Abstract
The mammalian skeletal system is densely innervated by both neural and vascular networks. Peripheral nerves in the skeleton include sensory and sympathetic nerves. The crosstalk between skeletal and neural tissues is critical for skeletal development and regeneration. The cellular processes of osteogenesis and angiogenesis are coupled in both physiological and pathophysiological contexts. The cellular and molecular regulation of osteogenesis and angiogenesis have yet to be fully defined. This review will provide a detailed characterization of the regulatory role of nerves and blood vessels during bone regeneration. Furthermore, given the importance of the spatial relationship between nerves and blood vessels in bone, we discuss neurovascular coupling during physiological and pathological bone formation. A better understanding of the interactions between nerves and blood vessels will inform future novel therapeutic neural and vascular targeting for clinical bone repair and regeneration.
Similar content being viewed by others
Introduction
Bone tissue is vital for all mammalian species as a living organ system that allows body structure and movement. Bone remodeling maintains bone strength and mineral-calcium homeostasis, and it involves bone cells, including bone-resorbing osteoclasts, bone-forming osteoblasts, osteocytes, and bone-lining cells1,2. The skeleton has a rich innervation of both sensory and sympathetic nerve fibers in conjunction with the bone vascular system3,4,5.
Although it has only recently begun to be in the spotlight, the regulatory role of the skeletal, nervous system, including sensory, sympathetic, and parasympathetic nerves, has been examined for many years3,4,5,6,7,8,9. Each peripheral nerve fiber subtype has distinct functions within bone10,11,12. In addition to performing the general roles of nerve fibers, such as providing motor control of muscle fibers, delivering sensory information from the periphery to the central nervous system (CNS), and regulating autonomic functions13, skeletal nerve fibers also provide paracrine stimuli, such as neuropeptide Y (NPY), leptin, calcitonin gene-related peptide (CGRP), and substance P (SP)3,14,15,16. Moreover, adrenergic receptors and other receptors for neuropeptides residing on osteoblasts and osteoclasts express axon guidance molecules such as semaphorins, netrins, and neurotrophins, which further potentiate nerve ingrowth17,18. Neurotrophins promote the survival of neurons and target innervation by activating the receptors TrkA and p7519. Semaphorins serve as axon guidance molecules during nervous system development20. The growth of peripheral nerve fibers and the initial innervation and neurotransmission into the bone tissue regulate bone remodeling and metabolism21. Furthermore, nerve ingrowth is an essential upstream mediator of endochondral ossification during bone growth22 and intramembranous bone formation during growth and repair7,8,23. These accumulating study findings suggest that the stimuli provided by peripheral afferent neurons represent an essential requirement for proper skeletal growth, homeostasis, and repair7,10,11.
Recent studies suggest that the bone vascular system plays a significant role in bone remodeling. Bone marrow arteries and capillaries are essential components of bone multicellular units24,25,26,27. The bone vasculature is also important for bone marrow homeostasis28,29. Declines in vascular function and perfusion are predicted to cause reduced bone volume, bone density, osteoblast activity, and increased osteoclastic resorption30,31,32. Accumulating evidence suggests that the skeletal, nervous, and vascular systems influence one another. Recent works have documented that the majority of sympathetic and parasympathetic nerves are located in association with blood vessels10,33. Moreover, nerves accompanying blood vessels innervate the primary and secondary ossification center during embryonic skeletal development, whereby CGRP-expressed sensory nerves are detected near osteoblasts and the area of high osteogenic activity34,35. However, the extent to which nerve-vessel coupling contributes to improvements in skeletal remodeling and metabolism remains an intriguing unanswered question. As such, this review presents evidence of the roles of neurovascular coupling in bone regeneration, as well as the clinical entity of heterotopic ossification (HO), and summarizes the potential clinical implications of neurovascular coupling in bone tissue.
Nerve and vascular distribution within the bone
The skeleton is densely innervated by sensory and sympathetic nerves. Peptidergic sensory nerve fibers are prevalent in the skeletal system of vertebrate species and express the neuropeptides CGRP and SP3,36. The general role of sensory nerves is to transmit internal and external information from the periphery to the CNS for processing of sensory information. The autonomic nervous system (ANS), including sympathetic and parasympathetic nerves, is a part of the efferent peripheral nervous system (PNS) that innervates and regulates all organs and involuntary functions in the body. Sympathetic nerves are abundant in bone and express tyrosine hydroxylase (TH)10,36,37. Both sensory and autonomic nerve fibers in bone are closely linked to bone metabolism and bone remodeling cellular activities.
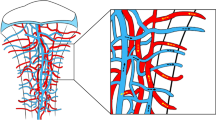
Although sensory and autonomic nerve fibers have been shown to have mixed effects, both types of nerve fibers are strongly associated with blood vessels during skeletal development and bone repair. During development, blood vessels run into the medullary cavity with their accompanying nerves along the shaft within the bone38,39, and the collection of sinusoidal vessels returns to the central nutrient vein along with nerves38,40. In cortical bone, sensory and sympathetic nerve fibers are largely confined to vascularized Haversian canals41. Moreover, CGRP+ and NF200+ sensory nerve fibers are associated with blood vessels located in the Haversian canals, and TH+ sympathetic nerve fibers are observed in the bone marrow and wrapping around blood vessels42. In our prior observations, Thy1-YFP pan-neuronal reporter activity closely paralleled CD31+ blood vessels during calvarial defect healing8. A similar nerve-vessel coupling was observed by Chartier et al., in which CGRP+ sensory nerves and TH+ sympathetic nerves were colocalized with CD31+ blood vessels in the periosteum41. Moreover, neuropeptide Y (NPY) fibers were identified around cerebral blood vessels43 and in the medullary cavity accompanying blood vessels in the long bones11.
