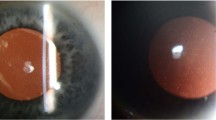
Abstract
Corneal dystrophies are broadly defined as inherited disorders that affect any layer of the cornea and are usually progressive, bilateral conditions that do not have systemic effects. The 2015 International Classification of Corneal Dystrophies classifies corneal dystrophies into four classes: epithelial and subepithelial dystrophies, epithelial–stromal TGFBI dystrophies, stromal dystrophies and endothelial dystrophies. Whereas some corneal dystrophies may result in few or mild symptoms and morbidity throughout a patient’s lifetime, others may progress and eventually result in substantial visual and ocular disturbances that require medical or surgical intervention. Corneal transplantation, either with full-thickness or partial-thickness donor tissue, may be indicated for patients with advanced corneal dystrophies. Although corneal transplantation techniques have improved considerably over the past two decades, these surgeries are still associated with postoperative risks of disease recurrence, graft failure and other complications that may result in blindness. In addition, a global shortage of cadaveric corneal graft tissue critically limits accessibility to corneal transplantation in some parts of the world. Ongoing advances in gene therapy, regenerative therapy and cell augmentation therapy may eventually result in the development of alternative, novel treatments for corneal dystrophies, which may substantially improve the quality of life of patients with these disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Qazi, Y., Wong, G., Monson, B., Stringham, J. & Ambati, B. K. Corneal transparency: genesis, maintenance and dysfunction. Brain Res. Bull. 81, 198–210 (2010).
Maurice, D. M. The structure and transparency of the cornea. J. Physiol. 136, 263–286 (1957).
DelMonte, D. W. & Kim, T. Anatomy and physiology of the cornea. J. Cataract Refract. Surg. 37, 588–598 (2011).
Weiss, J. S. Visual morbidity in thirty-four families with Schnyder crystalline corneal dystrophy (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 105, 616–648 (2007). The largest collection of SCD cases published to date.
Weiss, J. S. et al. IC3D classification of corneal dystrophies — edition 2. Cornea 34, 117–159 (2015). This manuscript describes the latest classification system for corneal dystrophies, published and endorsed by the Cornea Society.
Soh, Y. Q. et al. Predicative factors for corneal endothelial cell migration. Investig. Ophthalmol. Vis. Sci. 57, 338 (2016).
Soh, Y. Q. & Mehta, J. S. Regenerative therapy for Fuchs endothelial corneal dystrophy. Cornea 37, 523–527 (2018).
Bhogal, M., Lwin, C. N., Seah, X.-Y., Peh, G. & Mehta, J. S. Allogeneic Descemet’s membrane transplantation enhances corneal endothelial monolayer formation and restores functional integrity following Descemet’s stripping. Invest. Ophthalmol. Vis. Sci. 58, 4249–4260 (2017).
Peh, G. S. L. et al. Functional evaluation of two corneal endothelial cell-based therapies: tissue-engineered construct and cell injection. Sci. Rep. 9, 6087 (2019).
Kinoshita, S. et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N. Engl. J. Med. 378, 995–1003 (2018). First-in-human trial describing the successful treatment of bullous keratopathy, including cases of FECD, with intracameral injection of cultivated human corneal endothelial cells.
Yam, G. H.-F. et al. Safety and feasibility of intrastromal injection of cultivated human corneal stromal keratocytes as cell-based therapy for corneal opacities. Invest. Ophthalmol. Vis. Sci. 59, 3340–3354 (2018).
Peh, G. S. L. et al. Regulatory compliant tissue-engineered human corneal endothelial grafts restore corneal function of rabbits with bullous keratopathy. Sci. Rep. 7, 14149 (2017).
Mehta, J. S., Kocaba, V. & Soh, Y. Q. The future of keratoplasty: cell-based therapy, regenerative medicine, bioengineering keratoplasty, gene therapy. Curr. Opin. Ophthalmol. 30, 286–291 (2019).
Soh, Y. Q. et al. Trinucleotide repeat expansion length as a predictor of the clinical progression of Fuchs’ endothelial corneal dystrophy. PLoS One 14, e0210996 (2019).
Taketani, Y. et al. Repair of the TGFBI gene in human corneal keratocytes derived from a granular corneal dystrophy patient via CRISPR/Cas9-induced homology-directed repair. Sci. Rep. 7, 16713 (2017).
Boutboul, S. et al. A subset of patients with epithelial basement membrane corneal dystrophy have mutations in TGFBI/BIGH3. Hum. Mutat. 27, 553–557 (2006).
Reidy, J. J., Paulus, M. P. & Gona, S. Recurrent erosions of the cornea: epidemiology and treatment. Cornea 19, 767–771 (2000).
Suri, K. et al. Demographic patterns and treatment outcomes of patients with recurrent corneal erosions related to trauma and epithelial and Bowman layer disorders. Am. J. Ophthalmol. 156, 1082–1087.e2 (2013).
Waring, G. O., Rodrigues, M. M. & Laibson, P. R. Corneal dystrophies. I. Dystrophies of the epithelium, Bowman’s layer and stroma. Surv. Ophthalmol. 23, 71–122 (1978).
Werblin, T. P., Hirst, L. W., Stark, W. J. & Maumenee, I. H. Prevalence of map-dot-fingerprint changes in the cornea. Br. J. Ophthalmol. 65, 401–409 (1981).
Bozkurt, B. & Irkec, M. In vivo laser confocal microscopic findings in patients with epithelial basement membrane dystrophy. Eur. J. Ophthalmol. 19, 348–354 (2009).
Kaza, H., Barik, M. R., Reddy, M. M., Mittal, R. & Das, S. Gelatinous drop-like corneal dystrophy: a review. Br. J. Ophthalmol. 101, 10–15 (2017).
Fujiki, K., Nakayasu, K. & Kanai, A. Corneal dystrophies in Japan. J. Hum. Genet. 46, 431–435 (2001).
Kawasaki, S. & Kinoshita, S. Clinical and basic aspects of gelatinous drop-like corneal dystrophy. Dev. Ophthalmol. 48, 97–115 (2011).
Song, Y. et al. Prevalence of transforming growth factor β-induced gene corneal dystrophies in Chinese refractive surgery candidates. J. Cataract Refract. Surg. 43, 1489–1494 (2017).
