Abstract
Children are at risk of exposure to secondhand smoke. We aimed to evaluate the extent of their exposure to it in relation to their parents’ smoking status by using biomarkers relevant to smoking. We evaluated 847 school-age children (6–12 years) who lived with their parents, using data from the Korea National Health and Nutrition Examination Survey 2016–2018. Secondhand smoke exposure in children of non-smoking and smoking parents was assessed by measuring urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and cotinine concentrations. Overall, the parents of 482 (55.1%) children smoked and those of 392 (44.9%) children did not smoke. After adjusting for covariates, significantly higher concentrations of NNAL (β = 0.482, standard error [S.E.] = 0.065, P < 0.001) and cotinine (β = 0.472, S.E. = 0.06, P < 0.001) were found in children of smoking parents than in children of non-smoking parents. Children of parents who smoked a higher number of cigarettes showed higher NNAL and cotinine concentrations than children of non-smoking parents. Children with both parents who smoked showed the highest NNAL and cotinine concentrations. Children of smoking parents are at a higher risk of exposure to secondhand smoke. A smoke-free environment must be maintained to protect children from the harmful effects of secondhand smoke. Therefore, comprehensive national anti-smoking policies are required.
Similar content being viewed by others
Introduction
Individuals exposed to secondhand smoke (SHS) are subject to > 250 carcinogens and toxic chemicals1,2. Exposure to SHS is as harmful as smoking itself because it can cause diseases, such as lung cancer, cardiovascular disorders, and chronic diseases, among non-smokers3. Approximately 603,000 individuals, including children, die each year from SHS exposure, accounting for approximately 1.0% of the global mortality rate4.
Children are especially vulnerable to SHS5,6. Exposure to SHS in children leads to early death7 and increases the risk of sudden infant mortality syndrome8, acute respiratory infections, and severe asthma symptoms9,10,11. SHS exposure is also associated with severe adverse health effects, such as the slowing of lung growth in children. At the global level, around 40% of children are still exposed to SHS at home or other places frequently visited by children, and most exposures are related to parental smoking11. Recently, there have been considerable efforts to prevent children from being exposed to SHS. According to a nationwide survey in Korea, 98% of the respondents were aware that SHS exposure was harmful to children's health, and 94% reported that smoking was banned at home for this reason12.
Recently, however, there has been evidence that this effort to prevent SHS exposure does not provide sufficient protection form all of the effects of smoking13. This is due to concerns about thirdhand smoke (THS) exposure. THS exposure is described as the intake of chemicals produced by smoking that are absorbed into surfaces, such as walls, furniture, or house dust, and released back into the air over a long period of time. THS exposure could result in the bodily absorption of new toxic substances produced from reactions between chemicals12. Even if parents smoke out on a balcony or someplace several feet outside the house, the airborne smoke could enter the house and spread indoors, causing SHS or THS exposure14. Therefore, children can still be exposed to toxic substances from smoking.
The major biomarkers of SHS and THS include 4-(methylnitrosoamino)-(3-pyridyl)1-butanol (NNAL) and cotinine, which are metabolites of nicotine15. Cotinine is one of the most commonly used tobacco exposure biomarkers in SHS and THS studies and has the advantage of very high specificity and sensitivity in screening tests15,16. NNAL is one of the metabolites of N-nitrosamines, a carcinogen derived from nicotine, and has the advantage of a long half-life of approximately 3 weeks17. Many studies have investigated the association between parents’ smoking status, SHS and THS exposure biomarkers in children11,14,15,18. However, most previous studies have focused on adolescents in terms of smoking probability or on children with certain diseases13,19.
Thus, this study aimed to investigate the association between parental smoking and NNAL and cotinine concentrations, as biomarkers of SHS and THS exposure, in children. Furthermore, children’s NNAL and cotinine concentrations were investigated according to the number of cigarettes smoked and smoking patterns in parents. We targeted school-age children living with their parents and have a low likelihood of smoking on their own, based on the premise that children who live with at least one smoking parent are more likely to be exposed to SHS and THS.
Results
The mean age was 8.79 years (standard deviation: 1.9); 447 (51.1%) and 427 (48.9%) children were male and female, respectively. Table 1 shows the general characteristics of the study population. Of the 874 children, 392 (44.9%) and 482 (55.1%) had smoking parents and non-smoking parents, respectively. The median NNAL and cotinine concentrations were 1.4 (interquartile range [IQR] 1.9) and 0.4 (IQR 0.4) in children with parents who smoked, respectively. Meanwhile, the corresponding values were 0.8 (IQR 0.9) and 0.2 (IQR 0.3) in children with parents who did not smoke, respectively.