In summary, the skeleton is covered by both neural and vascular networks. Their intimate spatial relationship suggests that they modulate each other. However, the interaction between the nerves and vessels remains largely unknown, which has been a trending topic of research in recent years.
Neurovascular coupling during bone repair and regeneration
Nerve signals during fracture repair and regeneration processes
Primary sensory and sympathetic axons covering the periosteal bone are required for bone tissue repair and fracture healing by rearranging and reinnervating the nerve fibers into injury sites3,44. The nerve growth factor (NGF) is the most important neurotrophin and neuropeptide involved in the regulation of the growth, maintenance, proliferation, and survival of sensory neurons45. Tropomyosin receptor kinase A (TrkA) is the high-affinity neurotrophin receptor for NGF, and TrkA+ sensory neurons are extensively innervated in the periosteum42,46. A previous study in mouse long bone stress fracture repair showed that NGF expression within a nascent callus was considerably upregulated at 3 days and up to 7 days postfracture with a concomitant increase in axonal ingrowth and sprouting, followed by vascularization and ossification7. Consistent with this study, our recent work demonstrated that during calvarial bone defect healing, neurotrophic NGF and TrkA signaling play a crucial role in normal calvarial bone regeneration8. Moreover, Tomlinson et al. reported that communications between osteoblasts and sensory nerves via NGF-TrkA signaling are required for mechanical loading-induced bone formation47. In contrast, studies in murine injury models have revealed that surgical and chemical denervation of sensory nerves resulted in impaired fracture healing and decreased bone formation48,49,50,51. In sensory nerves, the disruption of TrkA catalytic activity by the small molecule 1NMPP1 impairs the sequential events of long bone fracture and flat bone defect healing. This disruption also leads to reduced osteoblast activity and abrogated blood vessels in the defect area7,8. In addition to NGF-TrkA signaling, common sensory nerves that express CGRP and SP have been shown to play a role in rat tibial fracture healing, whereby sensory nerve innervation was present on the periosteal layers and influenced bone regeneration by stimulating newly produced bony calli52,53. The regulatory role of sensory nerves in bone formation was also observed in both mammalian models and human patients with nerve dysfunction. Moreover, reduced peripheral innervation was associated with delayed fracture healing54, and the loss of the sensory nerves led to a reduction in new bone quality in rabbits55.
In regard to the ANS contribution to the bone healing process, in contrast to the sensory nerves, NPY-positive autonomic nerves were found to be decreased at the fracture or defect regions at early time points, while the presence of NPY-expressing nerve ingrowth and sprouting through Y1 receptor-associated signaling was observed. This accelerated the late phase of bone formation by increasing the osteogenic differentiation of mesenchymal stem cells (MSCs)56,57,58. A recent finding has demonstrated that the disruption of sympathetic nerve stimulation via sympathectomy hinders motor function and locomotion and delays fracture callus formation in the late phase of the bone healing process59. Although the relationship between the nervous system and the cells that drive bone formation is not fully understood, it is known that neurotransmitters can affect osteoblast formation60,61. In addition, osteoblasts are regulated by peripheral nerve-derived norepinephrine62,63, and bone contributes to the completeness of the microenvironment by accommodating nerves in the bones to provide bone stability13. Recently, our group used spatial transcriptomics of the developing cranium to reveal the importance of nerve signaling in the regulation of bone patterning and the differentiation of bone precursors, highlighting the important role of neural signaling in tissue regulation23.
Given our data and previously reported outcomes demonstrating the essential role of NGF-TrkA signaling, CGRP- and SP-associated sensory nerves, and autonomic nerve fibers in bone repair and remodeling, both sensory and autonomic innervation are employed for stimulating vascular ingrowth and sprouting, increasing osteogenic differentiation, and facilitating the delivery of osteogenic factors to the fracture site.
Vascular signals during bone repair and regeneration processes
Crucial roles of the vascular system for bone and bone marrow function, such as bone homeostasis and hematopoietic activity, have also been suggested. Bone blood vessels deliver O2, nutrients, and systemic hormones to bone and bone marrow, and they remove waste products. Bone blood vessels also transport immune and precursor cells to and from bone and bone marrow and are fundamental components of the bone multicellular unit24,25,64. In addition, blood vessels enriching bone plays a significant role in hematopoietic stem cell (HSC) niches, which are essential for bone homeostasis29. It is presumed that the dysfunction of bone and bone marrow is highly associated with dysfunction in the skeletal vascular system31,32,65. For example, declines in bone mass were found to be associated with diminished bone blood flow and impaired vasodilator function of bone blood vessels66,67,68 in rats and humans.