Mashima, Y. et al. Association of autosomal dominantly inherited corneal dystrophies with BIGH3 gene mutations in Japan. Am. J. Ophthalmol. 130, 516–517 (2000).
Cho, K. J. et al. TGFBI gene mutations in a Korean population with corneal dystrophy. Mol. Vis. 18, 2012–2021 (2012).
Lee, J. H. et al. Prevalence of granular corneal dystrophy type 2 (Avellino corneal dystrophy) in the Korean population. Ophthalmic Epidemiol. 17, 160–165 (2010).
Musch, D. C., Niziol, L. M., Stein, J. D., Kamyar, R. M. & Sugar, A. Prevalence of corneal dystrophies in the united states: estimates from claims data. Invest. Ophthalmol. Vis. Sci. 52, 6959–6963 (2011).
Chao-Shern, C. et al. Evaluation of TGFBI corneal dystrophy and molecular diagnostic testing. Eye 33, 874–881 (2019).
Munier, F. L. et al. BIGH3 mutation spectrum in corneal dystrophies. Invest. Ophthalmol. Vis. Sci. 43, 949–954 (2002).
Han, K. E. et al. Pathogenesis and treatments of TGFBI corneal dystrophies. Prog. Retinal Eye Res. 50, 67–88 (2016).
Kheir, V., Cortés-González, V., Zenteno, J. C. & Schorderet, D. F. Mutation update: TGFBI pathogenic and likely pathogenic variants in corneal dystrophies. Hum. Mutat. 40, 675–693 (2019).
Munier, F. L. et al. Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nat. Genet. 15, 247–251 (1997).
Pampukha, V. M., Drozhyna, G. I. & Livshits, L. A. TGFBI gene mutation analysis in families with hereditary corneal dystrophies from Ukraine. OPH 218, 411–414 (2004).
Al-Arfai, K. M., Yassin, S. A., Al-Beshri, A. S., Al-Jindan, M. Y. & Al-Tamimi, E. R. Indications and techniques employed for keratoplasty in the Eastern province of Saudi Arabia: 6 years of experience. Ann. Saudi Med. 35, 387–393 (2015).
Eye Bank Association of America. 2018 Eye Banking Statistical Report 1–108 (Eye Bank Association of America, 2019).
Eye Bank Association of America. 2016 Eye Banking Statistical Report 1–99 (Eye Bank Association of America, 2016).
Saadat, M., Ansari-Lari, M. & Farhud, D. D. Consanguineous marriage in Iran. Ann. Hum. Biol. 31, 263–269 (2004).
Zare, M. et al. Changing indications and surgical techniques for corneal transplantation between 2004 and 2009 at a tertiary referral center. Middle East Afr. J. Ophthalmol. 19, 323–329 (2012).
Zare, M. et al. Indications for corneal transplantation at a tertiary referral center in Tehran. J. Ophthalmic Vis. Res. 5, 82–86 (2010).
Yaylacioglu Tuncay, F. et al. Genetic analysis of CHST6 and TGFBI in Turkish patients with corneal dystrophies: five novel variations in CHST6. Mol. Vis. 22, 1267–1279 (2016).
Warren, J. F. et al. Novel mutations in the CHST6 gene associated with macular corneal dystrophy in Southern India. Arch. Ophthalmol. 121, 1608–1612 (2003).
Sultana, A., Klintworth, G. K., Thonar, E. J.-M. A., Vemuganti, G. K. & Kannabiran, C. Immunophenotypes of macular corneal dystrophy in India and correlation with mutations in CHST6. Mol. Vis. 15, 319–325 (2009).
Jonasson, F., Johannsson, J. H., Garner, A. & Rice, N. S. Macular corneal dystrophy in Iceland. Eye 3 (Pt 4), 446–454 (1989).
Jonasson, F. et al. Macular corneal dystrophy in Iceland. A clinical, genealogic, and immunohistochemical study of 28 patients. Ophthalmology 103, 1111–1117 (1996).
Schnyder, W. F. Mitteilung über einen neuen typus von familiärer hornhauterkrankung [German]. Schweiz. Med. Wschr. 10, 559–571 (1929).
Schnyder, W. F. Scheibenförmige kristalleinlagerungen in der hornhautmitte als erbleiden [German]. KIin. Monatsbl. Augenheilkd. 103, 494–502 (1939).
Weiss, J. S. Schnyder’s dystrophy of the cornea. A Swede-Finn connection. Cornea 11, 93–101 (1992).
Nickerson, M. L. et al. The UBIAD1 prenyltransferase links menaquione-4 synthesis to cholesterol metabolic enzymes. Hum. Mutat. 34, 317–329 (2013).
Yamada, M., Mochizuki, H., Kamata, Y., Nakamura, Y. & Mashima, Y. Quantitative analysis of lipid deposits from Schnyder’s corneal dystrophy. Br. J. Ophthalmol. 82, 444–447 (1998).
Weiss, J. S. Schnyder corneal dystrophy. Curr. Opin. Ophthalmol. 20, 292–298 (2009).
Hung, C., Ayabe, R. I., Wang, C., Frausto, R. F. & Aldave, A. J. Pre-Descemet corneal dystrophy and X-linked ichthyosis associated with deletion of Xp22.31 containing the STS gene. Cornea 32, 1283–1287 (2013).
Costagliola, C., Fabbrocini, G., Illiano, G. M., Scibelli, G. & Delfino, M. Ocular findings in X-linked ichthyosis: a survey on 38 cases. Ophthalmologica 202, 152–155 (1991).
Soh, Y. Q., Peh, G. S. & Mehta, J. S. Evolving therapies for Fuchs’ endothelial dystrophy. Regen. Med. 13, 97–115 (2018).
Luther, M. et al. TGC repeats in Intron 2 of the TCF4 gene have a good predictive power regarding to Fuchs endothelial corneal dystrophy [German]. Klin. Monbl. Augenheilkd. 233, 187–194 (2016).
Afshari, N. A. et al. Genome-wide association study identifies three novel loci in Fuchs endothelial corneal dystrophy. Nat. Commun. 8, 14898 (2017).
Soh, Y. Q., Kocaba, V., Pinto, M. & Mehta, J. S. Fuchs endothelial corneal dystrophy and corneal endothelial diseases: East meets West. Eye 34, 427–441 (2020).