Table 2 shows the association between children’s NNAL and cotinine concentrations and parents’ smoking status after adjusting for all confounding variables. There was a positive association between parents’ smoking status and children’s NNAL (β = 0.482, standard error [S.E.] = 0.065, P < 0.001) and cotinine (β = 0.472, S.E. = 0.06, P < 0.001) concentrations; the association was stronger for smoking parents than for non-smoking parents.
Table 3 shows the results of the subgroup analyses stratified by independent variables. Children’s sex and body mass index (BMI), their parents’ education level, household income, and type of housing showed positive associations with children’s NNAL and cotinine concentrations. Parents currently smoking and with an education higher than college level showed the weakest association with children’s NNAL (β = 0.407, S.E. = 0.079, P < 0.001) and cotinine (β = 0.414, S.E. = 0.066, P < 0.001) concentrations. Additionally, parents currently smoking and with the lowest income level tended to show the strongest association with children’s NNAL (β = 0.762, S.E. = 0.167, P < 0.001) and cotinine (β = 0.634, S.E. = 0.155, P < 0.001) concentrations.
Figure 1 shows the association between the number of cigarettes smoked and smoking patterns with the children’s NNAL and cotinine concentrations. The higher the number of cigarettes smoked, the higher the children’s NNAL and cotinine concentrations. Children of parents who smoked > 20 cigarettes had the highest NNAL (β = 0.825, S.E. = 0.096, P < 0.001) and cotinine (β = 0.604, S.E. = 0.085, P < 0.001) concentrations. Further, the children’s NNAL and cotinine concentrations were also high when only the father (NNAL: β = 0.444, S.E. = 0.066, P < 0.001; cotinine: β = 0.443, S.E. = 0.058, P < 0.001) or mother (NNAL: β = 0.738, S.E. = 0.244, P = 0.003; cotinine: β = 0.561, S.E. = 0.241, P = 0.009) smoked. When both parents smoked, the NNAL (β = 1.209, S.E. = 0.204, P < 0.001) and cotinine (β = 1.111, S.E. = 0.179, P < 0.001) concentrations were the highest (Supplementary Table S1).
Association of children’s 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and cotinine concentrations with the number of cigarettes smoked by the parents (a) and smoking pattern (b). Adjusted for children’s sex, age, body mass index, secondhand smoke exposure (house), and secondhand smoke exposure (public), and for parents’ household income, type of housing, region, age, education level, private health insurance, drinking status, and year of evaluation. The reference group is the group of parents who are non-smokers.
Discussion
Most studies on the association between parents’ smoking status, SHS and THS exposure biomarkers have focused on adolescents in terms of smoking probability or on children with certain diseases. In this study of school-age children in Korea, at least one out of two children was living with a parent who smoked, and the NNAL and cotinine concentrations were higher in children whose parents were smokers. Further analysis confirmed that the higher the number of cigarettes smoked by both parents, the higher the degree of SHS exposure in children. To the best of our knowledge, no previous study in Asia has evaluated both NNAL and cotinine levels in school-age children to determine the extent of SHS exposure.
Our results are similar to those of previous studies13,20. However, it should be noted that our study targeted school-age children. School-age children are particularly vulnerable to second-hand smoke compared to other ages11. The adverse effects of parents smoking on their children's health, such as respiratory symptoms, can be reduced as their children grow older, due to spending less time with the parents21. Adolescents tend to spend more time outside the home, school-age children spend a lot of time at home and stay close to their parents, suggesting that living with parents who smoke can be a strong predictor of increased exposure to substances included in cigarettes. The highest NNAL and cotinine concentrations were observed in children when both parents smoked. These results were similar to those of previous studies22,23. Additionally, although a direct correlation is difficult, compared to when both the parents were non-smokers, the children’s NNAL and cotinine concentrations were higher when only the mother smoked than when only the father smoked. Compared to children of non-smokers, children whose mothers alone smoked or whose both parents were smokers were 2–13 times more likely to be exposed to SHS at home24. However, a recent study showed that SHS exposure among adolescents is associated with paternal smoking17. In fact, worldwide smoking rates are higher for men than for women25. Thus, smoking abstinence by paternal figures is often chosen as the first strategy to reduce children’s exposure to indirect smoking13,26. However, for school-age children, time spent with the mother tends to be more than two-fold longer than the time spent with the father. Therefore, smoking abstinence in mothers should also be considered27. Consistent with the results of previous research24,28,29, we found that the higher the number of cigarettes smoked, the higher the children’s NNAL and cotinine concentrations, regardless of the child’s age. This could be because as the number of cigarettes smoked by the parents increased, the amount of harmful substances adhering to their clothes and skin also rose, indirectly exposing the children.