Normally, skeletal blood vessel sprouting and vascular function-related substances, such as nitric oxide, are augmented quickly following fracture incidence due to rapid increases in blood flow to the fracture region69. Blood supply is a challenge for bone repair and regeneration. To achieve proper bone repair, the local microcirculation must be intact. Many factors stimulate angiogenesis, some of which include fibroblast growth factors, transforming growth factors, platelet-derived growth factors, and vascular endothelial growth factors (VEGF)70,71. VEGF has been shown to increase angiogenesis, which increases vascular permeability and the recruitment of MSCs and osteoprogenitor cells. VEGF has the ability to attract MSCs and promote differentiation into osteogenic cell types. Studies have shown that when VEGF is inhibited, bone formation and invasion of the vasculature are decreased71,72. When the VEGF receptor is antagonized, this inhibits bone regeneration by bone morphogenetic protein (BMP) 4 and 2, as osteogenesis is induced by these osteogenic cytokines. Kaigler et al. showed that when VEGF is inhibited, angiogenesis and osteogenesis are inhibited73. However, new vascularization and bone regeneration occurred with the controlled release of VEGF. In addition, BMPs act synergistically with VEGF to enhance cell survival, cartilage formation, resorption, bone formation, and mineralization71,72. BMPs and VEGF synergistically enhance angiogenesis, osteogenic genes, and MSC responses to BMP6, while the ratio of VEGF/BMP is an important mediator. A high ratio of VEGF/BMP2 was found to produce well-formed mineralized bone but a decreased amount of bone compared to a low ratio of VEGF/BMP222.
Neurovascular crosstalk and potential sources of bone repair and regeneration
Neurovascular coupling is a combination of vascular smooth muscle cells, neurons, and astrocyte glial cells. Both neurons and astrocytes respond to increases in vasoactive metabolites that adjust blood flow. Glutamate released from presynaptic neurons in the presence of oxygen stimulates the activation of nitric oxide synthase (NOS), which produces NO to act directly as a dilator on parenchymal arterioles74. Astrocytes respond to glutamate in the presence of oxygen to activate a cascading pathway to produce arachidonic acid, which leads to the production of epoxyeicosatrienoic acid and prostaglandins75. By having dual stimuli, there is an even greater effect on the increase in blood flow76. The neurovascular theory for nerves in bone emerged in the 1980s following the observation that blood flow was increased in the joints of patients with diabetic neuropathy77. In addition, the crucial functions of both blood vessels and nerve stimuli in hematopoietic niches have been described. Nociceptive nerves are required to induce HSC mobilization, while sympathetic and sensory nerves collaboratively maintain HSC homeostasis in the bone marrow78,79.
After a bone is fractured, growth factors and cytokines recruit osteoprogenitor cells to the affected site80,81. As the bone remodels, angiogenesis allows for an invasion of vascular networks into the bone to restore normal circulation to remove/replace the necrotic bone tissue82, and nerve fibers are closely associated with blood vessels7,22. Our results showed that nerve ingrowth occurred before revascularization and contributed to vascular regrowth8. Moreover, impaired nerve reinnervation is accompanied by decreased vascularity and reduced osteogenic capacity, which delayed bone defect healing8. Single-cell RNA sequencing data from a trauma-induced HO model revealed that NGF was highly expressed in perivascular stem cells and that NGF-TrkA signaling increased angiogenesis and vascular regeneration9,83,84,85. The inhibition of TrkA signaling resulted in delayed vascular invasion in primary and secondary ossification centers, which further reduced the length and volume of the femur in a rodent model22. We also demonstrated that chemical genetic inhibition of TrkA signaling led to blunted sensory nerve reinnervation and diminished revascularization, thereby impairing fracture healing7.
VEGF is one of the primary mediators promoting angiogenesis while binding to VEGF receptors 1 and 2 promotes bone repair and has direct effects on the nervous systems in terms of axon branching and survival86. Conversely, NGF exhibits angiogenic properties87. The injection of NGF directly into the site of the defect enhances vascularization and osteogenesis84. SP could promote angiogenesis and osteogenic differentiation by upregulating VEGF expression and enhancing BMP2 signaling61,88,89. In addition, cholinergic neurons release the neuropeptide vasoactive intestinal peptide, which improves cranial defect healing and bone formation by increasing VEGF expression in MSCs and stimulating angiogenesis90.
Clinically, vascularization and neurotization are important in the care of bone trauma, tumors, and infection. A study by Fan et al. showed how tissue-engineered bone (TEB) is affected by implanted vessels and nerves by using a mouse model where the femur was exposed, and osteotomy was performed91. Bone tissue gradually increased in TEB along with the femoral blood vessels and saphenous nerve implantation at the defect site91. However, new bone formation was highest following saphenous nerve implantation, whereas vascular implantation led to more and better-quality blood vessels91. Interestingly, vascular implantation led to increased CGRP and NPY expression, which was presumably associated with the nerves in the walls of the blood vessels, which in turn stimulated neurotization and osteoblast activity and inhibited osteoclasts91. Moreover, the levels of CGRP and NPY were found at the highest levels with saphenous nerve implantation, which led to better neurotization of the TEB and more blood vessels91. Taken together, these findings demonstrate that the increase in vasculature was due to the capillary network within the nerve fibers, which led to an increase in osteogenesis.
Given the roles of nerve-vascular interactions and functions in bone repair and regeneration, therapeutic targeting of neural and vascular signals may be a promising therapeutic option for bone regeneration.