Liu, C. et al. Ultraviolet A light induces DNA damage and estrogen-DNA adducts in Fuchs endothelial corneal dystrophy causing females to be more affected. Proc. Natl Acad. Sci. USA 117, 573–583 (2020).
Jurkunas, U. V. Fuchs endothelial corneal dystrophy through the prism of oxidative stress. Cornea 37 (Suppl. 1), 50–54 (2018).
Zhang, X. et al. Association of smoking and other risk factors with Fuchs’ endothelial corneal dystrophy severity and corneal thickness. Invest. Ophthalmol. Vis. Sci. 54, 5829–5835 (2013).
Zoega, G. M. et al. Prevalence and risk factors for cornea guttata in the Reykjavik eye study. Ophthalmology 113, 565–569 (2006).
Krachmer, J. H., Purcell, J. J. Jr., Young, C. W. & Bucher, K. D. Corneal endothelial dystrophy. A study of 64 families. Arch. Ophthalmol. 96, 2036–2039 (1978).
Kitagawa, K. et al. Prevalence of primary cornea guttata and morphology of corneal endothelium in aging Japanese and Singaporean subjects. Ophthalmic Res. 34, 135–138 (2002).
Davidson, A. E. et al. Autosomal-dominant corneal endothelial dystrophies CHED1 and PPCD1 are allelic disorders caused by non-coding mutations in the promoter of OVOL2. Am. J. Hum. Genet. 98, 75–89 (2016).
Hong, T. et al. An Ovol2-Zeb1 mutual inhibitory circuit governs bidirectional and multi-step transition between epithelial and mesenchymal states. PLoS Computational Biol. 11, e1004569 (2015).
Biswas, S. et al. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum. Mol. Genet. 10, 2415–2423 (2001).
Kobayashi, A. et al. Analysis of COL8A2 gene mutation in Japanese patients with Fuchs’ endothelial dystrophy and posterior polymorphous dystrophy. Jpn. J. Ophthalmol. 48, 195–198 (2004).
Yellore, V. S. et al. No pathogenic mutations identified in the COL8A2 gene or four positional candidate genes in patients with posterior polymorphous corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 46, 1599–1603 (2005).
Frausto, R. F. et al. ZEB1 insufficiency causes corneal endothelial cell state transition and altered cellular processing. PLoS One 14, e0218279 (2019).
Liskova, P. et al. Ectopic GRHL2 expression due to non-coding mutations promotes cell state transition and causes posterior polymorphous corneal dystrophy 4. Am. J. Hum. Genet. 102, 447–459 (2018).
Chung, D. D. et al. Alterations in GRHL2-OVOL2-ZEB1 axis and aberrant activation of Wnt signaling lead to altered gene transcription in posterior polymorphous corneal dystrophy. Exp. Eye Res. 188, 107696 (2019).
Liskova, P. et al. High prevalence of posterior polymorphous corneal dystrophy in the Czech Republic; linkage disequilibrium mapping and dating an ancestral mutation. PLoS One 7, e45495 (2012).
Schmid, E. et al. A new, X-linked endothelial corneal dystrophy. Am. J. Ophthalmol. 141, 478–487 (2006).
Gipson, I. K., Spurr-Michaud, S. J. & Tisdale, A. S. Anchoring fibrils form a complex network in human and rabbit cornea. Invest. Ophthalmol. Vis. Sci. 28, 212–220 (1987).
Kabosova, A. et al. Compositional differences between infant and adult human corneal basement membranes. Invest. Ophthalmol. Vis. Sci. 48, 4989–4999 (2007).
Torricelli, A. A. M., Singh, V., Santhiago, M. R. & Wilson, S. E. The corneal epithelial basement membrane: structure, function, and disease. Invest. Ophthalmol. Vis. Sci. 54, 6390–6400 (2013).
Legrand, J. Dystrophie épithéliale cornéenne récidivante familiale [French]. Bull. Soc. Ophtalmol. 5, 384–387 (1963).
Remler, O. Hereditary recurrent erosion of the cornea [German]. Klin. Monatsbl. Augenheilkd. 183, 59 (1983).
Shindo, S. Familial recurrent corneal erosion [Japanese]. Nippon Ganka Gakkai Zasshi 72, 998–1004 (1968).
Wales, H. J. A family history of corneal erosions. Trans. Ophthalmol. Soc. N. Z. 8, 77–78 (1955).
Franceschetti, A. Hereditäre rezidivierende Erosion der Hornhaut. Z Augenheilk 66, 309–316 (1928).
Lisch, W. et al. Franceschetti hereditary recurrent corneal erosion. Am. J. Ophthalmol. 153, 1073–1081.e4 (2012).
Hammar, B., Björck, E., Lagerstedt, K., Dellby, A. & Fagerholm, P. A new corneal disease with recurrent erosive episodes and autosomal-dominant inheritance. Acta Ophthalmol. 86, 758–763 (2008).
Hammar, B. et al. Dystrophia Smolandiensis: a novel morphological picture of recurrent corneal erosions. Acta Ophthalmol. 88, 394–400 (2010).
Hammar, B. et al. Dystrophia Helsinglandica: a new type of hereditary corneal recurrent erosions with late subepithelial fibrosis. Acta Ophthalmol. 87, 659–665 (2009).
Neira, W. et al. Dystrophia Helsinglandica-corneal morphology, topography and sensitivity in a hereditary corneal disease with recurrent erosive episodes. Acta Ophthalmol. 88, 401–406 (2010).
Kuwabara, T. & Ciccarelli, E. C. Meesmann’s corneal dystrophy: a pathological study. Arch. Ophthalmol. 71, 676–682 (1964).
Irvine, A. D. et al. Mutations in cornea-specific keratin K3 or K12 genes cause Meesmann’s corneal dystrophy. Nat. Genet. 16, 184–187 (1997).
Allen, E. H. A. et al. Keratin 12 missense mutation induces the unfolded protein response and apoptosis in Meesmann epithelial corneal dystrophy. Hum. Mol. Genet. 25, 1176–1191 (2016).
McLean, W. H. I. & Moore, C. B. T. Keratin disorders: from gene to therapy. Hum. Mol. Genet. 20, R189–R197 (2011).
