rep. 2020;18:101088–101088.
Saglani S, Custovic A. Childhood asthma: advances using machine learning and mechanistic studies. Am J Respiratory Crit Care Med. 2019;199:414–22.
Burton A. Does the smoke ever really clear? Thirdhand smoke exposure raises new concerns. Environ health Perspect. 2011;119:A70–4.
Acuff L, Fristoe K, Hamblen J, Smith M, Chen J. Third-hand smoke: old smoke, new concerns. J Community Health. 2016;41:680–7.
Matt GE. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control. 2004;13:29–37.
Matt GE, Quintana PJE, Zakarian JM, Hoh E, Hovell MF, Mahabee-Gittens M, et al. When smokers quit: exposure to nicotine and carcinogens persists from thirdhand smoke pollution. Tob Control. 2017;26:548–56.
Bekö G, Morrison G, Weschler CJ, Koch HM, Pälmke C, Salthammer T, et al. Dermal uptake of nicotine from air and clothing: experimental verification. Indoor Air. 2018;28:247–57.
Leung LT, Ho SY, Wang MP, Lam TH. Secondhand smoke from multiple sources, thirdhand smoke and respiratory symptoms in Hong Kong adolescents. Nicotine Tob Res. 2018;20:192–8.
Gee IL, Semple S, Watson A, Crossfield A. Nearly 85% of tobacco smoke is invisible-a confirmation of previous claims. Tob Control. 2013;22:429.
Hang B, Wang P, Zhao Y, Sarker A, Chenna A, Xia Y, et al. Adverse health effects of thirdhand smoke: from cell to animal models. Int J Mol Sci. 2017;18:932.
Northrup TF, Jacob P, Benowitz NL, Hoh E, Quintana PJE, Hovell MF, et al. Thirdhand smoke: state of the science and a call for policy expansion. Public Health Rep. 2016;131:233–8.
Northrup TF, Matt GE, Hovell MF, Khan AM, Stotts AL. Thirdhand smoke in the homes of medically fragile children: assessing the impact of indoor smoking levels and smoking bans. Nicotine Tob Res. 2016;18:1290–8.
Díez-Izquierdo A, Cassanello-Peñarroya P, Lidón-Moyano C, Matilla-Santander N, Balaguer A, Martínez-Sánchez JM. Update on thirdhand smoke: a comprehensive systematic review. Environ Res. 2018;167:341–71.
Matt GE, Quintana PJE, Destaillats H, Gundel LA, Sleiman M, Singer BC, et al. Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect. 2011;119:1218–26.
Davis RA, Stiles MF, DeBethizy JD, Reynolds JH. Dietary nicotine: a source of urinary cotinine. Food Chem Toxicol. 1991;29:821–7.
Cui Y, Shooshtari S, Forget EL, Clara I, Cheung KF. Smoking during pregnancy: findings from the 2009–2010 Canadian Community Health Survey. PLoS ONE. 2014;9:e84640.
Acknowledgements
We are grateful to all the families who took part in this study, and the whole CHILD team, which includes interviewers, nurses, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, and receptionists. Thank you to the CHILD study team for dataset access, assistance, and analytic advice. Finally, thank you to the CHILD families, investigators, and staff involved in the CHILD study for devoting their valuable time, energy, and resources to the success of this national cohort.
Funding
No financial assistance was received in support of this secondary analysis study. The Canadian Institutes of Health Research (CIHR), and the Allergy, Genes, and Environment (AllerGen) Network of Centres of Excellence (NCE) provided core funding for CHILD. Additional support for the CHILD Study has been provided by Health Canada, Environment Canada, Canada Mortgage and Housing Corporation, the Sick Children’s Hospital Foundation, Don & Debbie Morrison, the Silver Thread Foundation, and the Childhood Asthma Foundation. JP has received graduate student research award funding from Asthma-Canada and AllerGen NCE, as well as graduate scholarships from Simon Fraser University in support of this project. MBA is supported by the Canada Research Chairs program. This work has been funded in part by the Canadian Institutes of Health Research (CIHR), Allergy, Genes, and Environment Network of Centers of Excellence (AllerGen NCE), Asthma Canada, and Simon Fraser University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parks, J., McLean, K.E., McCandless, L. et al. Assessing secondhand and thirdhand tobacco smoke exposure in Canadian infants using questionnaires, biomarkers, and machine learning. J Expo Sci Environ Epidemiol 32, 112–123 (2022). https://doi.org/10.1038/s41370-021-00350-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-021-00350-4