The role of neurovascular coupling in ectopic bone formation
Nerve signals during the ectopic bone formation process
In the context of HO, the observation of a coregulation of nerves and bone implicates nerves as a critical mediator in HO pathogenesis. HO has been frequently observed in those who have paroxysmal sympathetic hyperactivity92,93. Moreover, the incidence of HO is dramatically increased in patients with traumatic CNS injury94. This evidence suggests an association between HO and peripheral nerves. Peripheral nerves enable HO through the release of molecules such as SP and the induction of neuroinflammation95. In addition, Nguyen et al. reported BMP2 as a dual-function cytokine that promotes HO via osteoinductive action and neuroinflammation induction96. Salisbury et al. demonstrated that sensory nerves contribute to HO development and that the inhibition of sensory nerves led to reduced HO formation by suppressing BMP2-mediated SP and CGRP97. Altogether, these findings suggest that HO formation depends on peripheral innervation.
In addition to the nociceptor potential of sensory nerves, a large body of literature has demonstrated the role of sensory nerves in osteoanabolic potential, as well as in regulating bone regeneration and formation. In the context of HO, our recent studies demonstrated that vascular smooth muscle cell- and pericyte-induced NGF-mediated axon innervation accompanied posttraumatic HO in a murine traumatic HO model9. Notably, in this study, surgical denervation blunted axonal invasion and reduced chondrogenesis and ectopic bone formation. Similarly, either NGF deletion or TrkA inhibition phenocopied impaired axonal ingrowth and heterotopic bone formation.
Vascular signals during the ectopic bone formation process
It has long been known that angiogenesis and vascularization make a remarkable contribution to ectopic bone formation. The representative angiogenic factor VEGFA, for instance, influences vascularity in the form of ectopic bone formation98,99. Although this is not fully understood, the activity of a tissue ischemia marker, hypoxia-inducible factor (HIF), induced by a local hypoxic microenvironment, initiates the pathogenesis of HO100,101, which induces the expression of VEGF, thereby enhancing angiogenesis, cartilage differentiation, and ultimately HO formation99,102,103. Furthermore, vascular histomorphometric analysis in human HO samples demonstrated that the pathophysiological processes of osteogenesis and angiogenesis are coupled in a time- and space-dependent manner104. Macrophage- and brown adipocyte-derived VEGF promoted the occurrence and pathogenesis of HO99,105. More recently, by using a murine posttraumatic HO model, our group demonstrated that traumatic HO is highly vascular and identified the critical role of HIF1α in traumatic HO102. Moreover, the release of VEGFA from mesenchymal cells drives HO formation106. The effects of angiogenic factors can be recapitulated in vivo with a transgenic mouse model of HO. Dilling et al. reported an increase in endothelial cells and a corresponding increase in VEGF mRNA expression in the area of HO, which preceded the inception of mesenchymal contribution and cartilaginous tissue formation99. This suggests that vascularization plays a pivotal role in MSC condensation and chondrogenesis during HO progression.
Neurovascular crosstalk and potential sources of HO progression
Although neurovascular coupling is well demonstrated during skeletal development, until recently, it has remained largely unknown in the context of HO. Our studies revealed that experimental models of HO are hyperinnervated, and sensory nerve innervation is an early and necessary feature for HO9. Furthermore, by using the same posttraumatic HO model, our group identified neural-derived angiogenic factors and demonstrated a unifying mechanism for neurovascular crosstalk in the context of HO106. Hwang et al. demonstrated that HO lesions are highly vascularized and that mesenchymal-derived VEGFA drives ectopic bone formation106. These findings implicate the interaction between nerves and blood vessels during HO formation. More recently, by using the traumatic HO model, we demonstrated that progressive neurovascular infiltration accompanies posttraumatic HO107. Qin et al. demonstrated progressive ingrowth in overall Tubb3+ nerve fibers and CD31+ blood vessels by 1883% and 3468%, respectively, over the early period (up to 3 weeks) of HO progression. These findings suggest that increased nerve fiber innervation mirrors the upregulation of endothelial vascularity during the early phase of HO development107. The authors further investigated neurovascular coupling by using sciatic neurectomy, which greatly inhibited axonal ingrowth and reduced vascular ingrowth into the injury site. This observation was phenocopied by independent mechanisms, and chemical or genetic inhibition of axonal ingrowth led to similar deficits in injury site angiogenesis, suggesting that neuronal perturbation impairs nerve and vessel infiltration and potential neurovascular crosstalk107. Single-cell transcriptomic analysis identified that vascular and perivascular cell clusters were present at the injury site, whereas surgical denervation led to a reduction in the proliferative index and a reduction in overall VEGF signaling activity in vascular and perivascular cell clusters107. Furthermore, a combination of single-cell transcriptomic approaches within the dorsal root ganglia identified key neural-derived angiogenic paracrine factors that may mediate neurovascular coupling107.
Reciprocally, the potential mutual impact of vessels on nerves during HO was investigated by the same group. By using a previously reported animal model, vascular ingrowth and HO were inhibited by conditional deletion of Vegfa in Prrx1-Cre animals106. The deletion of Vegfa had significant effects on vascular density, as expected. In addition, a significant reduction in TUBB3+ axons was also found within the HO site (unpublished data). Taken together, these findings demonstrate that it is plausible to assert that nerves and blood vessels are closely interrelated and promote ectopic bone formation interdependently.