Lisch, W. & Weiss, J. S. Clinical and genetic update of corneal dystrophies. Exp. Eye Res. 186, 107715 (2019).
Lisch, W. et al. Lisch corneal dystrophy is genetically distinct from Meesmann corneal dystrophy and maps to Xp22.3. Am. J. Ophthalmol. 130, 461–468 (2000).
Jongkhajornpong, P. et al. Novel TACSTD2 mutation in gelatinous drop-like corneal dystrophy. Hum. Genome Var. 2, 15047 (2015).
Tsujikawa, M. et al. Identification of the gene responsible for gelatinous drop-like corneal dystrophy. Nat. Genet. 21, 420–423 (1999).
Kinoshita, S. et al. Epithelial barrier function and ultrastructure of gelatinous drop-like corneal dystrophy. Cornea 19, 551–555 (2000).
Nakatsukasa, M. et al. Tumor-associated calcium signal transducer 2 is required for the proper subcellular localization of claudin 1 and 7: implications in the pathogenesis of gelatinous drop-like corneal dystrophy. Am. J. Pathol. 177, 1344–1355 (2010).
Tsujikawa, M. Gelatinous drop-like corneal dystrophy. Cornea 31 (Suppl. 1), 37–40 (2012).
McDougall, A. R. A., Tolcos, M., Hooper, S. B., Cole, T. J. & Wallace, M. J. Trop2: from development to disease. Dev. Dyn. 244, 99–109 (2015).
Skonier, J. et al. cDNA cloning and sequence analysis of βig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-β. DNA Cell Biol. 11, 511–522 (1992).
Ma, C. et al. Extracellular matrix protein βig-h3/TGFBI promotes metastasis of colon cancer by enhancing cell extravasation. Genes. Dev. 22, 308–321 (2008).
Zajchowski, D. A. et al. Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res. 61, 5168–5178 (2001).
Park, S.-Y., Jung, M.-Y. & Kim, I.-S. Stabilin-2 mediates homophilic cell-cell interactions via its FAS1 domains. FEBS Lett. 583, 1375–1380 (2009).
Korvatska, E. et al. On the role of kerato-epithelin in the pathogenesis of 5q31-linked corneal dystrophies. Invest. Ophthalmol. Vis. Sci. 40, 2213–2219 (1999).
Selkoe, D. J. Presenilin, Notch, and the genesis and treatment of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 98, 11039–11041 (2001).
Huang, W.-J., Zhang, X. & Chen, W.-W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 4, 519–522 (2016).
Lim, K. L. et al. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J. Neurosci. 25, 2002–2009 (2005).
Chung, K. K. et al. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat. Med. 7, 1144–1150 (2001).
Scott, J. E. & Haigh, M. Identification of specific binding sites for keratan sulphate proteoglycans and chondroitin-dermatan sulphate proteoglycans on collagen fibrils in cornea by the use of cupromeronic blue in ‘critical-electrolyte-concentration’ techniques. Biochem. J. 253, 607–610 (1988).
Lewis, D. et al. Ultrastructural localization of sulfated and unsulfated keratan sulfate in normal and macular corneal dystrophy type I. Glycobiology 10, 305–312 (2000).
Zhang, J. et al. A comprehensive evaluation of 181 reported CHST6 variants in patients with macular corneal dystrophy. Aging 11, 1019–1029 (2019).
Musselmann, K. & Hassell, J. R. Focus on molecules: CHST6 (carbohydrate sulfotransferase 6; corneal N-acetylglucosamine-6-sulfotransferase). Exp. Eye Res. 83, 707–708 (2006).
Hassell, J. R., Newsome, D. A., Krachmer, J. H. & Rodrigues, M. M. Macular corneal dystrophy: failure to synthesize a mature keratan sulfate proteoglycan. Proc. Natl Acad. Sci USA 77, 3705–3709 (1980).
Aggarwal, S., Peck, T., Golen, J. & Karcioglu, Z. A. Macular corneal dystrophy: a review. Surv. Ophthalmol. 63, 609–617 (2018).
Klintworth, G. K. & Vogel, F. S. Macular corneal dystrophy. An inherited acid mucopolysaccharide storage disease of the corneal fibroblast. Am. J. Pathol. 45, 565–586 (1964).
Orr, A. et al. Mutations in the UBIAD1 gene, encoding a potential prenyltransferase, are causal for Schnyder crystalline corneal dystrophy. PLoS One 2, e685 (2007).
Nakagawa, K. et al. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 468, 117–121 (2010).
Jo, Y. et al. Schnyder corneal dystrophy-associated UBIAD1 inhibits ER-associated degradation of HMG CoA reductase in mice. Elife 8, e44396 (2019).
Weller, R. O. & Rodger, F. C. Crystalline stromal dystrophy: histochemistry and ultrastructure of the cornea. Br. J. Ophthalmol. 64, 46–52 (1980).
Hariri, M. et al. Biogenesis of multilamellar bodies via autophagy. Mol. Biol. Cell 11, 255–268 (2000).
Weiss, J. S. & Khemichian, A. J. Differential diagnosis of Schnyder corneal dystrophy. Dev. Ophthalmol. 48, 67–96 (2011).
Zhang, W. et al. Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment. Oncotarget 9, 5480–5491 (2018).
Mohan, R. R., Tovey, J. C. K., Gupta, R., Sharma, A. & Tandon, A. Decorin biology, expression, function and therapy in the cornea. Curr. Mol. Med. 11, 110–128 (2011).
Bredrup, C., Knappskog, P. M., Majewski, J., Rødahl, E. & Boman, H. Congenital stromal dystrophy of the cornea caused by a mutation in the decorin gene. Invest. Ophthalmol. Vis. Sci. 46, 420–426 (2005).
Kamma-Lorger, C. S. et al. Role of decorin core protein in collagen organisation in congenital stromal corneal dystrophy (CSCD). PLoS One 11, e0147948 (2016).
Nicholson, D. H., Green, W. R., Cross, H. E., Kenyon, K. R. & Massof, D. A clinical and histopathological study of François-Neetens speckled corneal dystrophy. Am. J. Ophthalmol. 83, 554–560 (1977).
Gee, J. A. et al. Identification of novel PIKFYVE gene mutations associated with Fleck corneal dystrophy. Mol. Vis. 21, 1093–1100 (2015).
