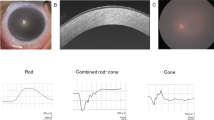
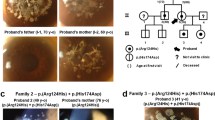
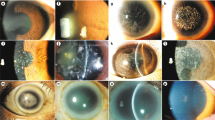
Abstract
Harboyan syndrome or corneal dystrophy and progressive deafness (MIM #217400) is characterized by congenital hereditary endothelial dystrophy (CHED) and progressive, sensorineural hearing loss. Mutations in SLC4A11 are responsible for this rare genetic syndrome. Eight patients from seven unrelated families affected with Harboyan Syndrome with mean follow-up of 12.0 ± 0.9 years were thoroughly investigated for the ocular, hearing, and kidney function abnormalities and the outcome of penetrating keratoplasty (PK). Mutation analysis of SLC4A11 was performed. All patients presented with bilateral cloudy corneas since birth. Sensorineural hearing loss was detected in all patients. Seven patients (11 eyes) underwent PK with the median age at surgery of 10.1 years (7.1–22.9). The overall corneal graft survival rate after primary PK was 72.7% (8/11 eyes). The mean graft survival time was 94.6 months (95% CI 83.1–126.0). All patients had unremarkable kidney function. The c.2264G>A (p.Arg755Gln) mutation in SCL4A11 was detected in most patients (87.5%). All unrelated Karen tribe patients had p.Arg755Gln mutation, suggestive of founder effect. We found the allele frequency of this variant in the Karen population to be 0.01. The c.2263C>T (p.Arg755Trp) mutation was found in one patient with mild phenotype and the novel truncating protein mutation c.2127delG (p.Gly710fsx*25) in SCL4A11 was identified in two Thai sisters. Visual outcome and graft survival after PK were satisfactory. Our study shows that all studied patients with SLC4A11 mutations had CHED and sensorineural hearing loss, and SLC4A11 mutations were not related to the onset and severity of hearing loss or outcome of keratoplasty.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Harboyan G, Mamo J, Kaloustian VD, Karam F. Congenital corneal dystrophy. Progressive sensorineural deafness in a family. Arch Ophthalmol. 1971;85:27–32.
Desir J, Abramowicz M. Congenital hereditary endothelial dystrophy with progressive sensorineural deafness (Harboyan syndrome). Orphanet J Rare Dis. 2008;3:28.
Weiss JS, Moller HU, Aldave AJ, Seitz B, Bredrup C, Kivela T, et al. IC3D classification of corneal dystrophies–edition 2. Cornea. 2015;34:117–59.
Mehta JS, Hemadevi B, Vithana EN, Arunkumar J, Srinivasan M, Prajna V, et al. Absence of phenotype-genotype correlation of patients expressing mutations in the SLC4A11 gene. Cornea. 2010;29:302–6.
Vithana EN, Morgan PE, Ramprasad V, Tan DT, Yong VH, Venkataraman D, et al. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum Mol Genet. 2008;17:656–66.
Pushkin A, Kurtz I. SLC4 base (HCO3−, CO32−) transporters: classification, function, structure, genetic diseases, and knockout models. Am J Physiol Ren Physiol. 2006;290:F580–99.
Vilas GL, Morgan PE, Loganathan SK, Quon A, Casey JR. A biochemical framework for SLC4A11, the plasma membrane protein defective in corneal dystrophies. Biochemistry. 2011;50:2157–69.
Parker MD, Ourmozdi EP, Tanner MJ. Human BTR1, a new bicarbonate transporter superfamily member and human AE4 from kidney. Biochem Biophys Res Commun. 2001;282:1103–9.
Malhotra D, Loganathan SK, Chiu AM, Lukowski CM, Casey JR. Human corneal expression of SLC4A11, a gene mutated in endothelial corneal dystrophies. Sci Rep. 2019;9:9681.
Jalimarada SS, Ogando DG, Vithana EN, Bonanno JA. Ion transport function of SLC4A11 in corneal endothelium. Invest Ophthalmol Vis Sci. 2013;54:4330–40.
Kao L, Azimov R, Shao XM, Frausto RF, Abuladze N, Newman D, et al. Multifunctional ion transport properties of human SLC4A11: comparison of the SLC4A11-B and SLC4A11-C variants. Am J Physiol Cell Physiol. 2016;311:C820–30.