Summary
Nerve and blood vessel ingrowth are spatially coordinated and functionally interdependent during skeletal development and regeneration. The mechanisms of neurovascular coupling are complex, and a variety of cells and molecules participate in neurovascular crosstalk. Many regulatory effects remain unknown. In this review, we discussed the role of nerves and blood vessels in the regulation of bone remodeling and repair and explored the interaction between nerves and vessels during physiological and pathological scenarios. Nerves densely innervate bone, and increased nerve density coincides with bone remodeling and regeneration11. Moreover, nerve innervation is normally followed by vascularization during bone fracture repair. In contrast, the inhibition of nerve signaling is accompanied by blunted revascularization and delayed fracture healing7. The secretion of VEGF by sensory nerves is the major factor influencing vascular growth and differentiation108. NGF-TrkA signaling promotes vascularization by inducing sensory innervation during embryonic development22. Furthermore, NGF leads to endothelial cell proliferation and inflammation109. NGF-expressing cells may play a key role in neurovascular coupling, which remains to be further investigated. On the other hand, VEGF promotes reinnervation and stimulates axonal outgrowth in the peripheral nervous system110. In this regard, further research on the neurotrophic activity of VEGF in bone remodeling and fracture healing is needed.
A better understanding of neurovascular coupling and underlying cellular and molecular drivers could advance the identification of novel targets for the treatment and clinical prevention of diseases such as heterotopic bone formation, osteoarthritis, and even bone tumors. Research focusing on neurovascular coupling during bone regeneration could also contribute to the development of novel differentiation factors and biomaterials for bone repair.
References
Frost, H. M. A determinant of bone architecture. The minimum effective strain. Clin. Orthop. Relat. Res. 175, 286–292 (1983).
Hauge, E. M., Qvesel, D., Eriksen, E. F., Mosekilde, L. & Melsen, F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J. Bone Miner. Res. 16, 1575–1582 (2001).
Mach, D. B. et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 113, 155–166 (2002).
Hara-Irie, F., Amizuka, N. & Ozawa, H. Immunohistochemical and ultrastructural localization of CGRP-positive nerve fibers at the epiphyseal trabecules facing the growth plate of rat femurs. Bone 18, 29–39 (1996).
Chenu, C. Role of innervation in the control of bone remodeling. J. Musculoskelet. Neuronal Interact. 4, 132–134 (2004).
Bajayo, A. et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc. Natl Acad. Sci. USA 109, 15455–15460 (2012).
Li, Z. et al. Fracture repair requires TrkA signaling by skeletal sensory nerves. J. Clin. Invest. 129, 5137–5150 (2019).
Meyers, C. A. et al. A neurotrophic mechanism directs sensory nerve transit in cranial bone. Cell Rep. 31, 107696 (2020).
Lee, S. et al. NGF-TrkA signaling dictates neural ingrowth and aberrant osteochondral differentiation after soft tissue trauma. Nat. Commun. 12, 4939 (2021).
Brazill, J. M., Beeve, A. T., Craft, C. S., Ivanusic, J. J. & Scheller, E. L. Nerves in bone: evolving concepts in pain and anabolism. J. Bone Miner. Res. 34, 1393–1406 (2019).
Tomlinson, R. E., Christiansen, B. A., Giannone, A. A. & Genetos, D. C. The role of nerves in skeletal development, adaptation, and aging. Front. Endocrinol. 11, 646 (2020).
Qiao, Y. et al. The role of nervous system in adaptive response of bone to mechanical loading. J. Cell Physiol. 234, 7771–7780 (2019).
Wan, Q. Q. et al. Crosstalk between bone and nerves within bone. Adv. Sci. 8, 2003390 (2021).
Ducy, P. et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100, 197–207 (2000).
Baldock, P. A. et al. Hypothalamic Y2 receptors regulate bone formation. J. Clin. Invest. 109, 915–921 (2002).
Kodama, D., Hirai, T., Kondo, H., Hamamura, K. & Togari, A. Bidirectional communication between sensory neurons and osteoblasts in an in vitro coculture system. FEBS Lett. 591, 527–539 (2017).
Togari, A. et al. Expression of mRNAs for neuropeptide receptors and beta-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci. Lett. 233, 125–128 (1997).
Togari, A., Mogi, M., Arai, M., Yamamoto, S. & Koshihara, Y. Expression of mRNA for axon guidance molecules, such as semaphorin-III, netrins and neurotrophins, in human osteoblasts and osteoclasts. Brain Res. 878, 204–209 (2000).
Huang, E. J. & Reichardt, L. F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 (2001).
Neufeld, G. et al. The semaphorins and their receptors as modulators of tumor progression. Drug Resist. Update 29, 1–12 (2016).
Hanoun, M., Maryanovich, M., Arnal-Estape, A. & Frenette, P. S. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron 86, 360–373 (2015).
Tomlinson, R. E. et al. NGF-TrkA signaling by sensory nerves coordinates the vascularization and ossification of developing endochondral bone. Cell Rep. 16, 2723–2735 (2016).