Kawasaki, S. et al. A novel mutation (p.Glu1389AspfsX16) of the phosphoinositide kinase, FYVE finger containing gene found in a Japanese patient with fleck corneal dystrophy. Mol. Vis. 18, 2954–2960 (2012).
Li, S. et al. Mutations in PIP5K3 are associated with François-Neetens Mouchetée fleck corneal dystrophy. Am. J. Hum. Genet. 77, 54–63 (2005).
Kim, M. J. et al. Posterior amorphous corneal dystrophy is associated with a deletion of small leucine-rich proteoglycans on chromosome 12. PLoS One 9, e95037 (2014).
Aldave, A. J. et al. Linkage of posterior amorphous corneal dystrophy to chromosome 12q21.33 and exclusion of coding region mutations in KERA, LUM, DCN, and EPYC. Invest. Ophthalmol. Vis. Sci. 51, 4006–4012 (2010).
Fernandez-Sasso, D., Acosta, J. E. & Malbran, E. Punctiform and polychromatic pre-Descemet’s dominant corneal dystrophy. Br. J. Ophthalmol. 63, 336–338 (1979).
Alió Del Barrio, J. L. et al. Punctiform and polychromatic pre-Descemet corneal dystrophy: clinical evaluation and identification of the genetic basis. Am. J. Ophthalmol. 212, 88–97 (2020).
Henríquez-Recine, M. A. et al. Heredity and in vivo confocal microscopy of punctiform and polychromatic pre-Descemet dystrophy. Graefes Arch. Clin. Exp. Ophthalmol. 256, 1661–1667 (2018).
Curran, R. E., Kenyon, K. R. & Green, W. R. Pre-Descemet’s membrane corneal dystrophy. Am. J. Ophthalmol. 77, 711–716 (1974).
Grayson, M. & Wilbrandt, H. Pre-Descemet dystrophy. Am. J. Ophthalmol. 64, 276–282 (1967).
Alafaleq, M., Georgeon, C., Grieve, K. & Borderie, V. M. Multimodal imaging of pre-Descemet corneal dystrophy. Eur. J. Ophthalmol. https://doi.org/10.1177/1120672119862505 (2019).
Kempster, R. C., Hirst, L. W., Cruz de la, Z. & Green, W. R. Clinicopathologic study of the cornea in X-linked Ichthyosis. Arch. Ophthalmol. 115, 409–415 (1997).
Rudolf, M., Grösch, S. & Geerling, G. Recurrent bilateral corneal erosions and opacities in corneal stroma. Pre-Descemet dystrophy in X chromosome recessive ichthyosis [German]. Ophthalmologe 99, 962–963 (2002).
Tiepolo, L. et al. Assignment by deletion mapping of the steroid sulfatase X-linked ichthyosis locus to Xp223. Hum. Genet. 54, 205–206 (1980).
Diociaiuti, A. et al. X-linked ichthyosis: clinical and molecular findings in 35 Italian patients. Exp. Dermatol. 28, 1156–1163 (2019).
Mootha, V. V. et al. TCF4 triplet repeat expansion and nuclear RNA foci in Fuchs’ endothelial corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 56, 2003–2011 (2015).
Wieben, E. D. et al. Trinucleotide repeat expansion in the transcription factor 4 (TCF4) gene leads to widespread mRNA splicing changes in Fuchs’ endothelial corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 58, 343–352 (2017). The authors of this paper were the first to describe the strong association between a trinucleotide repeat expansion in TCF4 and FECD; in this paper, they describe the pathophysiological link between the repeat expansion sequence and disease phenotype.
Azizi, B. et al. p53-regulated increase in oxidative-stress-induced apoptosis in Fuchs endothelial corneal dystrophy: a native tissue model. Invest. Ophthalmol. Vis. Sci. 52, 9291–9297 (2011).
Jurkunas, U. V., Bitar, M. S., Funaki, T. & Azizi, B. Evidence of oxidative stress in the pathogenesis of Fuchs endothelial corneal dystrophy. Am. J. Pathol. 177, 2278–2289 (2010).
Halilovic, A. et al. Menadione-induced DNA damage leads to mitochondrial dysfunction and fragmentation during rosette formation in Fuchs endothelial corneal dystrophy. Antioxid. Redox Signal. 24, 1072–1083 (2016).
Benischke, A.-S. et al. Activation of mitophagy leads to decline in Mfn2 and loss of mitochondrial mass in Fuchs endothelial corneal dystrophy. Sci. Rep. 7, 6656 (2017).
Miyai, T. et al. Activation of PINK1-Parkin-mediated mitophagy degrades mitochondrial quality control proteins in Fuchs endothelial corneal dystrophy. Am. J. Pathol. 189, 2061–2076 (2019).
Matthaei, M. et al. Fuchs endothelial corneal dystrophy: clinical, genetic, pathophysiologic, and therapeutic aspects. Annu. Rev. Vis. Sci. 5, 151–175 (2019).
Kim, E. C. et al. Screening and characterization of drugs that protect corneal endothelial cells against unfolded protein response and oxidative stress. Invest. Ophthalmol. Vis. Sci. 58, 892–900 (2017).
Toyono, T. et al. MicroRNA-29b overexpression decreases extracellular matrix mRNA and protein production in human corneal endothelial cells. Cornea 35, 1466–1470 (2016).
Matthaei, M. et al. Transcript profile of cellular senescence-related genes in Fuchs endothelial corneal dystrophy. Exp. Eye Res. 129, 13–17 (2014).
Miyajima, T. et al. Loss of NQO1 generates genotoxic estrogen-DNA adducts in Fuchs endothelial corneal dystrophy. Free. Radic. Biol. Med. 147, 69–79 (2020).
Chaurasia, S., Mittal, R., Bichappa, G., Ramappa, M. & Murthy, S. I. Clinical characterization of posterior polymorphous corneal dystrophy in patients of Indian ethnicity. Int. Ophthalmol. 37, 945–952 (2017).
Shiraishi, A., Zheng, X., Sakane, Y., Hara, Y. & Hayashi, Y. In vivo confocal microscopic observations of eyes diagnosed with posterior corneal vesicles. Jpn. J. Ophthalmol. 60, 425–432 (2016).