In the subgroup analysis, the association trend was significant according to sex (male and female), BMI (underweight, normal weight, and overweight), household income (Q1, Q2, Q3, and Q4), and parents’ education level (middle school or lower and college or higher). In the case of BMI, the NNAL and cotinine concentrations showed a tendency to be highest in the overweight group, but only the cotinine concentration was statistically significant. This is mainly due to the fact that NNAL is produced by smoking, whereas cotinine may be affected by diet30. We assumed that obese children consume more food than children of a normal weight, and that the amount of cotinine accumulated through food intake may influence the statistical significance. More detailed research on cotinine and food intake is needed in the future. NNAL and cotinine concentrations were both highest in children from the low-income group. People from this group have less awareness regarding the risks of exposure to SHS; thus, these children may be more vulnerable31. The children of parents with a higher level of education had lower NNAL and cotinine concentrations. This supports the premise that education level has a greater influence on SHS exposure than income, and individuals with higher education levels are less likely to smoke and, in cases where they do, are more likely to quit31,32.
Our study shows that even after controlling SHS exposure at home and in public places, many children are still likely to have high levels of NNAL, cotinine concentration due to their parents' smoking habits. This indicates that while the prohibition of smoking at home and in public show a highly negative correlation with children’s exposure to SHS29,31, these policies alone cannot fully protect them from the adverse effects of SHS exposure due to parental smoking. This can be explained based on the results of previous studies in which children living with smoker parents had higher cotinine and NNAL concentration than children with non-smoker parents even if they do not smoke at home15,33. In other words, these results are the effect of THS, which allows children to inhale harmful substances by combining household fibers, clothing, sedimentation dust and surfaces with toxic substances related to external cigarettes, even if parents control their exposure at home15. Parents’ smoking may cause substances such as tobacco-specific nitrosamine (TSNA) to be adsorbed by all indoor home surfaces, which then release these substances into the air34,35. Several studies have warned that THS is as damaging as SHS because it releases harmful substances similar to those released through SHS, causing DNA mutations and damage34,36.
The World Health Organization states that there is no safe level of exposure to SHS and THS a pollutant that causes serious illnesses in adults and children. Hence, the only effective way to protect the population from the harmful effects of exposure to SHS and THS is to maintain a 100% non-smoking environment37. Implementing physical measures or anti-smoking measures at home, such as the opening of windows or doors or removing cigarette smoke using a ventilator fan, is ineffective in preventing children’s exposure to cigarette smoke. This is because only a completely non-smoking environment can prevent SHS and THS exposure in the home11,33. Parents’ smoking cessation eliminates threats to their own and their children’s health, so relevant policies should be encouraged. For this, it is first necessary to raise awareness of the risks of SHS and THS. It was found that the rate of smoking cessation attempts increased after the campaign to revitalize the hazard awareness of SHS and THS38. Even if the adverse effects of THS cannot be completely eliminated, these efforts can increase openness to laws prohibiting smoking in the home, and furthermore, the rate of successful cessation will be even higher if quit smoking intervention policies are implemented together38,39. Raising awareness of SHS and THS could be an effective strategy to protect children from tobacco exposure.
This study has some limitations. First, we used cross-sectional data. Therefore, the cause and effect and the direction of the relationships observed cannot be determined. Second, the results of this study were based on self-reported data. In this self-report, a vaper may report him or herself as a smoker despite not using flammable cigarettes, or a “social smoker” might report as a non-smoker. Thus, the number of cigarettes smoked may have been underestimated or overestimated, and some survey questions may be subject to recall bias. As a result, we cannot eliminate the likelihood that some smokers will be classified as nonsmokers or nonsmokers as smokers. Third, despite our efforts to control for confounding factors, not all covariates affecting NNAL and cotinine concentrations may have been considered. Lastly, This study sample derived from KNHANES was limited because NNAL test was randomly conducted to only a portion of the participants, thus we gathered 3 years of KNHANES data (2016–2018).