Tower, R. J. et al. Spatial transcriptomics reveals a role for sensory nerves in preserving cranial suture patency through modulation of BMP/TGF-beta signaling. Proc. Natl Acad. Sci. USA 118, e2103087118 (2021).
Parfitt, A. M. The mechanism of coupling: a role for the vasculature. Bone 26, 319–323 (2000).
Jilka, R. L. Biology of the basic multicellular unit and the pathophysiology of osteoporosis. Med. Pediatr. Oncol. 41, 182–185 (2003).
Stabley, J. N., Moningka, N. C., Behnke, B. J. & Delp, M. D. Exercise training augments regional bone and marrow blood flow during exercise. Med. Sci. Sports Exerc. 46, 2107–2112 (2014).
Chang, B. & Liu, X. Osteon: structure, turnover, and regeneration. Tissue Eng. Part B Rev. 28, 261–278 (2022).
Kumar, N., Saraber, P., Ding, Z. & Kusumbe, A. P. Diversity of vascular niches in bones and joints during homeostasis, ageing, and diseases. Front. Immunol. 12, 798211 (2021).
Lafage-Proust, M. H., Prisby, R., Roche, B. & Vico, L. Bone vascularization and remodeling. Jt. Bone Spine 77, 521–524 (2010).
Collin-Osdoby, P. Role of vascular endothelial cells in bone biology. J. Cell Biochem. 55, 304–309 (1994).
Colleran, P. N. et al. Alterations in skeletal perfusion with simulated microgravity: a possible mechanism for bone remodeling. J. Appl. Physiol. (1985) 89, 1046–1054 (2000).
Griffith, J. F. et al. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology 236, 945–951 (2005).
Tabarowski, Z., Gibson-Berry, K. & Felten, S. Y. Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem. 98, 453–457 (1996).
Bidegain, M., Roos, B. A., Hill, E. L. & Howard, G. A. & Balkan, W. Calcitonin gene-related peptide (CGRP) in the developing mouse limb. Endocr. Res. 21, 743–755 (1995).
Sisask, G., Silfversward, C. J., Bjurholm, A. & Nilsson, O. Ontogeny of sensory and autonomic nerves in the developing mouse skeleton. Auton. Neurosci. 177, 237–243 (2013).
Hill, E. L. & Elde, R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 264, 469–480 (1991).
McCorry, L. K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 71, 78 (2007).
Chen, M. et al. Skeleton-vasculature chain reaction: a novel insight into the mystery of homeostasis. Bone Res. 9, 21 (2021).
Ramasamy, S. K. Structure and functions of blood vessels and vascular niches in bone. Stem Cells Int. 2017, 5046953 (2017).
Ramasamy, S. K. et al. Blood flow controls bone vascular function and osteogenesis. Nat. Commun. 7, 13601 (2016).
Chartier, S. R., Mitchell, S. A. T., Majuta, L. A. & Mantyh, P. W. The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience 387, 178–190 (2018).
Castaneda-Corral, G. et al. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience 178, 196–207 (2011).
Edvinsson, L., Copeland, J. R., Emson, P. C., McCulloch, J. & Uddman, R. Nerve fibers containing neuropeptide Y in the cerebrovascular bed: immunocytochemistry, radioimmunoassay, and vasomotor effects. J. Cereb. Blood Flow. Metab. 7, 45–57 (1987).
Simoes, M. G. et al. Denervation impairs regeneration of amputated zebrafish fins. BMC Dev. Biol. 14, 49 (2014).
Ebendal, T. Function and evolution in the NGF family and its receptors. J. Neurosci. Res. 32, 461–470 (1992).
Mantyh, P. W. The neurobiology of skeletal pain. Eur. J. Neurosci. 39, 508–519 (2014).
Tomlinson, R. E. et al. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc. Natl Acad. Sci. USA 114, E3632–E3641 (2017).
Heffner, M. A., Anderson, M. J., Yeh, G. C., Genetos, D. C. & Christiansen, B. A. Altered bone development in a mouse model of peripheral sensory nerve inactivation. J. Musculoskelet. Neuronal Interact. 14, 1–9 (2014).
Madsen, J. E. et al. Fracture healing and callus innervation after peripheral nerve resection in rats. Clin. Orthop. Relat. Res. 351, 230–240 (1998).
Apel, P. J. et al. Effect of selective sensory denervation on fracture-healing: an experimental study of rats. J. Bone Jt. Surg. Am. 91, 2886–2895 (2009).
Garces, G. L. & Santandreu, M. E. Longitudinal bone growth after sciatic denervation in rats. J. Bone Jt. Surg. Br. 70, 315–318 (1988).
Hukkanen, M. et al. Rapid proliferation of calcitonin gene-related peptide-immunoreactive nerves during healing of rat tibial fracture suggests neural involvement in bone growth and remodelling. Neuroscience 54, 969–979 (1993).
Li, J. et al. Occurrence of substance P in bone repair under different load comparison of straight and angulated fracture in rat tibia. J. Orthop. Res. 28, 1643–1650 (2010).
Santavirta, S. et al. Immunologic studies of nonunited fractures. Acta Orthop. Scand. 63, 579–586 (1992).
Cao, J. et al. Sensory nerves affect bone regeneration in rabbit mandibular distraction osteogenesis. Int. J. Med. Sci. 16, 831–837 (2019).