Aldave, A. J., Han, J. & Frausto, R. F. Genetics of the corneal endothelial dystrophies: an evidence-based review. Clin. Genet. 84, 139–119 (2013).
Patel, S. P. & Parker, M. D. SLC4A11 and the pathophysiology of congenital hereditary endothelial dystrophy. Biomed. Res. Int. 2015, 475392 (2015).
Vilas, G. L. et al. Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum. Mol. Genet. 22, 4579–4590 (2013).
Lopez, I. A. et al. Slc4a11 gene disruption in mice: cellular targets of sensorineuronal abnormalities. J. Biol. Chem. 284, 26882–26896 (2009).
Desir, J. & Abramowicz, M. Congenital hereditary endothelial dystrophy with progressive sensorineural deafness (Harboyan syndrome). Orphanet J. Rare Dis. 3, 28 (2008).
Feder, R. S. et al. Subepithelial mucinous corneal dystrophy. Clinical and pathological correlations. Arch. Ophthalmol. 111, 1106–1114 (1993).
Pole, C. et al. High-resolution optical coherence tomography findings of Lisch epithelial corneal dystrophy. Cornea 35, 392–394 (2016).
Patel, D. V., Grupcheva, C. N. & McGhee, C. N. J. Imaging the microstructural abnormalities of Meesmann corneal dystrophy by in vivo confocal microscopy. Cornea 24, 669–673 (2005).
Klintworth, G. K. Corneal dystrophies. Orphanet J. Rare Dis. 4, 7 (2009).
Javadi, M.-A., Rezaei-Kanavi, M., Javadi, A. & Naghshgar, N. Meesmann corneal dystrophy; a clinico-pathologic, ultrastructural and confocal scan report. J. Ophthalmic Vis. Res. 5, 122–126 (2010).
Kurbanyan, K., Sejpal, K. D., Aldave, A. J. & Deng, S. X. In vivo confocal microscopic findings in Lisch corneal dystrophy. Cornea 31, 437 (2012).
Ide, T. et al. A spectrum of clinical manifestations of gelatinous drop-like corneal dystrophy in Japan. Am. J. Ophthalmol. 137, 1081–1084 (2004).
Kobayashi, A. & Sugiyama, K. In vivo laser confocal microscopy findings for Bowman’s layer dystrophies (Thiel-Behnke and Reis-Bücklers corneal dystrophies). Ophthalmology 114, 69–75 (2007).
Werner, L. P., Werner, L., Dighiero, P., Legeais, J. M. & Renard, G. Confocal microscopy in Bowman and stromal corneal dystrophies. Ophthalmology 106, 1697–1704 (1999).
Nowinska, A. K. et al. Comparative study of anterior eye segment measurements with spectral swept-source and time-domain optical coherence tomography in eyes with corneal dystrophies. Biomed. Res. Int. 2015, 805367 (2015).
Chaurasia, S., Ramappa, M. & Mishra, D. K. Clinical diversity in macular corneal dystrophy: an optical coherence tomography study. Int. Ophthalmol. 39, 2883–2888 (2019).
Rubinstein, Y. et al. Macular corneal dystrophy and posterior corneal abnormalities. Cornea 35, 1605–1610 (2016).
Sarosiak, A. et al. Clinical diversity in patients with Schnyder corneal dystrophy — a novel and known UBIAD1 pathogenic variants. Graefes Arch. Clin. Exp. Ophthalmol. 256, 2127–2134 (2018).
Lisch, W. et al. Schnyder’s dystrophy. Progression and metabolism. Ophthalmic Paediatr. Genet. 7, 45–56 (1986).
Weiss, J. S. et al. Genetic analysis of 14 families with Schnyder crystalline corneal dystrophy reveals clues to UBIAD1 protein function. Am. J. Med. Genet. A 146A, 271–283 (2008).
Lin, B. R. et al. Identification of the first de novo UBIAD1 gene mutation associated with Schnyder corneal dystrophy. J. Ophthalmol. 2016, 1968493 (2016).
Witschel, H., Fine, B. S., Grützner, P. & McTigue, J. W. Congenital hereditary stromal dystrophy of the cornea. Arch. Ophthalmol. 96, 1043–1051 (1978).
Jiao, X. et al. Genetic linkage of Francois-Neetens fleck (Mouchetée) corneal dystrophy to chromosome 2q35. Hum. Genet. 112, 593–599 (2003).
Akova, Y. A., Unlü, N. & Duman, S. Fleck dystrophy of the cornea; a report of cases from three generations of a family. Eur. J. Ophthalmol. 4, 123–125 (1994).
Moshegov, C. N., Hoe, W. K., Wiffen, S. J. & Daya, S. M. Posterior amorphous corneal dystrophy: a new pedigree with phenotypic variation. Ophthalmology 103, 474–478 (1996).
Kontadakis, G. A., Kymionis, G. D., Kankariya, V. P., Papadiamantis, A. G. & Pallikaris, A. I. Corneal confocal microscopy findings in sporadic cases of pre-Descemet corneal dystrophy. Eye Contact Lens 40, e8–e12 (2014).
Lagrou, L., Midgley, J. & Romanchuk, K. G. Punctiform and polychromatophilic dominant pre-Descemet corneal dystrophy. Cornea 35, 572–575 (2016).
Soh, Y. Q. & Mehta, J. S. Selective endothelial removal for Peters anomaly. Cornea 37, 382–385 (2018).
Acar, B. T., Bozkurt, K. T., Duman, E. & Acar, S. Bilateral cloudy cornea: is the usual suspect congenital hereditary endothelial dystrophy or stromal dystrophy? BMJ Case Rep. 2016, bcr2015214094 (2016).
Yu Chan, J. Y., Choy, B. N., Ng, A. L. & Shum, J. W. Review on the management of primary congenital glaucoma. J. Curr. Glaucoma Pract. 9, 92–99 (2015).
Wacker, K., McLaren, J. W., Amin, S. R., Baratz, K. H. & Patel, S. V. Corneal high-order aberrations and backscatter in Fuchs’ endothelial corneal dystrophy. Ophthalmology 122, 1645–1652 (2015).
Fritz, M. et al. Diurnal variation in corneal edema in Fuchs endothelial corneal dystrophy. Am. J. Ophthalmol. 207, 351–355 (2019).