Zhang W, Ogando DG, Bonanno JA, Obukhov AG. Human SLC4A11 Is a Novel NH3/H+ co-transporter. J Biol Chem. 2015;290:16894–905.
Loganathan SK, Schneider HP, Morgan PE, Deitmer JW, Casey JR. Functional assessment of SLC4A11, an integral membrane protein mutated in corneal dystrophies. Am J Physiol Cell Physiol. 2016;311:C735–48.
Vilas GL, Loganathan SK, Liu J, Riau AK, Young JD, Mehta JS, et al. Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum Mol Genet. 2013;22:4579–90.
Loganathan SK, Casey JR. Corneal dystrophy-causing SLC4A11 mutants: suitability for folding-correction therapy. Hum Mutat. 2014;35:1082–91.
Li S, Kim E, Ogando DG, Bonanno JA. Corneal endothelial pump coupling to lactic acid efflux in the rabbit and mouse. Invest Ophthalmol Vis Sci. 2020;61:7.
Malhotra D, Jung M, Fecher-Trost C, Lovatt M, Peh GSL, Noskov S, et al. Defective cell adhesion function of solute transporter, SLC4A11, in endothelial corneal dystrophies. Hum Mol Genet. 2020;29:97–116.
Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2136–46.
Groger N, Frohlich H, Maier H, Olbrich A, Kostin S, Braun T, et al. SLC4A11 prevents osmotic imbalance leading to corneal endothelial dystrophy, deafness, and polyuria. J Biol Chem. 2010;285:14467–74.
Han SB, Ang HP, Poh R, Chaurasia SS, Peh G, Liu J, et al. Mice with a targeted disruption of Slc4a11 model the progressive corneal changes of congenital hereditary endothelial dystrophy. Invest Ophthalmol Vis Sci. 2013;54:6179–89.
World Health Organization. Grades of hearing impairment. 1991. http://www.who.int/pbd/deafness/hearing_impairment_grades/en/. Accessed 18 Dec 2018.
Jiao X, Sultana A, Garg P, Ramamurthy B, Vemuganti GK, Gangopadhyay N, et al. Autosomal recessive corneal endothelial dystrophy (CHED2) is associated with mutations in SLC4A11. J Med Genet. 2007;44:64–8.
Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–5.
Arakawa T, Kobayashi-Yurugi T, Alguel Y, Iwanari H, Hatae H, Iwata M, et al. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science. 2015;350:680–4.
Badior KE, Alka K, Casey JR. SLC4A11 three-dimensional homology model rationalizes corneal dystrophy-causing mutations. Hum Mutat. 2017;38:279–88.
Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DT, Mohamed MD, et al. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nat Genet. 2006;38:755–7.
Ramprasad VL, Ebenezer ND, Aung T, Rajagopal R, Yong VH, Tuft SJ, et al. Novel SLC4A11 mutations in patients with recessive congenital hereditary endothelial dystrophy (CHED2). Mutation in brief #958. Online. Hum Mutat. 2007;28:522–3.
Sultana A, Garg P, Ramamurthy B, Vemuganti GK, Kannabiran C. Mutational spectrum of the SLC4A11 gene in autosomal recessive congenital hereditary endothelial dystrophy. Mol Vis. 2007;13:1327–32.
Hemadevi B, Veitia RA, Srinivasan M, Arunkumar J, Prajna NV, Lesaffre C, et al. Identification of mutations in the SLC4A11 gene in patients with recessive congenital hereditary endothelial dystrophy. Arch Ophthalmol. 2008;126:700–8.
Vilas GL, Loganathan SK, Quon A, Sundaresan P, Vithana EN, Casey J. Oligomerization of SLC4A11 protein and the severity of FECD and CHED2 corneal dystrophies caused by SLC4A11 mutations. Hum Mutat. 2012;33:419–28.
Alka K, Casey JR. Molecular phenotype of SLC4A11 missense mutants: Setting the stage for personalized medicine in corneal dystrophies. Hum Mutat. 2018;39:676–90.
Ellory JC, Guizouarn H, Borgese F, Bruce LJ, Wilkins RJ, Stewart GW. Review. Leaky Cl–HCO3- exchangers: cation fluxes via modified AE1. Philos Trans R Soc Lond Ser B, Biol Sci. 2009;364:189–94.