Gu, X. C., Zhang, X. B., Hu, B., Zi, Y. & Li, M. Neuropeptide Y accelerates post-fracture bone healing by promoting osteogenesis of mesenchymal stem cells. Neuropeptides 60, 61–66 (2016).
Long, H., Ahmed, M., Ackermann, P., Stark, A. & Li, J. Neuropeptide Y innervation during fracture healing and remodeling. A study of angulated tibial fractures in the rat. Acta Orthop. 81, 639–646 (2010).
Sousa, D. M. et al. Neuropeptide Y modulates fracture healing through Y1 receptor signaling. J. Orthop. Res. 31, 1570–1578 (2013).
Niedermair, T., Straub, R. H., Brochhausen, C. & Grassel, S. Impact of the sensory and sympathetic nervous system on fracture healing in ovariectomized mice. Int. J. Mol. Sci. 21, 405 (2020).
Sullivan, M. P., Torres, S. J., Mehta, S. & Ahn, J. Heterotopic ossification after central nervous system trauma: a current review. Bone Jt. Res. 2, 51–57 (2013).
Wang, X. D. et al. The neural system regulates bone homeostasis via mesenchymal stem cells: a translational approach. Theranostics 10, 4839–4850 (2020).
Takeda, S. et al. Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 305–317 (2002).
Elefteriou, F. et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434, 514–520 (2005).
Sims, N. A. & Martin, T. J. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 3, 481 (2014).
Prisby, R. D. Mechanical, hormonal and metabolic influences on blood vessels, blood flow and bone. J. Endocrinol. 235, R77–R100 (2017).
Prisby, R. D. et al. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO Bioavailability in rats. J. Bone Miner. Res. 22, 1280–1288 (2007).
Stabley, J. N., Prisby, R. D., Behnke, B. J. & Delp, M. D. Type 2 diabetes alters bone and marrow blood flow and vascular control mechanisms in the ZDF rat. J. Endocrinol. 225, 47–58 (2015).
Dominguez, J. M., Prisby, R. D., Muller-Delp, J. M., Allen, M. R. & Delp, M. D. Increased nitric oxide-mediated vasodilation of bone resistance arteries is associated with increased trabecular bone volume after endurance training in rats. Bone 46, 813–819 (2010).
Corbett, S. A. et al. Nitric oxide in fracture repair. Differential localisation, expression and activity of nitric oxide synthases. J. Bone Jt. Surg. Br. 81, 531–537 (1999).
Gerber, H. P. et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 5, 623–628 (1999).
Peng, H. et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J. Clin. Invest. 110, 751–759 (2002).
Peng, H. R. et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J. Bone Miner. Res. 20, 2017–2027 (2005).
Kaigler, D., Wang, Z., Horger, K., Mooney, D. J. & Krebsbach, P. H. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J. Bone Miner. Res. 21, 735–744 (2006).
Busija, D. W., Bari, F., Domoki, F. & Louis, T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res. Rev. 56, 89–100 (2007).
Porter, J. T. & McCarthy, K. D. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J. Neurosci. 16, 5073–5081 (1996).
Phillips, A. A., Chan, F. H., Zheng, M. M., Krassioukov, A. V. & Ainslie, P. N. Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J. Cereb. Blood Flow. Metab. 36, 647–664 (2016).
Edmonds, M. E., Clarke, M. B., Newton, S., Barrett, J. & Watkins, P. J. Increased uptake of bone radiopharmaceutical in diabetic neuropathy. Q. J. Med. 57, 843–855 (1985).
Gao, X. et al. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature 589, 591–596 (2021).
Bohm, L., Helbing, D. L., Oraha, N. & Morrison, H. The peripheral nervous system in hematopoietic stem cell aging. Mech. Ageing Dev. 191, 111329 (2020).
Dickson, K., Katzman, S., Delgado, E. & Contreras, D. Delayed unions and nonunions of open tibial fractures. Correlation with arteriography results. Clin. Orthop. Relat. Res. 302, 189–193 (1994).
Hankenson, K. D., Dishowitz, M., Gray, C. & Schenker, M. Angiogenesis in bone regeneration. Injury 42, 556–561 (2011).
Glowacki, J. Angiogenesis in fracture repair. Clin. Orthop. Relat. Res. 355, S82–S89 (1998).
Graiani, G. et al. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice. Diabetologia 47, 1047–1054 (2004).
Vera, C., Tapia, V., Vega, M. & Romero, C. Role of nerve growth factor and its TRKA receptor in normal ovarian and epithelial ovarian cancer angiogenesis. J. Ovarian Res. 7, 82 (2014).
Ahluwalia, A., Jones, M. K., Brzozowski, T. & Tarnawski, A. S. Nerve growth factor is critical requirement for in vitro angiogenesis in gastric endothelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G981–G987 (2016).
Rosenstein, J. M., Mani, N., Khaibullina, A. & Krum, J. M. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J. Neurosci. 23, 11036–11044 (2003).
Cantarella, G. et al. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 16, 1307–1309 (2002).
Hong, H. S. et al. A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat. Med. 15, 425–435 (2009).
Fu, S. et al. Neuropeptide substance P improves osteoblastic and angiogenic differentiation capacity of bone marrow stem cells in vitro. Biomed. Res. Int. 2014, 596023 (2014).