Read, S. A. & Collins, M. J. Diurnal variation of corneal shape and thickness. Optom. Vis. Sci. 86, 170–180 (2009).
Soliman, A. Z., Xing, C., Radwan, S. H., Gong, X. & Mootha, V. V. Correlation of severity of Fuchs endothelial corneal dystrophy with triplet repeat expansion in TCF4. JAMA Ophthalmol. 133, 1386–1391 (2015).
Eghrari, A. O. et al. CTG18.1 expansion in TCF4 increases likelihood of transplantation in Fuchs corneal dystrophy. Cornea 36, 40–43 (2017).
Liskova, P., Filipec, M., Merjava, S., Jirsova, K. & Tuft, S. J. Variable ocular phenotypes of posterior polymorphous corneal dystrophy caused by mutations in the ZEB1 gene. Ophthalmic Genet. 31, 230–234 (2010).
Cibis, G. W., Krachmer, J. A., Phelps, C. D. & Weingeist, T. A. The clinical spectrum of posterior polymorphous dystrophy. Arch. Ophthalmol. 95, 1529–1537 (1977).
Lefebvre, V., Sowka, J. W. & Frauens, B. J. The clinical spectrum between posterior polymorphous dystrophy and iridocorneal endothelial syndromes. Optometry 80, 431–436 (2009).
Krachmer, J. H. Posterior polymorphous corneal dystrophy: a disease characterized by epithelial-like endothelial cells which influence management and prognosis. Trans. Am. Ophthalmol. Soc. 83, 413–475 (1985).
Liskova, P., Palos, M., Hardcastle, A. J. & Vincent, A. L. Further genetic and clinical insights of posterior polymorphous corneal dystrophy 3. JAMA Ophthalmol. 131, 1296–1303 (2013).
Ho, C. L. & Walton, D. S. Primary congenital glaucoma: 2004 update. J. Pediatr. Ophthalmol. Strabismus 41, 271–288 (2004).
Tan, Y.-L., Chua, J. & Ho, C.-L. Updates on the surgical management of pediatric glaucoma. Asia Pac. J. Ophthalmol. 5, 85–92 (2016).
Singh, R. P. et al. Alcohol delamination of the corneal epithelium for recalcitrant recurrent corneal erosion syndrome: a prospective study of efficacy and safety. Br. J. Ophthalmol. 91, 908–911 (2007).
Watson, S. L. & Leung, V. Interventions for recurrent corneal erosions. Cochrane Database Syst. Rev. 7, CD001861 (2018).
Lee, W.-S., Lam, C. K. & Manche, E. E. Phototherapeutic keratectomy for epithelial basement membrane dystrophy. Clin. Ophthalmol. 11, 15–22 (2016).
Jalbert, I. & Stapleton, F. Management of symptomatic Meesmann dystrophy. Optom. Vis. Sci. 86, E1202–E1206 (2009).
Bourne, W. M. Soft contact lens wear decreases epithelial microcysts in Meesmann’s corneal dystrophy. Trans. Am. Ophthalmol. Soc. 84, 170–182 (1986).
Zarei-Ghanavati, M. & Liu, C. Keratoprosthesis: current choices and future development. Asia Pac. J. Ophthalmol. 8, 429–431 (2019).
Lekhanont, K., Jongkhajornpong, P., Chuephanich, P., Inatomi, T. & Kinoshita, S. Boston type 1 keratoprosthesis for gelatinous drop-like corneal dystrophy. Optom. Vis. Sci. 93, 640–646 (2016).
Avadhanam, V., Messina, M., Said, D. G. & Dua, H. S. Alcohol delamination of corneal epithelium in recurrent granular dystrophy. Ophthalmology 123, 2050–2052 (2016).
Seitz, B. & Lisch, W. Stage-related therapy of corneal dystrophies. Dev. Ophthalmol. 48, 116–153 (2011).
Dinh, R., Rapuano, C. J., Cohen, E. J. & Laibson, P. R. Recurrence of corneal dystrophy after excimer laser phototherapeutic keratectomy. Ophthalmology 106, 1490–1497 (1999).
Stewart, O. G., Pararajasegaram, P., Cazabon, J. & Morrell, A. J. Visual and symptomatic outcome of excimer phototherapeutic keratectomy (PTK) for corneal dystrophies. Eye 16, 126–131 (2002).
Reddy, J. C., Rapuano, C. J., Hammersmith, K. M. & Nagra, P. K. Clinical outcomes of surgical intervention for stromal corneal dystrophies. Invest. Ophthalmol. Vis. Sci. 53, 6052–6052 (2012).
Lewis, D. R., Price, M. O., Feng, M. T. & Price, F. W. Jr. Recurrence of granular corneal dystrophy type 1 after phototherapeutic keratectomy, lamellar keratoplasty, and penetrating keratoplasty in a single population. Cornea 36, 1227–1232 (2017).
Marcon, A. S., Cohen, E. J., Rapuano, C. J. & Laibson, P. R. Recurrence of corneal stromal dystrophies after penetrating keratoplasty. Cornea 22, 19–21 (2003).
Küchle, M., Green, W. R., Völcker, H. E. & Barraquer, J. Reevaluation of corneal dystrophies of Bowman’s layer and the anterior stroma (Reis-Bücklers and Thiel-Behnke types): a light and electron microscopic study of eight corneas and a review of the literature. Cornea 14, 333–354 (1995).
Reddy, J. C. et al. Clinical outcomes and risk factors for graft failure after deep anterior lamellar keratoplasty and penetrating keratoplasty for macular corneal dystrophy. Cornea 34, 171–176 (2015).
Köksal, M., Kargi, S., Gürelik, G. & Akata, F. Phototherapeutic keratectomy in Schnyder crystalline corneal dystrophy. Cornea 23, 311–313 (2004).
Paparo, L. G. et al. Phototherapeutic keratectomy for Schnyder’s crystalline corneal dystrophy. Cornea 19, 343–347 (2000).
Freddo, T. F., Polack, F. M. & Leibowitz, H. M. Ultrastructural changes in the posterior layers of the cornea in Schnyder’s crystalline dystrophy. Cornea 8, 170–177 (1989).
Mehta, J. S. et al. Surgical management and genetic analysis of a Chinese family with the S171P mutation in the UBIAD1 gene, the gene for Schnyder corneal dystrophy. Br. J. Ophthalmol. 93, 926–931 (2009).