Despite these limitations, our study has important implications. This study evaluated the Association between parental smoking and children's exposure to SHS and THS using a well-defined nationally representative data in Korea. Our findings also support previous results. We targeted school-age children and thus minimized the bias related to smoking status. Further, we controlled for both SHS exposure in public and at home. These factors were not well-considered in previous studies. Furthermore, while analyses based on cotinine measurements were commonly performed in previous studies, our research is meaningful in that we additionally analyzed the concentration of NNAL, which has a longer half-life.
Our study demonstrated that children with parents who smoked are at a higher risk of exposure to SHS and THS, implying that individual efforts to avoid smoking in the presence of children may be an insufficient alternative. The best way to protect children from toxic substances from exposure to smoking is to quit smoking. This requires comprehensive anti-smoking arbitration policies, such as improving awareness of how to protect children from smoking substances.
Methods
Study population
This study was based on data from the 2016–2018 KNHANES VII and the secondary analysis of a large dataset. The KNHANES is a nationwide population-based cross-sectional survey conducted annually since 1998 under the direction of the Centers for Disease Control and Prevention of the Ministry of Health and Welfare to accurately assess the national health and nutritional status40.
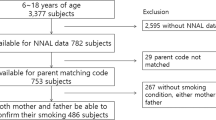
The total number of respondents for the 2016–2018 KNHANES was 24,269. Participants included single parents, but if they did not match the criteria for a parent–child relationship (n = 1,384); had no data on age (n = 21,019) for those between 6 and 12 years; and those without data on NNAL levels, cotinine levels, or other independent variables (n = 992) were excluded. Finally, a total of 847 participants were included in the study (Fig. 2). KNHANES data is publicly accessible and ethical approval is not required for the use of the data. This data were collected with prior consent before participating in the survey and respondents' information was completely anonymized for use for research purposes40.
Variables
The dependent variables were NNAL and cotinine concentrations, which are biomarkers of indirect smoking exposure. They were used to quantify the children’s degree of exposure to SHS. NNAL and cotinine concentrations during the KNHANES were measured in the urine and were analyzed using high-performance liquid chromatography−tandem mass spectrometry using Agilent 1,200 Series with Triple Quadrupole 5500 (AB Sciex, USA17,20. The limit of detection (LOD) was 0.27399 ng/mL for cotinine and 0.1006 pg/mL for NNAL, and was calculated by dividing it by 2 referring to prior study41,42. In addition, a random subsample (1/2 or 1/3 of the total sample) of subjects aged 6 years or older was used for gathering NNAL data42. The main independent variable was the parents’ smoking status, classified as “smoker” if any one parent replied “yes” or as “non-smoker” if both parents replied “no” to the question “Do you currently identify yourself as a smoker?” Independent variables that were considered to be potential confounding variables included sociodemographic, economic, and health-related characteristics, as well as the survey year. Sociodemographic characteristics included children’s sex, age, parents’ age, education level, type of housing, and region. Economic characteristics included the parental household income and ownership of private health insurance. Health-related characteristics included parents’ drinking status and children’s exposure to SHS at home and in public.
Statistical analysis
Univariate linear regression was used to assess the relationship between children’s NNAL and cotinine concentrations and parents’ smoking status; sociodemographic, economic, and health-related variables; and survey year. Prior to the multiple logistic regression analysis, we performed a log-transformation of the NNAL and cotinine values to ensure normality. Multiple regression analysis was performed while controlling for covariates to analyze the association between parental smoking status and log-transformed NNAL and cotinine concentrations in children. We performed subgroup analyses stratified by the parents’ smoking status and multiple regression analysis to examine the associations of children’s NNAL and cotinine concentrations according to the children’s sex and BMI and the parental household income and education level. Furthermore, after adjusting for covariates, we classified the number of cigarettes smoked (0, < 10, 10–19, and ≥ 20) and smoking patterns (non-smoking parents, father only, mother only, both parents) and determined their associations with children’s NNAL and cotinine concentrations using multiple regression analysis. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Inc.). Statistical results were considered significant at a P-value of < 0.05.
