Abstract
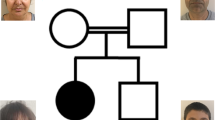
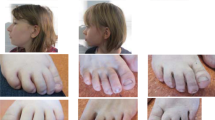
We describe five members of a consanguineous Pakistani family (Family I) plus two affected children from families of different ethnic origins presenting with neurodevelopmental disorders with overlapping features. All affected individuals from families have intellectual disability (ID), ranging from mild to profound, and reduced motor and cognitive skills plus variable features including short stature, microcephaly, developmental delay, hypotonia, dysarthria, deafness, visual problems, enuresis, encopresis, behavioural anomalies, delayed pubertal onset and facial dysmorphism. We first mapped the disease locus in the large family (Family I), and by exome sequencing identified homozygous ZNF407 c.2814_2816dup (p.Val939dup) in four affected members where DNA samples were available. By exome sequencing we detected homozygous c.2405G>T (p.Gly802Val) in the affected member of Family II and compound heterozygous variants c.2884C>G (p.Arg962Gly) and c.3642G>C (p.Lys1214Asn) in the affected member of Family III. Homozygous c.5054C>G (p.Ser1685Trp) has been reported in two brothers with an ID syndrome. Affected individuals we present did not exhibit synophrys, midface hypoplasia, kyphosis, 5th finger camptodactyly, short 4th metatarsals or limited knee mobility observed in the reported family.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data will be made available upon request.
References
Leonard H, Wen X. The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment Retard Dev Disabil Res Rev. 2002;8:117–34.
Ropers HH. Genetics of early onset cognitive impairment. Annu Rev Genom Hum Genet. 2010;11:161–87.
van Karnebeek CD, Jansweijer MC, Leenders AG, Offringa M, Hennekam RC. Diagnostic investigations in individuals with mental retardation: a systematic literature review of their usefulness. Eur J Hum Genet. 2005;13:6–25.
Mefford HC, Batshaw ML, Hoffman EP. Genomics, intellectual disability, and autism. N Engl J Med. 2012;366:733–43.
Association AP. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
Kambouris M, Maroun RC, Ben-Omran T, Al-Sarraj Y, Errafii K, Ali R, et al. Mutations in zinc finger 407 [ZNF407] cause a unique autosomal recessive cognitive impairment syndrome. Orphanet J Rare Dis. 2014;9:80.
Buchner DA, Charrier A, Srinivasan E, Wang L, Paulsen MT, Ljungman M, et al. Zinc finger protein 407 (ZFP407) regulates insulin-stimulated glucose uptake and glucose transporter 4 (Glut4) mRNA. J Biol Chem. 2015;290:6376–86.
Charrier A, Wang L, Stephenson EJ, Ghanta SV, Ko CW, Croniger CM, et al. Zinc finger protein 407 overexpression upregulates PPAR target gene expression and improves glucose homeostasis in mice. Am J Physiol Endocrinol Metab. 2016;311:E869–80.
Ren CM, Liang Y, Wei F, Zhang YN, Zhong SQ, Gu H, et al. Balanced translocation t(3;18)(p13;q22.3) and points mutation in the ZNF407 gene detected in patients with both moderate non-syndromic intellectual disability and autism. Biochim Biophys Acta. 2013;1832:431–8.
Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat. 2015;36:928–30.
Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–4.
Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;Chapter 7:Unit7.20.
Choi Y, Chan AP. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics.2015;31:2745–7.
Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–2.
Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019;47:D886–94.
Ioannidis NM, Rothstein JH, Pejaver V, Middha S, McDonnell SK, Baheti S, et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016;99:877–85.
Jagadeesh KA, Wenger AM, Berger MJ, Guturu H, Stenson PD, Cooper DN, et al. M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nat Genet. 2016;48:1581–6.
Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–81.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Cody JD, Hasi M, Soileau B, Heard P, Carter E, Sebold C, et al. Establishing a reference group for distal 18q-: clinical description and molecular basis. Hum Genet. 2014;133:199–209.
Yıldırım Y, Ouriachi T, Woehlbier U, Ouahioune W, Balkan M, Malik S, et al. Linked homozygous BMPR1B and PDHA2 variants in a consanguineous family with complex digit malformation and male infertility. Eur J Hum Genet. 2018;26:876–85.
Kelly AM, Shaw NJ, Thomas AM, Pynsent PB, Baker DJ. Growth of Pakistani children in relation to the 1990 growth standards. Arch Dis Child. 1997;77:401–5.
Chen WY, Lin YT, Chen Y, Chen KC, Kuo BI, Tsao PC. et al. Reference equations for predicting standing height of children by using arm span or forearm length as an index. J Chin Med Assoc. 2018;81:649–56.
Thakur R, Gautam RK. Pre and post pubertal growth difference among boys and girls of 5–18 years of age: a cross sectional study among Central Indian Population. Hum Biol Rev. 2017;6:164–87.
Acknowledgements
We thank the participants for their cooperation and Dr. Michelle Morrow for critical reading of the paper. This study was supported by the Scientific and Technological Research Council of Turkey (114Z829 to AT) and URF-QAU Pakistan (2014–2015 to SM).
Author information
Authors and Affiliations
Contributions
QZ, MS, SM, LK and JS performed clinical evaluations; ÇÇ, AT, MJGS and NS performed genetic studies and analysed data; MK performed bioinformatics analysis; AT, SM and MK wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
MJGS is an employee of GeneDx, Inc. Other authors declare that they have no conflicts of interest.
Ethical approval
The study protocol was approved by the Ethical Review Committee of Quaid-i-Azam University (DAS/12-HG-07) and the Istanbul Technical University Human Research Ethical Review Board (MBG.22/2014).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zahra, Q., Çakmak, Ç., Koprulu, M. et al. Biallelic ZNF407 mutations in a neurodevelopmental disorder with ID, short stature and variable microcephaly, hypotonia, ocular anomalies and facial dysmorphism. J Hum Genet 65, 1115–1123 (2020). https://doi.org/10.1038/s10038-020-0812-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-020-0812-0