Abstract
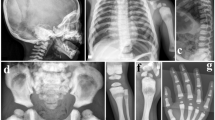
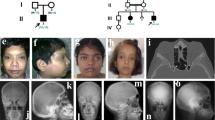
Dysosteosclerosis (DOS) is a rare sclerosing bone dysplasia characterized by osteosclerosis and platyspondyly. DOS is genetically heterogeneous and causally associated with mutations in three genes, SLC29A3, CSF1R, and TNFRSF11A. TNFRSF11A has been known as the causal gene for osteopetrosis, autosomal recessive 7, and is recently reported to cause DOS in three cases, which show a complex genotype-phenotype relationship. The phenotypic spectrum of TNFRSF11A-associated sclerosing bone dysplasia remains unclear and needs to be characterized further in more cases with molecular genetic diagnosis. Here, we report another TNFRSF11A-associated DOS case with a homozygous missense mutation (p.R129C). The mutation effect is different from the previous three cases, in which truncated or elongated RANK proteins were generated in isoform specific manner, thus enriching our understanding of the genotype-phenotype association in TNFRSF11A-associated sclerosing bone dysplasia. Besides DOS, our case presented with intracranial extramedullary hematopoiesis, which is an extremely rare condition and has not been identified in any other sclerosing bone dysplasias with molecular genetic diagnosis. Our findings provide the fourth case of TNFRSF11A-associated DOS and further expand its phenotypic spectrum.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Elçioglu NH, Vellodi A, Hall CM. Dysosteosclerosis: a report of three new cases and evolution of the radiological findings. J Med Genet. 2002;39:603–7.
Campeau PM, Lu JT, Sule G, Jiang MM, Bae Y, Madan S, et al. Whole-exome sequencing identifies mutations in the nucleoside transporter gene SLC29A3 in dysosteosclerosis, a form of osteopetrosis. Hum Mol Genet. 2012;21:4904–9.
Turan S, Mumm S, Gottesman GS, Abali S, Serpil B, Atay Z, et al. Dysosteosclerosis from a unique mutation in SLC29A3. Bone Abstr. 2015;4:97.
Howaldt A, Nampoothiri S, Quell LM, Ozden A, Fischer-Zirnsak B, Collet C, et al. Sclerosing bone dysplasias with hallmarks of dysosteosclerosis in four patients carrying mutations in SLC29A3 and TCIRG1. Bone. 2019;120:495–503.
Guo L, Bertola DR, Takanohashi A, Saito A, Segawa Y, Yokota T, et al. Bi-Allelic CSF1R mutations cause skeletal dysplasia of Dysosteosclerosis-Pyle disease spectrum and degenerative encephalopathy with brain malformation. Am J Hum Genet. 2019;104:925–35.
Guo L, Elcioglu NH, Karalar OK, Topkar MO, Wang Z, Sakamoto Y, et al. Dysosteosclerosis is also caused by TNFRSF11A mutation. J Hum Genet. 2018;63:769–74.
Xue JY, Wang Z, Shinagawa S, Ohashi H, Otomo N, Elcioglu NH, et al. TNFRSF11A-associated dysosteosclerosis: a report of the second case and characterization of the phenotypic spectrum. J Bone Min Res. 2019;34:1873–9.
Xue JY, Wang Z, Smithson SF, Burren CP, Matsumoto N, Nishimura G, et al. The third case of TNFRSF11A-associated dysosteosclerosis with a mutation producing elongating proteins. J Hum Genet. 2020;9:1–7.
Guerrini MM, Sobacchi C, Cassani B, Abinun M, Kilic SS, Pangrazio A, et al. Human osteoclast-poor osteopetrosis with hypogammaglobulinemia due to TNFRSF11A (RANK) mutations. Am J Hum Genet. 2008;83:64–76.
Pangrazio A, Cassani B, Guerrini MM, Crockett JC, Marrella V, Zammataro L, et al. RANK-dependent autosomal recessive osteopetrosis: characterization of five new cases with novel mutations. J Bone Min Res. 2012;27:342–51.
Sandeep S, Ravikanth R, Philip B. Magnetic resonance imaging findings in intracranial extramedullary hematopoiesis in myelofibrosis with myeloid metaplasia: a case report. Int J Adv Med. 2016;3:1074–6.
Haidar S, Ortiz-Neira C, Shroff M, Gilday D, Blaser S. Intracranial involvement in extramedullary hematopoiesis: case report and review of the literature. Pediatr Radiol. 2005;35:630–4.
Aljerdah SA. Case Report: intracranial Extra-Medullary Hematopoiesis in a Child with Osteopetrosis. Med J Cairo Univ. 2018;86:2507–10.
Liu C, Walter TS, Huang P, Zhang S, Zhu X, Wu Y, et al. Structural and functional insights of RANKL-RANK interaction and signaling. J Immunol. 2010;184:6910–9.
Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561:195–200.
Spranger JW, Brill PW, Hall C, Superti-Furga A, Unger S. Bone dysplasias: an atlas of genetic disorders of skeletal development. NewYork: Oxford University Press; 2018.
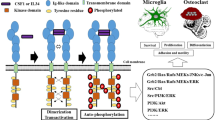
Xue JY, Ikegawa S, Guo L. Genetic disorders associated with the RANKL/OPG/RANK pathway. J Bone Min Metab. 2020;17:1–9.
Cook G, Sharp RA. Spinal cord compression due to extramedullary haemopoiesis in myelofibrosis. J Clin Pathol. 1994;47:464–5.
Acknowledgements
We thank Mrs Tomoko Kusadokoro for the technical support. This study is supported by grants from the Japan Society for the Promotion of Science (SI, No. 18H02932; NMi, No JP19H03621). Japan Agency For Medical Research and Development (SI, No. 20bm0804006h and 20ek0109486h; NMa, JP20ek0109486, JP20ek0109301, JP20ek0109348, and JP20kk0205012). CAMS Initiative Fund for Medical Sciences (ZW, 2016-I2M-3-003) and RIKEN Incentive Research Projects (ZW, 201801062228).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xue, JY., Simsek-Kiper, P.O., Utine, G.E. et al. Expanding the phenotypic spectrum of TNFRSF11A-associated dysosteosclerosis: a case with intracranial extramedullary hematopoiesis. J Hum Genet 66, 607–611 (2021). https://doi.org/10.1038/s10038-020-00891-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-020-00891-w
This article is cited by
-
From HDLS to BANDDOS: fast-expanding phenotypic spectrum of disorders caused by mutations in CSF1R
Journal of Human Genetics (2021)