Data availability
The datasets generated during and/or analyzed during the current study are available in the Korea National Health and Nutrition Examination Survey (KNHANES) 2016–2018, https://knhanes.cdc.go.kr/knhanes/main.do.
References
International Agency for Reasearch on Cancer IARC monographs on the evaluation of carcinogenic risks to humans, Vol. 83 (2004).
Talhout, R. et al. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 8, 613–628 (2011).
Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. (2010).
Öberg, M., Jaakkola, M. S., Woodward, A., Peruga, A. & Prüss-Ustün, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. The Lancet 377, 139–146 (2011).
Kang, S., Joo, J., Jang, S. & Park, E. Association of exposure to secondhand smoke at home with early age at menarche in South Korea. Public Health 185, 144–149 (2020).
Homa, D. M. et al. Vital signs: disparities in nonsmokers’ exposure to secondhand smoke—United States, 1999–2012. MMWR Morb. Mortal. Wkly Rep. 64, 103 (2015).
Diver, W. R., Jacobs, E. J. & Gapstur, S. M. Secondhand smoke exposure in childhood and adulthood in relation to adult mortality among never smokers. Am. J. Prev. Med. 55, 345–352 (2018).
Anderson, H. R. & Cook, D. G. Passive smoking and sudden infant death syndrome review of the epidemiological evidence. Thorax 52, 1003–1009 (1997).
California Environmental Protection Agency. Air Resources Board Proposed Identification of Environmental Tobacco Smoke as a Toxic Air Contaminant (University of California, Berkeley, 2005).
Cook, D. G. & Strachan, D. P. Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax 54, 357–366 (1999).
US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. (U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2006).
Kim, J. et al. Factors associated with beliefs among adults in Korea about the health effects of Thirdhand smoke on Children. J. Environ. Health Sciences 44, 90–97 (2018).
Park, M. B. Living with parents who smoke predicts levels of toxicant exposure in children. Sci. Rep. 10, 11173 (2020).
Gehring, U. et al. Comparison of parental reports of smoking and residential air nicotine concentrations in children. Occup. Environ. Med. 63, 766–772 (2006).
Torres, S., Merino, C., Paton, B., Correig, X. & Ramírez, N. Biomarkers of exposure to secondhand and thirdhand tobacco smoke: Recent advances and future perspectives. Int. J. Environ. Res. Public Health 15, 2693 (2018).
Kang, Y. H., Lee, Y.-J., Kong, S.-Y., Lee, D. H. & Yun, Y.-H. Usefulness of urinary cotinine test to discriminate between smokers and nonsmokers in Korean adolescents. Korean J. Lab. Med. 24, 155–159 (2004).
Vardavas, C. I. et al. Exposure to different sources of second-hand smoke during pregnancy and its effect on urinary cotinine and tobacco-specific nitrosamine (NNAL) concentrations. Tobacco Control 22, 194–200 (2013).
Rosen, L. J. et al. Parental receptivity to child biomarker testing for tobacco smoke exposure: A qualitative study. Patient Educ. Couns. 98, 1439–1445 (2015).
Oddoze, C. et al. Urinary cotinine and exposure to parental smoking in a population of children with asthma. Clin. Chem. 45, 505–509 (1999).
Marano, C., Schober, S. E., Brody, D. J. & Zhang, C. Secondhand tobacco smoke exposure among children and adolescents: United States, 2003–2006. Pediatrics 124, 1299–1305 (2009).
Cook, D. G. & Strachan, D. P. Health effects of passive smoking. 3. Parental smoking and prevalence of respiratory symptoms and asthma in school age children. Thorax 52, 1081–1094 (1997).
Sims, M. et al. Trends in and predictors of second-hand smoke exposure indexed by cotinine in children in England from 1996 to 2006. Addiction 105, 543–553 (2010).
Rudatsikira, E., Siziya, S., Dondog, J. & Muula, A. S. Prevalence and correlates of environmental tobacco smoke exposure among adolescents in Mangolia. Indian J. Pediatr. 74, 1089–1093 (2007).
Orton, S., Jones, L. L., Cooper, S., Lewis, S. & Coleman, T. Predictors of children’s secondhand smoke exposure at home: A systematic review and narrative synthesis of the evidence. PLoS ONE 9, e112690 (2014).
Hawkes, S., Buse, K. & Yoon, S.-Y. Gender-responsive tobacco control: evidence and options for policies and programmes. in Secretariat of the WHO Framework Convention on Tobacco Control (World Health Organization, 2018).