Shi, L. et al. Vasoactive intestinal peptide stimulates bone marrow-mesenchymal stem cells osteogenesis differentiation by activating wnt/beta-catenin signaling pathway and promotes rat skull defect repair. Stem Cells Dev. 29, 655–666 (2020).
Fan, J. J., Mu, T. W., Qin, J. J., Bi, L. & Pei, G. X. Different effects of implanting sensory nerve or blood vessel on the vascularization, neurotization, and osteogenesis of tissue-engineered bone in vivo. Biomed. Res. Int. 2014, 412570 (2014).
Li, L. & Tuan, R. S. Mechanism of traumatic heterotopic ossification: In search of injury-induced osteogenic factors. J. Cell Mol. Med. 24, 11046–11055 (2020).
Sato, T., Watanabe, M., Onoda, Y., Oyanagi, T. & Kushimoto, S. Heterotopic ossification in a patient with paroxysmal sympathetic hyperactivity following multiple trauma complicated with vitamin D deficiency: a case report. Surg. Case Rep. 6, 293 (2020).
Forsberg, J. A. et al. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J. Bone Jt. Surg. Am. 91, 1084–1091 (2009).
Genet, F. et al. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J. Pathol. 236, 229–240 (2015).
Nguyen, V. et al. BMP-2-induced bone formation and neural inflammation. J. Orthop. 14, 252–256 (2017).
Salisbury, E. et al. Sensory nerve induced inflammation contributes to heterotopic ossification. J. Cell Biochem. 112, 2748–2758 (2011).
Hankenson, K. D., Gagne, K. & Shaughnessy, M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev. 94, 3–12 (2015).
Dilling, C. F. et al. Vessel Formation Is Induced Prior to the Appearance of Cartilage in BMP-2-Mediated Heterotopic Ossification. J. Bone Miner. Res. 25, 1147–1156 (2010).
Winkler, S. et al. The impact of hypoxia on mesenchymal progenitor cells of human skeletal tissue in the pathogenesis of heterotopic ossification. Int. Orthop. 39, 2495–2501 (2015).
Olmsted-Davis, E. et al. Hypoxic adipocytes pattern early heterotopic bone formation. Am. J. Pathol. 170, 620–632 (2007).
Agarwal, S. et al. Inhibition of Hif1 alpha prevents both trauma-induced and genetic heterotopic ossification. Proc. Natl Acad. Sci. USA 113, E338–E347 (2016).
Huang, Y., Wang, X. & Lin, H. The hypoxic microenvironment: a driving force for heterotopic ossification progression. Cell Commun. Signal. 18, 20 (2020).
Cocks, M. et al. Vascular patterning in human heterotopic ossification. Hum. Pathol. 63, 165–170 (2017).
Huang, Y. et al. Macrophages in heterotopic ossification: from mechanisms to therapy. NPJ Regen. Med. 6, 70 (2021).
Hwang, C. et al. Mesenchymal VEGFA induces aberrant differentiation in heterotopic ossification. Bone Res. 7, 36 (2019).
Qin, Q. et al. Neuron-to-vessel signaling is a required feature of aberrant stem cell commitment after soft tissue trauma. Bone Res. 10, 43 (2022).
Mukouyama, Y. S., Shin, D., Britsch, S., Taniguchi, M. & Anderson, D. J. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109, 693–705 (2002).
Raychaudhuri, S. K., Raychaudhuri, S. P., Weltman, H. & Farber, E. M. Effect of nerve growth factor on endothelial cell biology: proliferation and adherence molecule expression on human dermal microvascular endothelial cells. Arch. Dermatol. Res. 293, 291–295 (2001).
Sondell, M., Lundborg, G. & Kanje, M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J. Neurosci. 19, 5731–5740 (1999).
Acknowledgements
A.W.J. was funded by the NIH/NIAMS (R01 AR070773, R01 AR079171), NIH/NIDCR (R21 DE027922), USAMRAA through the Peer-Reviewed Medical Research Program (W81XWH-18-1-0121, W81XWH-18-1-0336, W81XWH-20-1-0302, W81XWH-20-1-0795) and Broad Agency Announcement (W81XWH-18-10613), the American Cancer Society (Research Scholar Grant, RSG-18-027-01-CSM), and the Maryland Stem Cell Research Foundation. B.L. was funded by the NIH/NIAMS (R01 AR078324-01) and USAMRAA through the Peer-Reviewed Medical Research Program (W81XWH2010795).
Author information
Authors and Affiliations
Contributions
All authors contributed to the development of this review, searched and reviewed the relevant literature, and wrote and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
A.W.J. is a paid consultant for Novadip and Lifesprout LLC. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict-of-interest policies. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, Q., Lee, S., Patel, N. et al. Neurovascular coupling in bone regeneration. Exp Mol Med 54, 1844–1849 (2022). https://doi.org/10.1038/s12276-022-00899-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-022-00899-6
This article is cited by
-
ALPL regulates pro-angiogenic capacity of mesenchymal stem cells through ATP-P2X7 axis controlled exosomes secretion
Journal of Nanobiotechnology (2024)
-
Neuro–bone tissue engineering: emerging mechanisms, potential strategies, and current challenges
Bone Research (2023)