Liu J, Seet LF, Koh LW, Venkatraman A, Venkataraman D, Mohan RR, et al. Depletion of SLC4A11 causes cell death by apoptosis in an immortalized human corneal endothelial cell line. Invest Ophthalmol Vis Sci. 2012;53:3270–9.
Ogando DG, Choi M, Shyam R, Li S, Bonanno JA. Ammonia sensitive SLC4A11 mitochondrial uncoupling reduces glutamine induced oxidative stress. Redox Biol. 2019;26:101260.
Mullaney PB, Risco JM, Teichmann K, Millar L. Congenital hereditary endothelial dystrophy associated with glaucoma. Ophthalmology. 1995;102:186–92.
Ramamurthy B, Sachdeva V, Mandal AK, Vemuganti GK, Garg P, Sangwan VS. Coexistent congenital hereditary endothelial dystrophy and congenital glaucoma. Cornea. 2007;26:647–9.
Patel SP, Parker MD. SLC4A11 and the pathophysiology of congenital hereditary endothelial dystrophy. Biomed Res Int. 2015;2015:475392.
Lopez IA, Rosenblatt MI, Kim C, Galbraith GC, Jones SM, Kao L, et al. Slc4a11 gene disruption in mice: cellular targets of sensorineuronal abnormalities. J Biol Chem. 2009;284:26882–96.
Abramowicz MJ, Albuquerque-Silva J, Zanen A. Corneal dystrophy and perceptive deafness (Harboyan syndrome): CDPD1 maps to 20p13. J Med Genet. 2002;39:110–2.
Siddiqui S, Zenteno JC, Rice A, Chacon-Camacho O, Naylor SG, Rivera-de la Parra D, et al. Congenital hereditary endothelial dystrophy caused by SLC4A11 mutations progresses to Harboyan syndrome. Cornea. 2014;33:247–51.
Oghalai JS. The cochlear amplifier: augmentation of the traveling wave within the inner ear. Curr Opin Otolaryngol Head Neck Surg. 2004;12:431–8.
Liskova P, Dudakova L, Tesar V, Bednarova V, Kidorova J, Jirsova K, et al. Detailed assessment of renal function in a proband with Harboyan syndrome caused by a novel homozygous SLC4A11 nonsense mutation. Ophthalmic Res. 2015;53:30–5.
Ashar JN, Ramappa M, Vaddavalli PK. Paired-eye comparison of Descemet’s stripping endothelial keratoplasty and penetrating keratoplasty in children with congenital hereditary endothelial dystrophy. Br J Ophthalmol. 2013;97:1247–9.
Mittal V, Mittal R, Sangwan VS. Successful Descemet stripping endothelial keratoplasty in congenital hereditary endothelial dystrophy. Cornea. 2011;30:354–6.
AlArrayedh H, Collum L, Murphy CC. Outcomes of penetrating keratoplasty in congenital hereditary endothelial dystrophy. Br J Ophthalmol. 2018;102:19–25.
Tan DT, Janardhanan P, Zhou H, Chan YH, Htoon HM, Ang LP, et al. Penetrating keratoplasty in Asian eyes: the Singapore Corneal Transplant Study. Ophthalmology. 2008;115:975–82.e1.
Chaurasia S, Ramappa M, Annapurna M, Kannabiran C. Coexistence of congenital hereditary endothelial dystrophy and fuchs endothelial corneal dystrophy associated with SLC4A11 mutations in affected families. Cornea. 2020;39:354–7.
Acknowledgements
We thank our patients and their families for their kind cooperation and for allowing us to use their medical information for the benefit of others. This work was supported by The Thailand Research Fund; and the Faculties of Dentistry and Medicine, Chiang Mai University; and Genomics Thailand Research Grant of Health Systems Research Institute (HSRI) of Thailand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were reviewed and approved by the Research and Ethics Committee, Faculty of Medicine, Chiang Mai University (Study Code: OPT-2561-06011) and followed the tenets of the Declaration of Helsinki. Informed consent was obtained from the patients or their parents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tananuvat, N., Tananuvat, R., Chartapisak, W. et al. Harboyan syndrome: novel SLC4A11 mutation, clinical manifestations, and outcome of corneal transplantation. J Hum Genet 66, 193–203 (2021). https://doi.org/10.1038/s10038-020-00834-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-020-00834-5