Wang, Y. et al. Relationship between caregivers’ smoking at home and urinary levels of cotinine in children. Int. J. Environ. Res. Public Health 11, 12499–12513 (2014).
Sayer, L. C., Bianchi, S. M. & Robinson, J. P. Are parents investing less in children? Trends in mothers’ and fathers’ time with children. Am. J. Soc. 110, 1–43 (2004).
Hughes, S. C. et al. Children’s exposure to secondhand smoke at home in Seoul, Korea. Asian Pac. J. Cancer Prev. 9, 491–495 (2008).
Fernández, M. F. et al. Trends in children’s exposure to second-hand smoke in the INMA-Granada cohort: An evaluation of the Spanish anti-smoking law. Environ. Res. 138, 461–468 (2015).
Haufroid, V. & Lison, D. Urinary cotinine as a tobacco-smoke exposure index: A minireview. Int. Arch. Occup. Environ. Health 71, 162–168 (1998).
Gatzke-Kopp, L. M. et al. Magnitude and chronicity of environmental smoke exposure across infancy and early childhood in a sample of low-income children. Nicotine Tob. Res. 21, 1665–1672 (2019).
Yi, O. et al. Association between environmental tobacco smoke exposure of children and parental socioeconomic status: A cross-sectional study in Korea. Nicotine Tob. Res. 14, 607–615 (2011).
Hoh, E. et al. Environmental tobacco smoke as a source of polycyclic aromatic hydrocarbons in settled household dust. Environ. Sci. Technol. 46, 4174–4183 (2012).
Sleiman, M. et al. Inhalable constituents of thirdhand tobacco smoke: Chemical characterization and health impact considerations. Environ. Sci. Technol. 48, 13093–13101 (2014).
Ferrante, G. et al. Third-hand smoke exposure and health hazards in children. Monaldi Arch. Chest Dis. 79, 1 (2013).
Jacob, P. III. et al. Thirdhand smoke: New evidence, challenges, and future directions. Chem. Res. Toxicol. 30, 270–294 (2017).
World Health Organization. Protection from Exposure to Second-Hand Tobacco Smoke: Policy Recommendations. (2007).
Rosen, L. J., Noach, M. B., Winickoff, J. P. & Hovell, M. F. Parental smoking cessation to protect young children: A systematic review and meta-analysis. Pediatrics 129, 141–152 (2012).
Winickoff, J. P. et al. Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics 123, e74–e79 (2009).
Kweon, S. et al. Data resource profile: The Korea national health and nutrition examination survey (KNHANES). Int. J. Epidemiol. 43, 69–77 (2014).
Krishnamoorthy, K., Mallick, A. & Mathew, T. Model-based imputation approach for data analysis in the presence of non-detects. Ann. Occup. Hyg. 53, 249–263 (2009).
Korea Ministry of Health and Welfare. The Report of the Korean National Health and Nutritional Examination Survey in 2016–2018 (Korea Ministry of Health and Welfare, Sejong, 2020).
Author information
Authors and Affiliations
Contributions
S.H.J., B.N.J., S.H.K., J.H.J., and E.-C.P. contributed to the study concept and design. S.H.J., J.H.J. analyzed and interpreted the data. S.H.J., B.N.J. wrote the manuscript. S.H.J., B.N.J., S.H.K., J.H.J., and E.-C.P. critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jeong, S.H., Jang, B.N., Kang, S.H. et al. Association between parents’ smoking status and tobacco exposure in school-age children: assessment using major urine biomarkers. Sci Rep 11, 4536 (2021). https://doi.org/10.1038/s41598-021-84017-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84017-y
This article is cited by
-
Carcinogenic and tobacco smoke-derived particulate matter biomarker uptake and associated healthcare patterns among children
Pediatric Research (2023)
-
Do parental Smoking Behaviors Affect Children's Thinness, Stunting, and Overweight Status in Indonesia? Evidence from a Large-Scale Longitudinal Survey
Journal of Family and Economic Issues (2023)
-
Associations between environmental tobacco smoke exposure and oral health symptoms in adolescents
BMC Oral Health (2022)
-
Prevalence and correlates of current tobacco use and non-user susceptibility to using tobacco products among school-going adolescents in 22 African countries: a secondary analysis of the 2013-2018 global youth tobacco surveys
Archives of Public Health (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.