Zhu, A. Y., Marquezan, M. C., Kraus, C. L. & Prescott, C. R. Pediatric corneal transplants: review of current practice patterns. Cornea 37, 973–980 (2018).
Trief, D., Marquezan, M. C., Rapuano, C. J. & Prescott, C. R. Pediatric corneal transplants. Curr. Opin. Ophthalmol. 28, 477–484 (2017).
Eghrari, A. O. et al. Automated retroillumination photography analysis for objective assessment of Fuchs corneal dystrophy. Cornea 36, 44–47 (2017).
AlArrayedh, H., Collum, L. & Murphy, C. C. Outcomes of penetrating keratoplasty in congenital hereditary endothelial dystrophy. Br. J. Ophthalmol. 102, 19–25 (2018).
Özdemir, B. et al. Penetrating keratoplasty in congenital hereditary endothelial dystrophy. Cornea 31, 359–365 (2012).
Schaumberg, D. A., Moyes, A. L., Gomes, J. A. & Dana, M. R. Corneal transplantation in young children with congenital hereditary endothelial dystrophy. Multicenter Pediatric Keratoplasty Study. Am. J. Ophthalmol. 127, 373–378 (1999).
Mohebbi, M., Nabavi, A., Fadakar, K. & Hashemi, H. Outcomes of Descemet-stripping automated endothelial keratoplasty in congenital hereditary endothelial dystrophy. Eye Contact Lens 46, 57–62 (2020).
Madi, S., Santorum, P. & Busin, M. Descemet stripping automated endothelial keratoplasty in pediatric age group. Saudi J. Ophthalmol. 26, 309–313 (2012).
Ashar, J. N., Ramappa, M. & Vaddavalli, P. K. Paired-eye comparison of Descemet’s stripping endothelial keratoplasty and penetrating keratoplasty in children with congenital hereditary endothelial dystrophy. Br. J. Ophthalmol. 97, 1247–1249 (2013).
Ashar, J. N., Madhavi Latha, K. & Vaddavalli, P. K. Descemet’s stripping endothelial keratoplasty (DSEK) for children with congenital hereditary endothelial dystrophy: surgical challenges and 1-year outcomes. Graefes Arch. Clin. Exp. Ophthalmol. 250, 1341–1345 (2012).
Yang, F. et al. Descemet stripping endothelial keratoplasty in pediatric patients with congenital hereditary endothelial dystrophy. Am. J. Ophthalmol. 209, 132–140 (2020).
Anwar, H. M. & El-Danasoury, A. Endothelial keratoplasty in children. Curr. Opin. Ophthalmol. 25, 340–346 (2014).
Quantock, A. J., Nishida, K. & Kinoshita, S. Histopathology of recurrent gelatinous drop-like corneal dystrophy. Cornea 17, 215–221 (1998).
Ang, M., Soh, Y., Htoon, H. M., Mehta, J. S. & Tan, D. Five-year graft survival comparing Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology 123, 1646–1652 (2016).
Venkatraman, A. et al. Effect of osmolytes on in-vitro aggregation properties of peptides derived from TGFBIp. Sci. Rep. 10, 4011 (2020).
Courtney, D. G. et al. Development of allele-specific gene-silencing siRNAs for TGFBI Arg124Cys in lattice corneal dystrophy type I. Invest. Ophthalmol. Vis. Sci. 55, 977–985 (2014).
Yuan, C., Zins, E. J., Clark, A. F. & Huang, A. J. W. Suppression of keratoepithelin and myocilin by small interfering RNAs (siRNA) in vitro. Mol. Vis. 13, 2083–2095 (2007).
Christie, K. A. et al. Towards personalised allele-specific CRISPR gene editing to treat autosomal dominant disorders. Sci. Rep. 7, 16174 (2017).
Kim, E. K., Kim, S. & Maeng, Y.-S. Generation of TGFBI knockout ABCG2+/ABCB5+ double-positive limbal epithelial stem cells by CRISPR/Cas9-mediated genome editing. PLoS One 14, e0211864 (2019).
Courtney, D. G. et al. siRNA silencing of the mutant keratin 12 allele in corneal limbal epithelial cells grown from patients with Meesmann’s epithelial corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 55, 3352–3360 (2014).
Liao, H. et al. Development of allele-specific therapeutic siRNA in Meesmann epithelial corneal dystrophy. PLoS One 6, e28582 (2011).
Szabó, D. J. et al. Ex vivo 3D human corneal stroma model for Schnyder corneal dystrophy - role of autophagy in its pathogenesis and resolution. Histol. Histopathol. 33, 455–462 (2018).
Peh, G. S. L. et al. Propagation of human corneal endothelial cells: a novel dual media approach. Cell Transpl. 24, 287–304 (2015). One of the first published methods to reliably culture human corneal endothelial cells at significant scale while minimizing the loss of endothelial cell properties during culture.
Peh, G. S. L., Beuerman, R. W., Colman, A., Tan, D. T. & Mehta, J. S. Human corneal endothelial cell expansion for corneal endothelium transplantation: an overview. Transplantation 91, 811–819 (2011).
Wahlig, S., Kocaba, V. & Mehta, J. S. Cultured cells and ROCK inhibitor for bullous keratopathy. N. Engl. J. Med. 379, 1184 (2018).
Zarouchlioti, C. et al. Antisense therapy for a common corneal dystrophy ameliorates TCF4 repeat expansion-mediated toxicity. Am. J. Hum. Genet. 102, 528–539 (2018).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Feng, Z. et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 23, 1229–1232 (2013). This article and that of Jinek et al. are pioneering publications describing the potential use of the CRISPR–Cas9 platform for human gene therapy.
Acknowledgements
The authors thank W. Y. Ng, F. L. Lian, V. Phua and K. Sandhanam from the Singapore National Eye Centre for their help in obtaining the clinical images used in this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to all sections of the Primer, with J.S.M. coordinating the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Disease Primers thanks C. Rapuano, S. Tuft and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soh, Y.Q., Kocaba, V., Weiss, J.S. et al. Corneal dystrophies. Nat Rev Dis Primers 6, 46 (2020). https://doi.org/10.1038/s41572-020-0178-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-020-0178-9